Assessment of Functional Recovery and Subjective Donor-Site Morbidity Following Radial Forearm Flap Reconstruction in Small- to Moderate-Sized Palatal Defects
Abstract
:Introduction
Patients and Methods
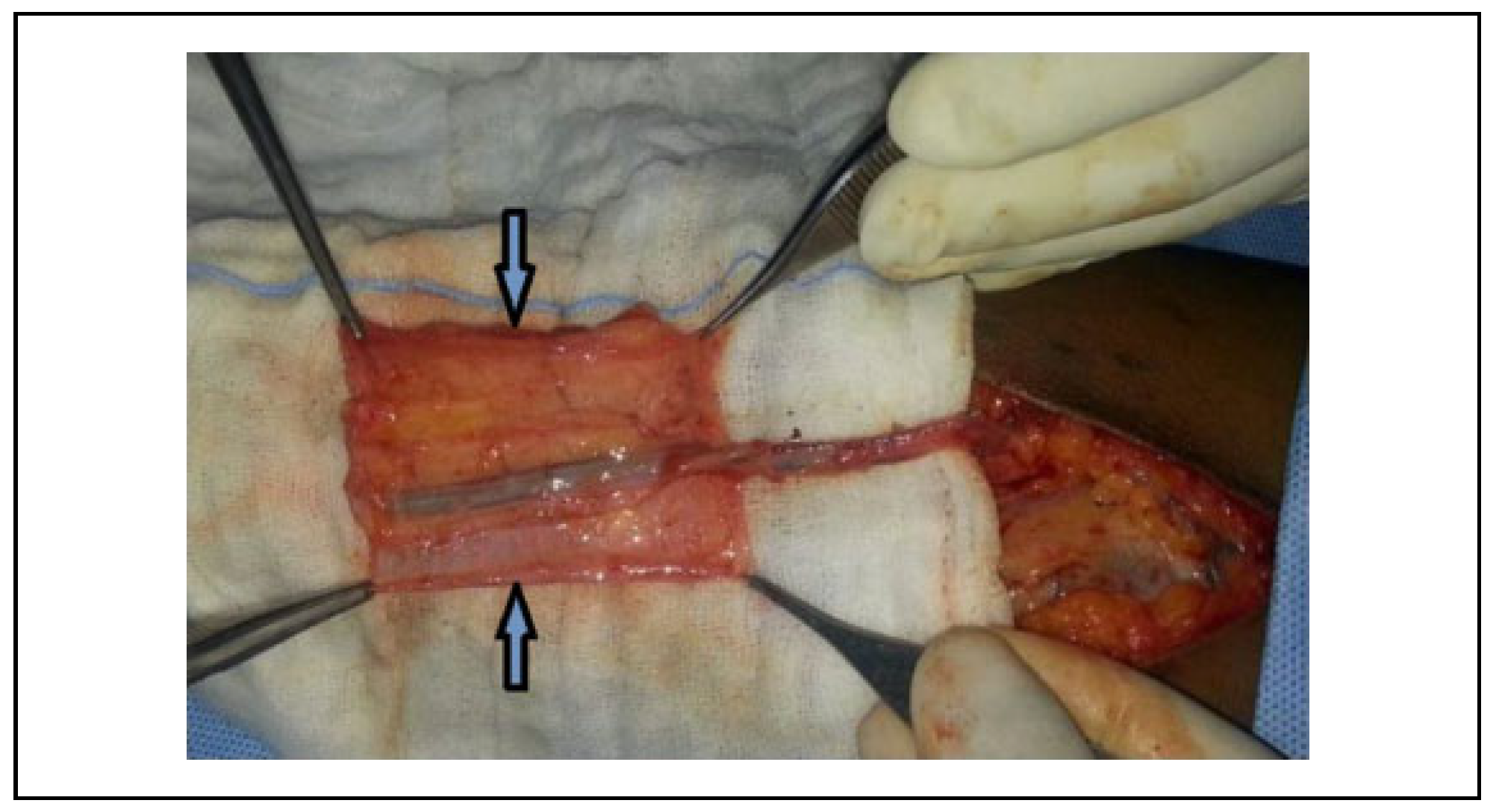
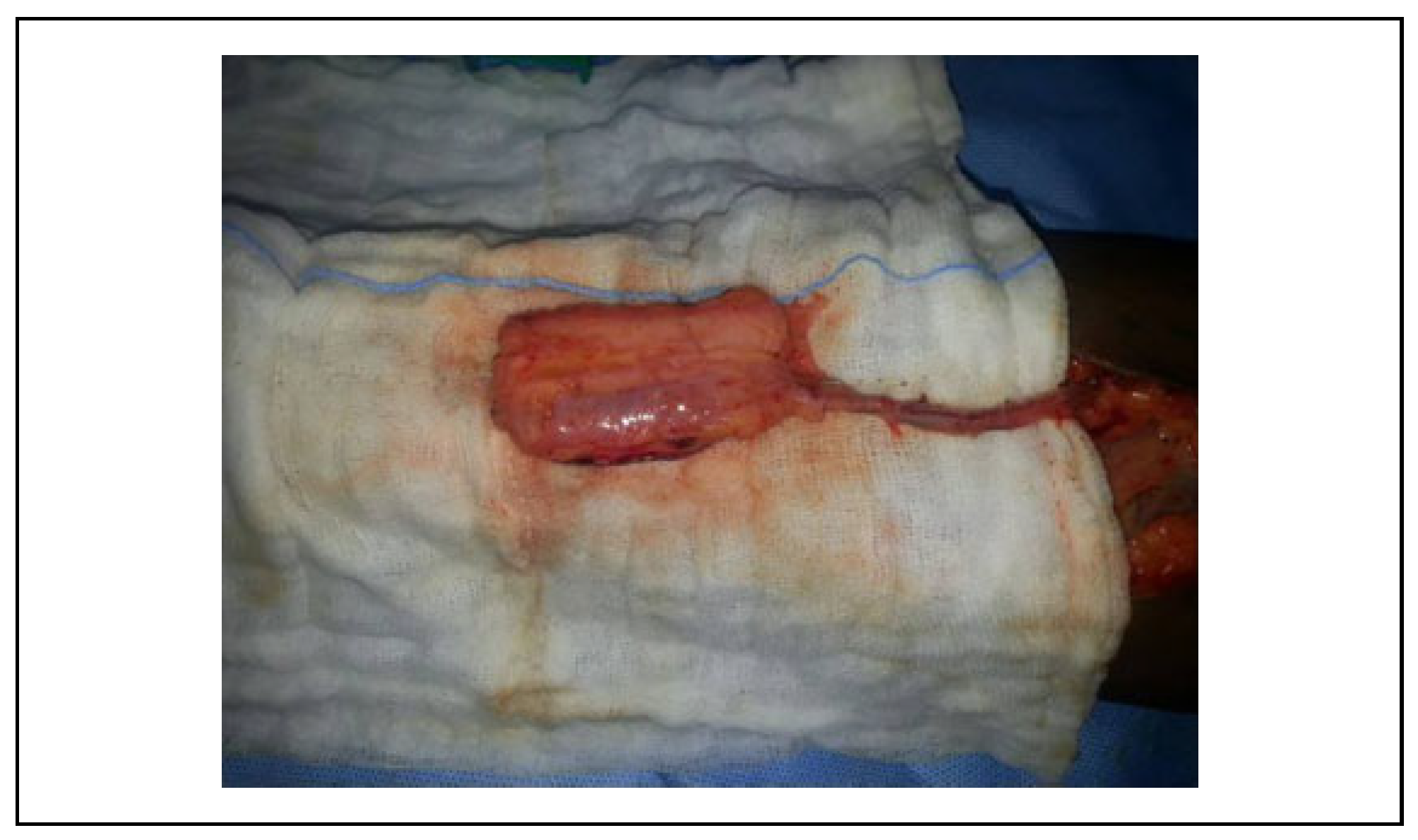
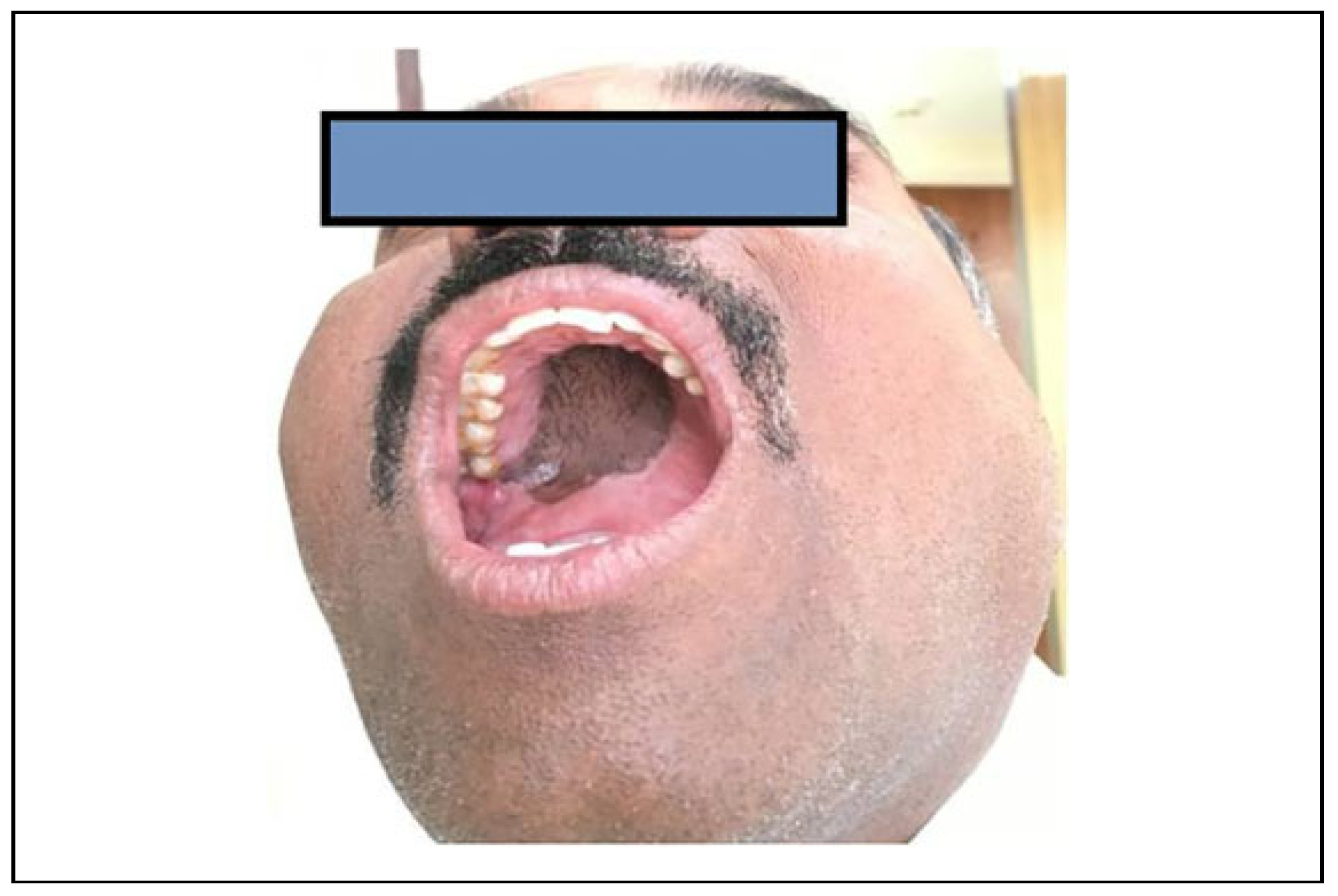
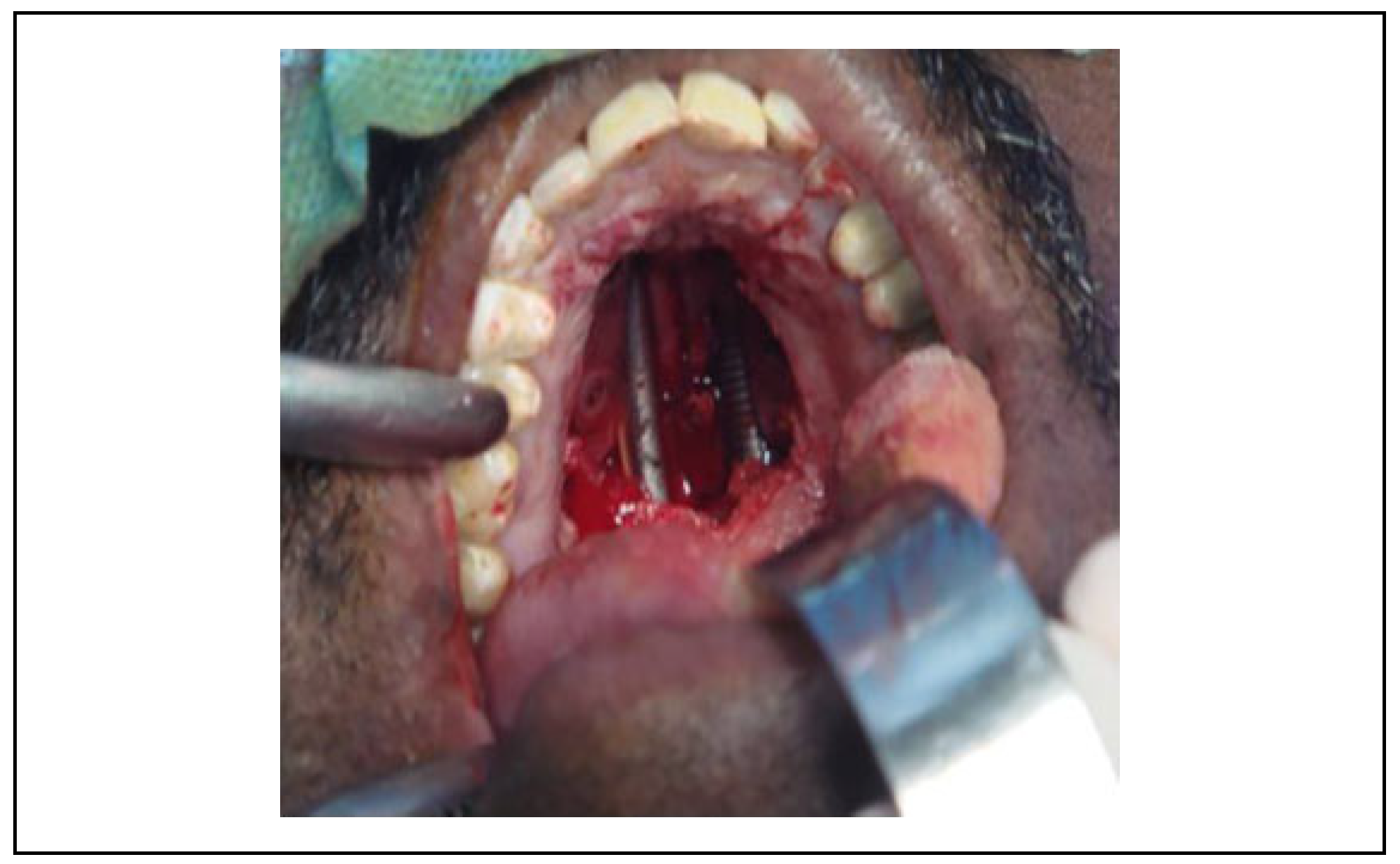
Surgical Technique
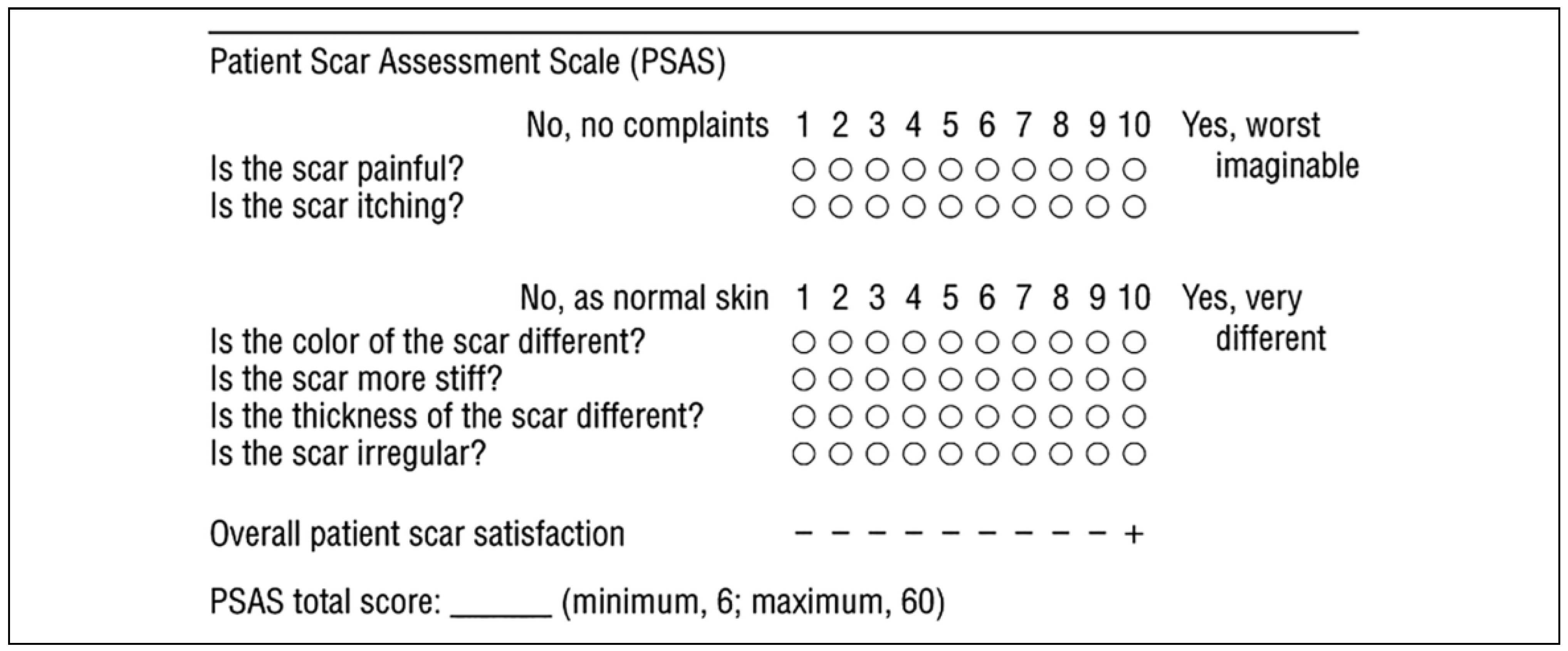
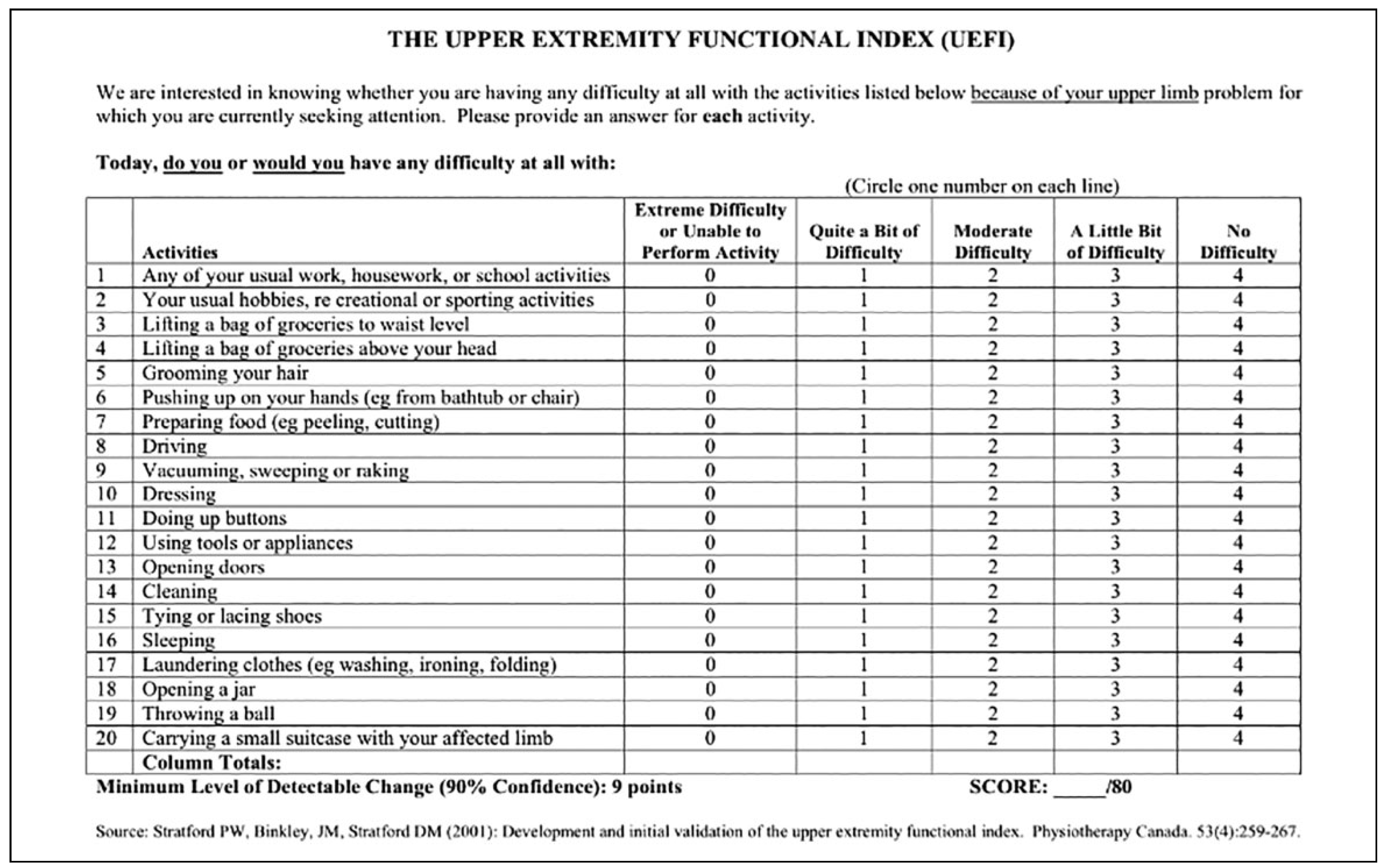
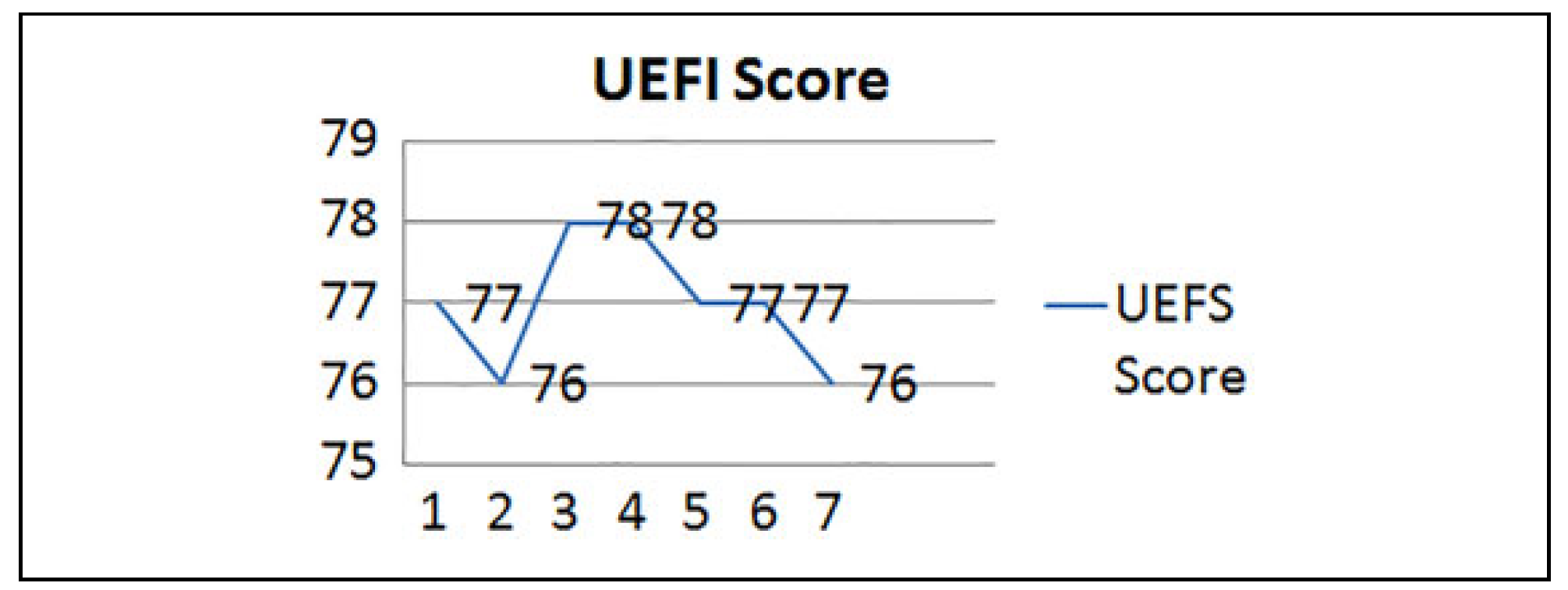
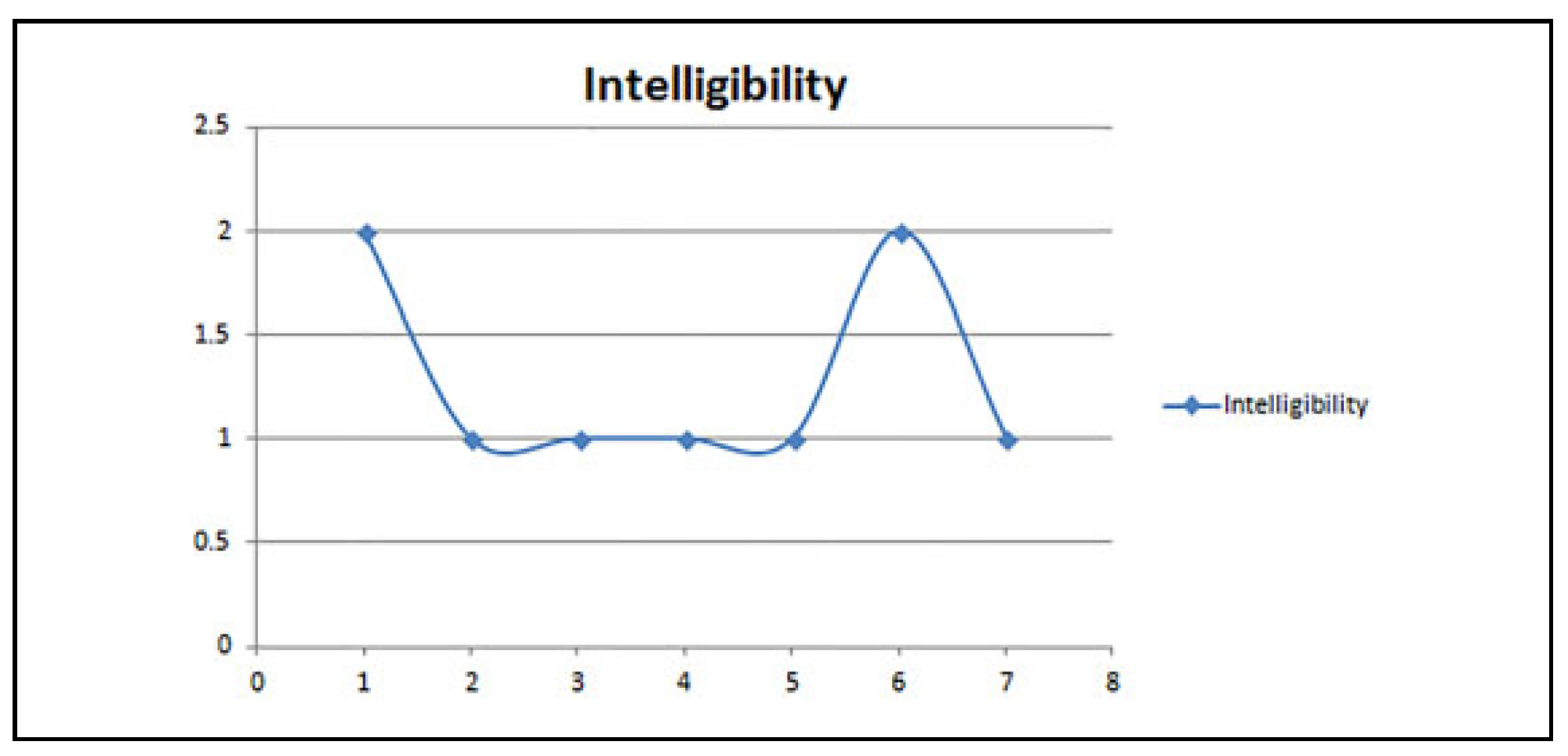
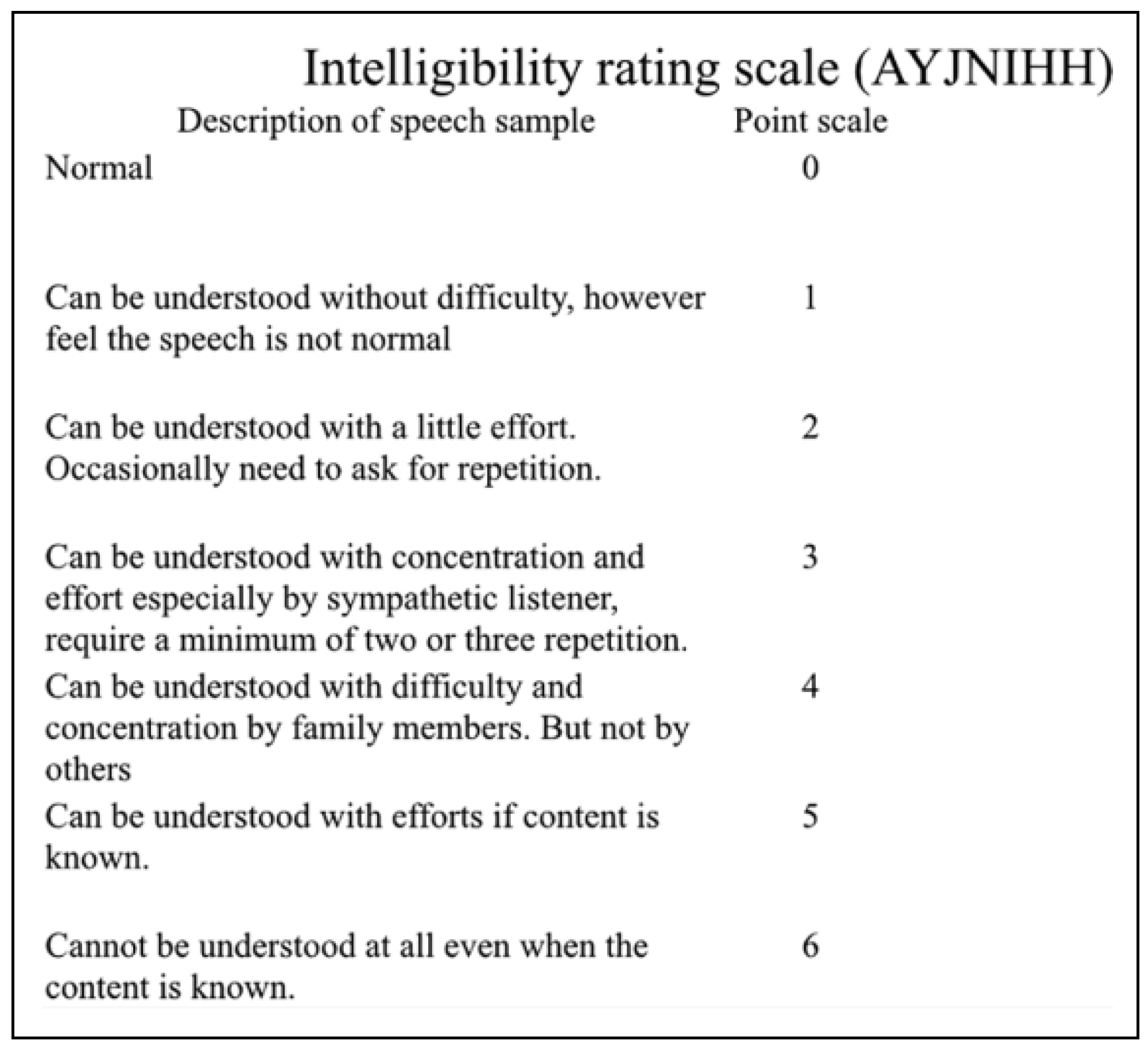
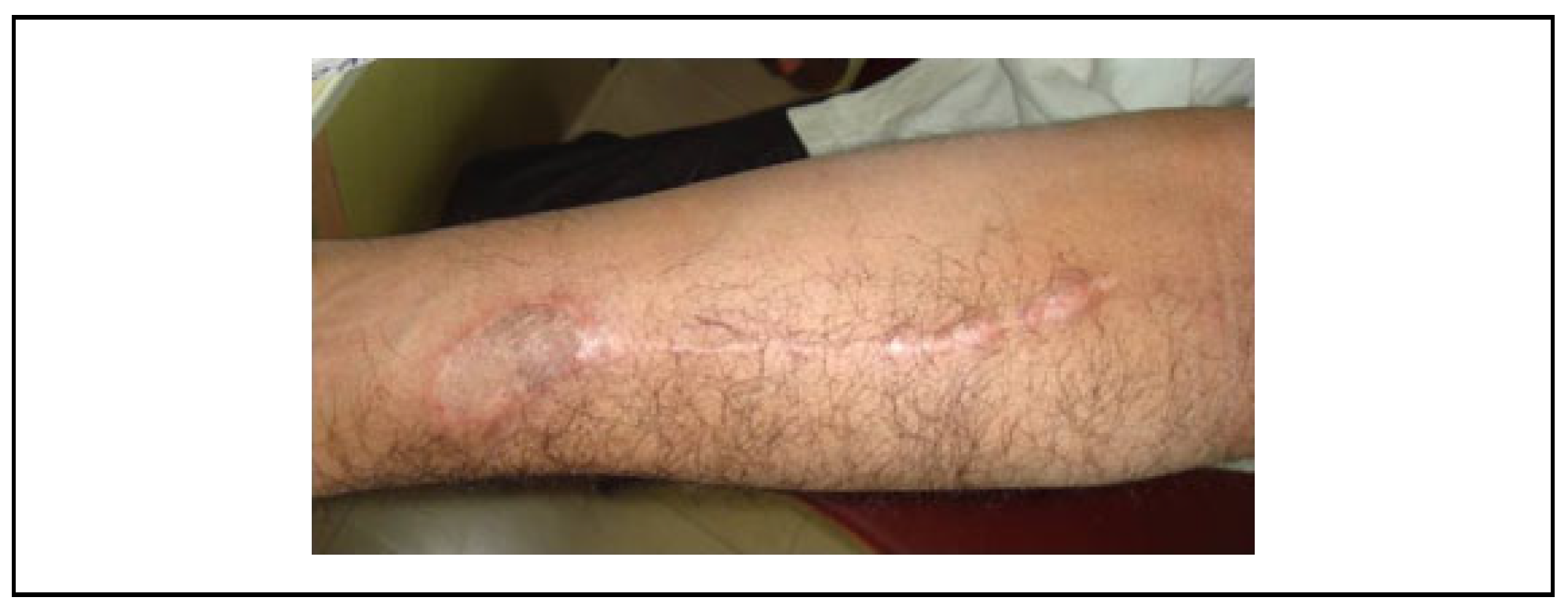
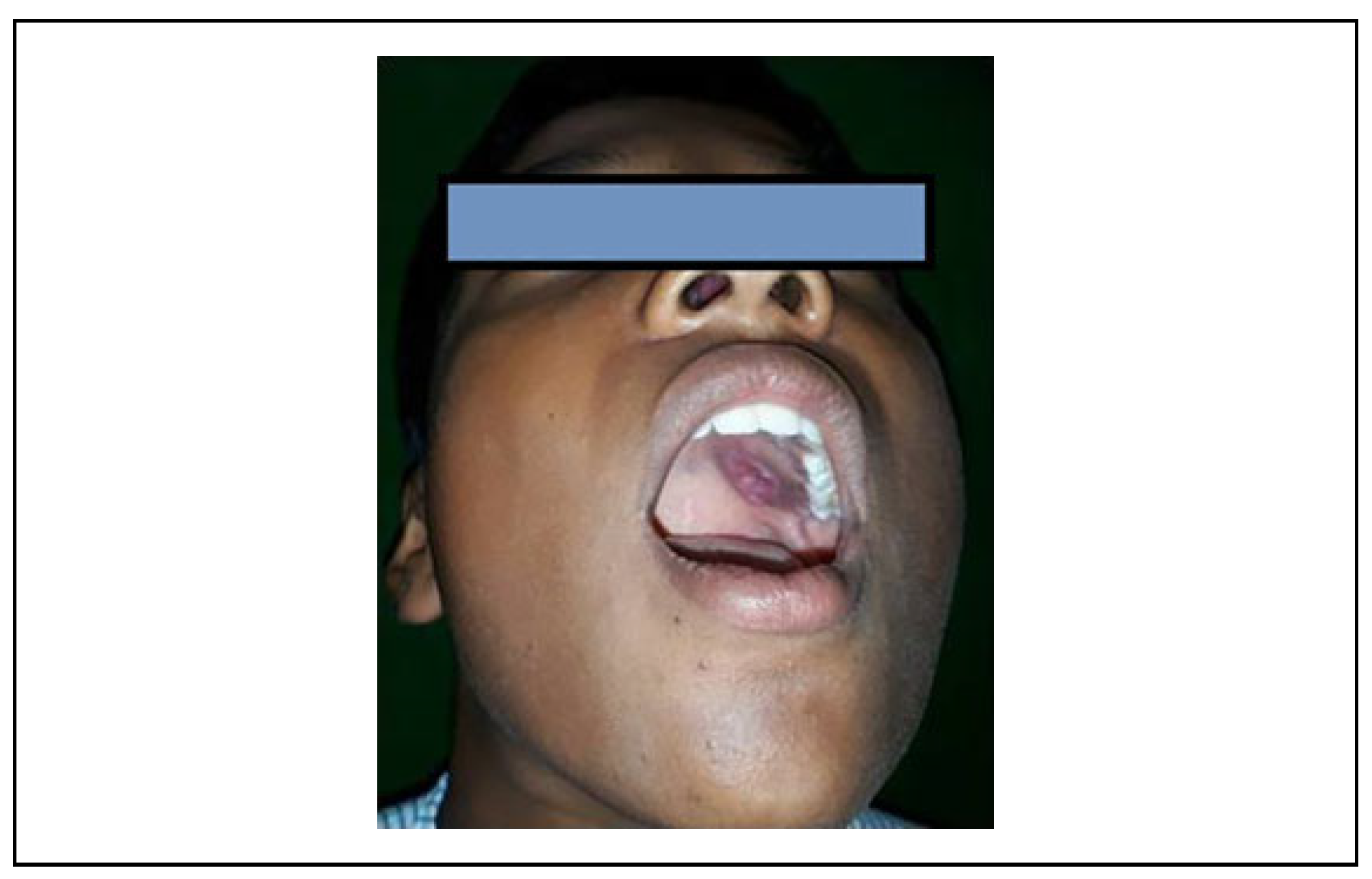
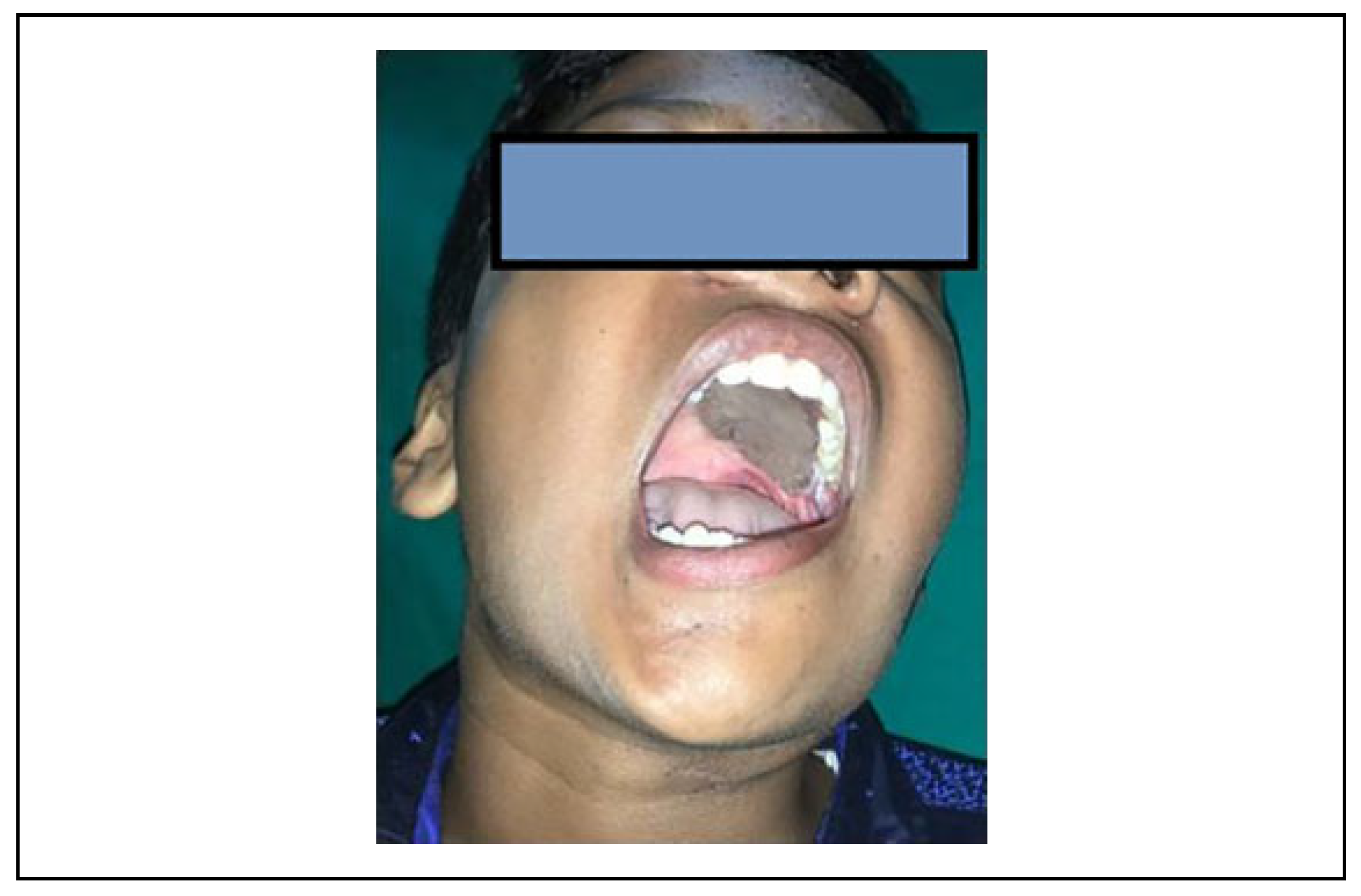
Results
Discussion


Conclusion
Funding
Conflicts of Interest
References
- Lissaois, B. Immediate reconstruction of the hard palate following maxillectomy for carcinoma. Int Surg. 1975, 60, 477–478. [Google Scholar] [PubMed]
- Borges, A.F. Palatal defect reconstruction with hinged nasal septum flap. Ann Plast Surg. 1983, 10, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Biorklund, A.; Koch, H.; Pettersson, K.I. A new method for strengthening palatal closure defects of the hard palate. Acta Otolaryngol. 1976, 82, 147–150. [Google Scholar] [PubMed]
- Carreirao, S.; Lessa, S. Tongue flaps and the closing of large fistulas of the hard palate. Ann Plast Surg. 1980, 4, 182–190. [Google Scholar] [PubMed]
- Konno, A.; Togawa, K.; Iizuka, K. Primary reconstruction after total or extended total maxillectomy for maxillary cancer. Plast Reconstr Surg. 1981, 67, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, C.; Martorell, V.; Colmenero, B.; Sierra, I. Temporalis myofascial flap for maxillofacial reconstruction. J Oral Maxillofac Surg. 1991, 49, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Santamaria, E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg. 2000, 105, 2331–2346; discussion 2347–2348. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.C.; Nagaraj, P.K.; Vasudevan, S.; Rao, A.Y.; Nagireddy, S.R.; Batth, R.S. Extended adipofascial wrap around radial forearm flap for hard palate reconstruction. Indian J Plast Surg. 2018, 51, 306–308. [Google Scholar] [PubMed]
- Gillespie, C.A.; Kenan, P.D.; Ferguson, B.J. Hard palate reconstruction in maxillectomy. Laryngoscope. 1986, 96, 443–444. [Google Scholar] [PubMed]
- Shestak, K.C.; Schusterman, M.A.; Jones, N.F.; Johnson, J.T. Immediate microvascular reconstruction of combined palatal and midfacial defects using soft tissue only. Microsurgery. 1988, 9, 128–131. [Google Scholar] [PubMed]
- Genden, E.M.; Wallace, D.I.; Okay, D.; Urken, M.L. Reconstruction of the hard palate using the radial forearm free flap: Indications and outcomes. Head Neck. 2004, 26, 808–814. [Google Scholar] [PubMed]
- Genden, E.M.; Okay, D.; Stepp, M.T.; et al. Comparison of functional and quality of life outcomes in patients with and without palatomaxillary reconstruction: A preliminary report. Arch Otolaryngol Head Neck Surg. 2003, 129, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.S.; Wei, F.C.; Chang, S.C.; Yang, K.H.; Chen, I.H. Donor site morbidity after Suprafascial elevation of the radial forearm flap: A prospective study in 95 consecutive cases. Plast Reconstr Surg. 1999, 103, 132–137. [Google Scholar] [PubMed]
- De Witt, C.A.; de Bree, R.; Verdonck-de Leeuw, I.M.; Quak, J.J.; Leemans, C.R. Donor site morbidity of the fasciocutaneous radial forearm flap: What does the patient really bother? Eur Arch Otorhinolaryngol. 2007, 264, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chen, H.C.; Huang, Y.L.; Mardini, S.; Feng, G.M. Comparison of the radial forearm flap and the thinned anterolateral thigh cutaneous flap for reconstruction of tongue defects: An evaluation of donor-site morbidity. Plast Reconstr Surg. 2004, 114, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, Y.; Enepekides, D.J.; Torgerson, C.; Higgins, K.M. Radial forearm free flap donor site morbidityulnar-based transposition flap vs split-thickness skin graft. Arch Otolaryngol Head Neck Surg. 2012, 138, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Noma, H.; Takeda, E.; Hashimoto, S. Morphologic changes in forearm flaps of the oral cavity. J Oral Maxillofac Surg. 2000, 58, 495–499. [Google Scholar] [PubMed]
- Draaijers, L.J.; Tempelman, F.R.; Botman, Y.A.; et al. The patient and observer scar assessment scale: A reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004, 113, 1960–1965; discussion 1966–7. [Google Scholar] [PubMed]
- Stratford, P.W.; Binkley, J.M.; Stratford, D.M. Development and initial validation of the upper extremity functional index. Physiotherapy Canada. 2001, 53, 259–267. [Google Scholar]

© 2020 by the author. The Author(s) 2020.
Share and Cite
Chandrappa, A.B.; Batth, R.S.; Vasudevan, S.; Yellambalase, A.N.R.; Kumar, P.N.; Reddy, S.; Seles, J.N. Assessment of Functional Recovery and Subjective Donor-Site Morbidity Following Radial Forearm Flap Reconstruction in Small- to Moderate-Sized Palatal Defects. Craniomaxillofac. Trauma Reconstr. 2020, 13, 71-77. https://doi.org/10.1177/1943387520904879
Chandrappa AB, Batth RS, Vasudevan S, Yellambalase ANR, Kumar PN, Reddy S, Seles JN. Assessment of Functional Recovery and Subjective Donor-Site Morbidity Following Radial Forearm Flap Reconstruction in Small- to Moderate-Sized Palatal Defects. Craniomaxillofacial Trauma & Reconstruction. 2020; 13(1):71-77. https://doi.org/10.1177/1943387520904879
Chicago/Turabian StyleChandrappa, Ashok B., Ritu S. Batth, Srikanth Vasudevan, Anantheswar N. R. Yellambalase, Pradeep N. Kumar, Sudarshan Reddy, and J. Nidya Seles. 2020. "Assessment of Functional Recovery and Subjective Donor-Site Morbidity Following Radial Forearm Flap Reconstruction in Small- to Moderate-Sized Palatal Defects" Craniomaxillofacial Trauma & Reconstruction 13, no. 1: 71-77. https://doi.org/10.1177/1943387520904879
APA StyleChandrappa, A. B., Batth, R. S., Vasudevan, S., Yellambalase, A. N. R., Kumar, P. N., Reddy, S., & Seles, J. N. (2020). Assessment of Functional Recovery and Subjective Donor-Site Morbidity Following Radial Forearm Flap Reconstruction in Small- to Moderate-Sized Palatal Defects. Craniomaxillofacial Trauma & Reconstruction, 13(1), 71-77. https://doi.org/10.1177/1943387520904879


