Prosthetics in Facial Reconstruction
Abstract
:Methods
Presurgical Planning
Imaging Modalities and Prosthesis Design
Materials
Retention Systems
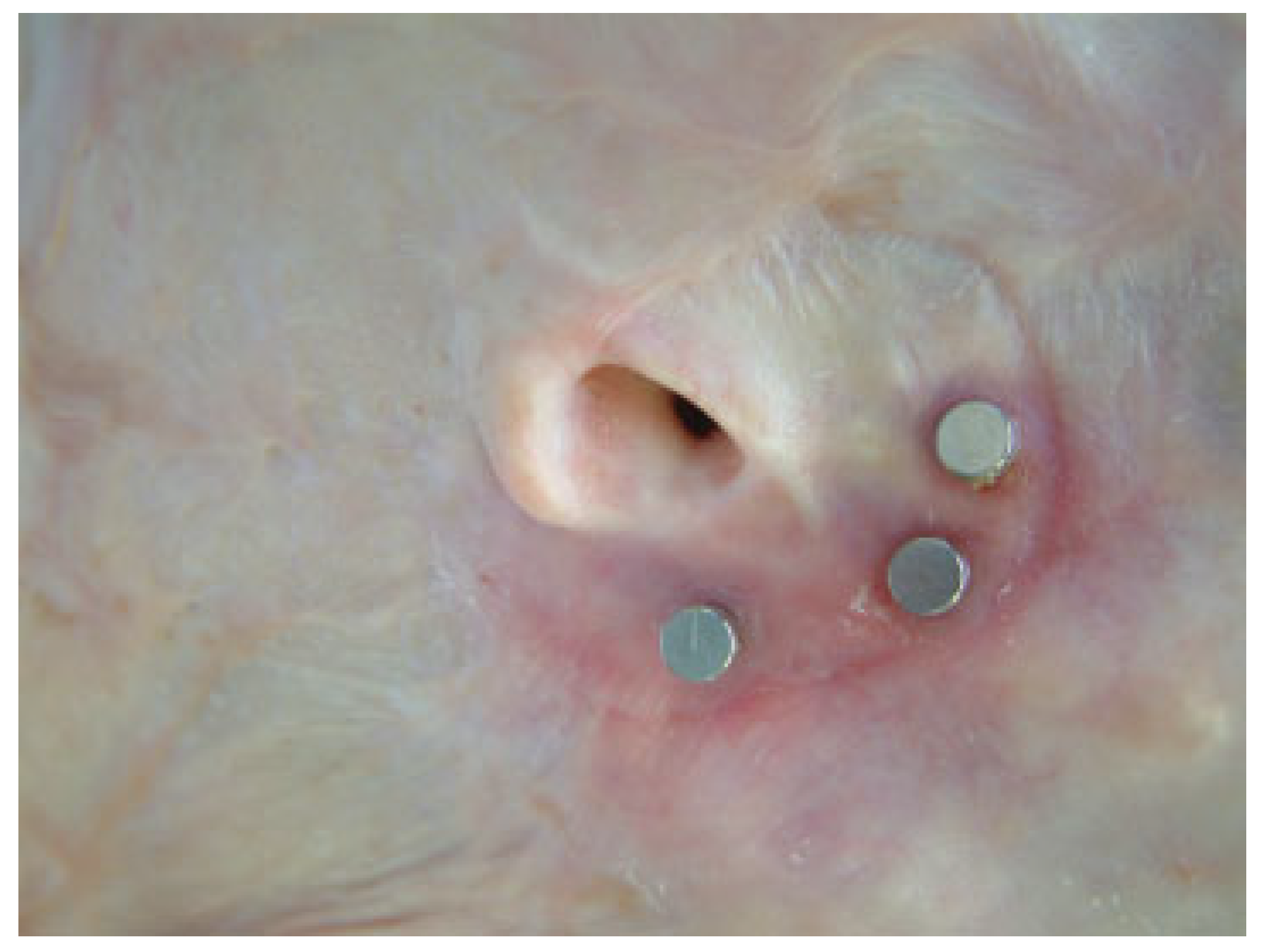
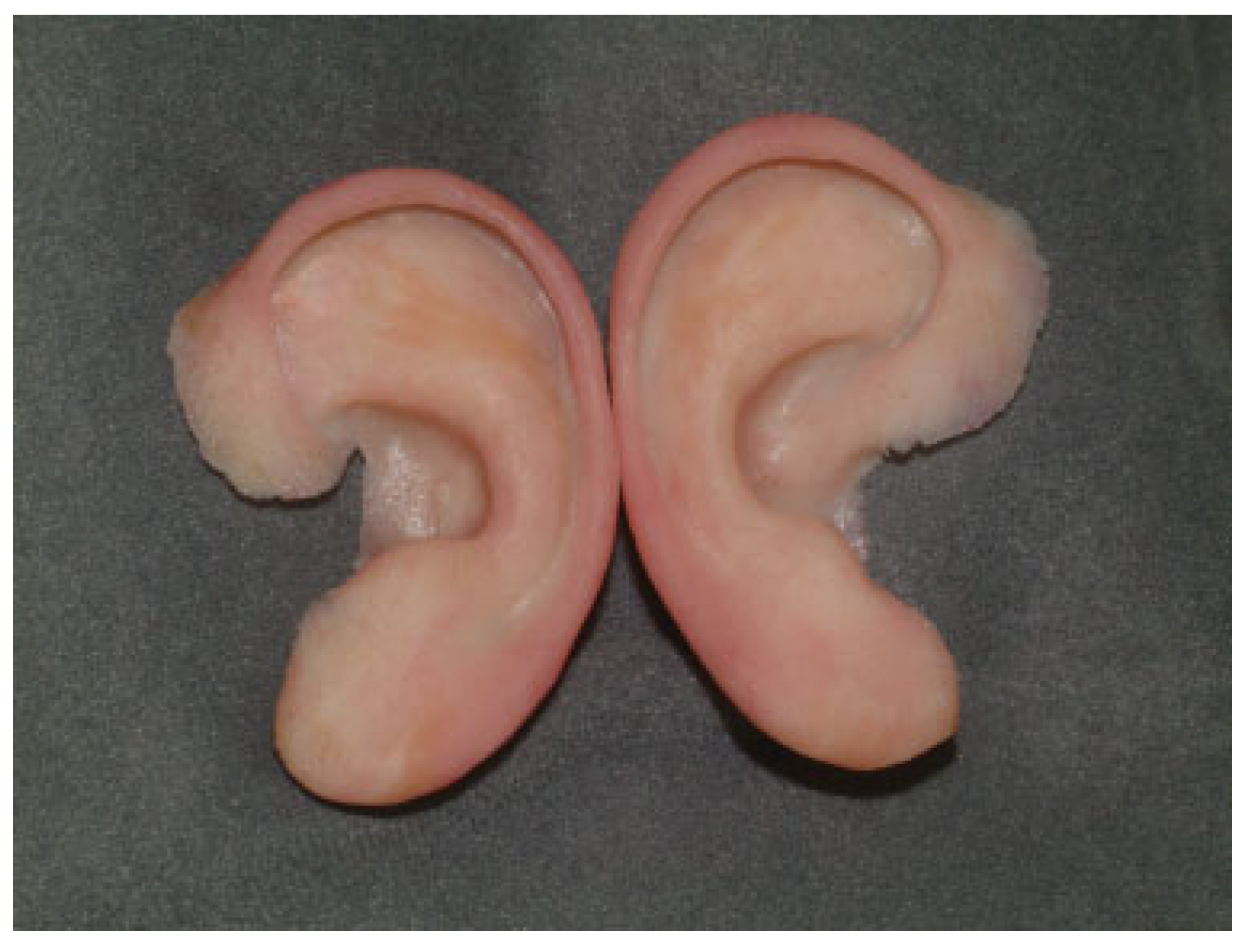
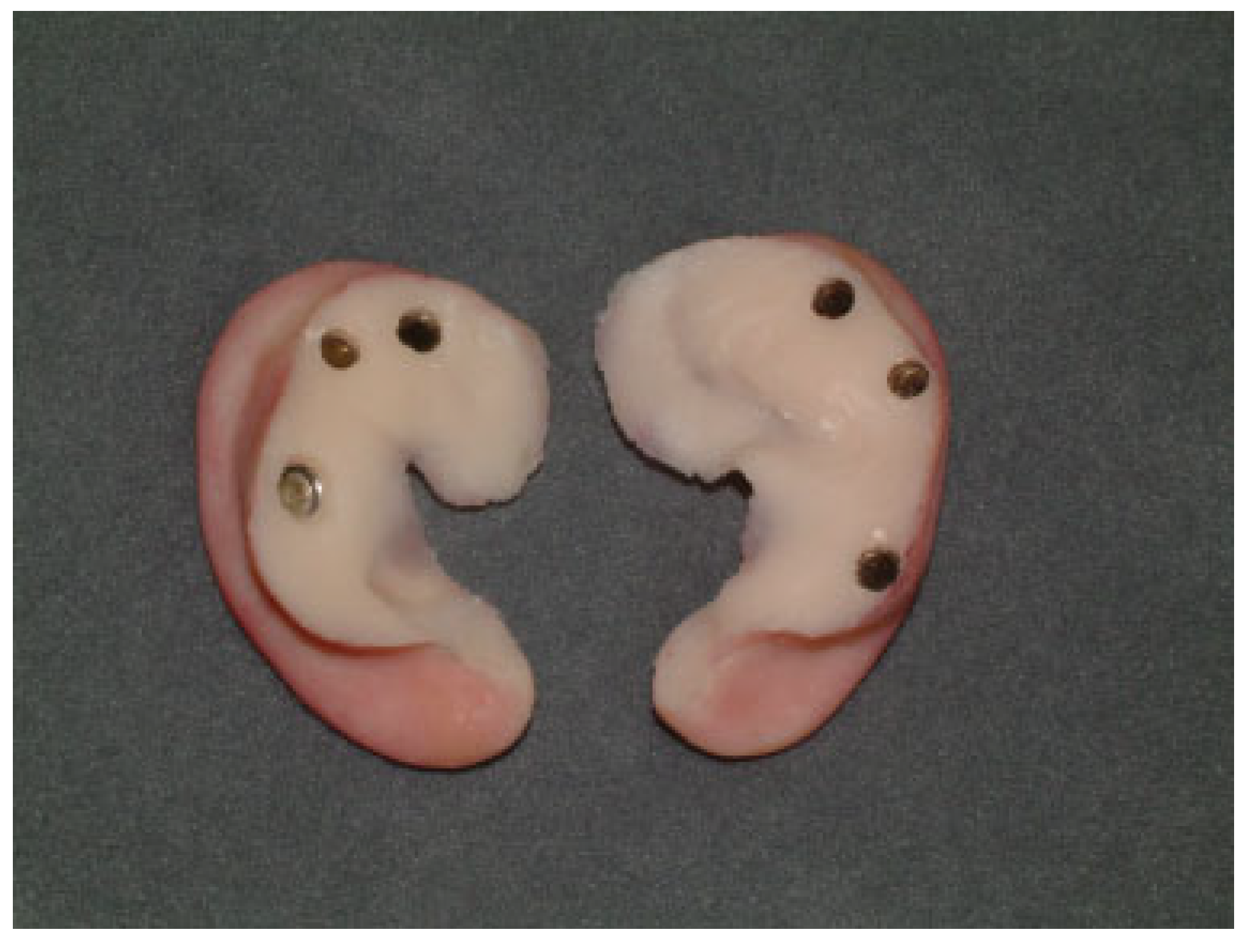
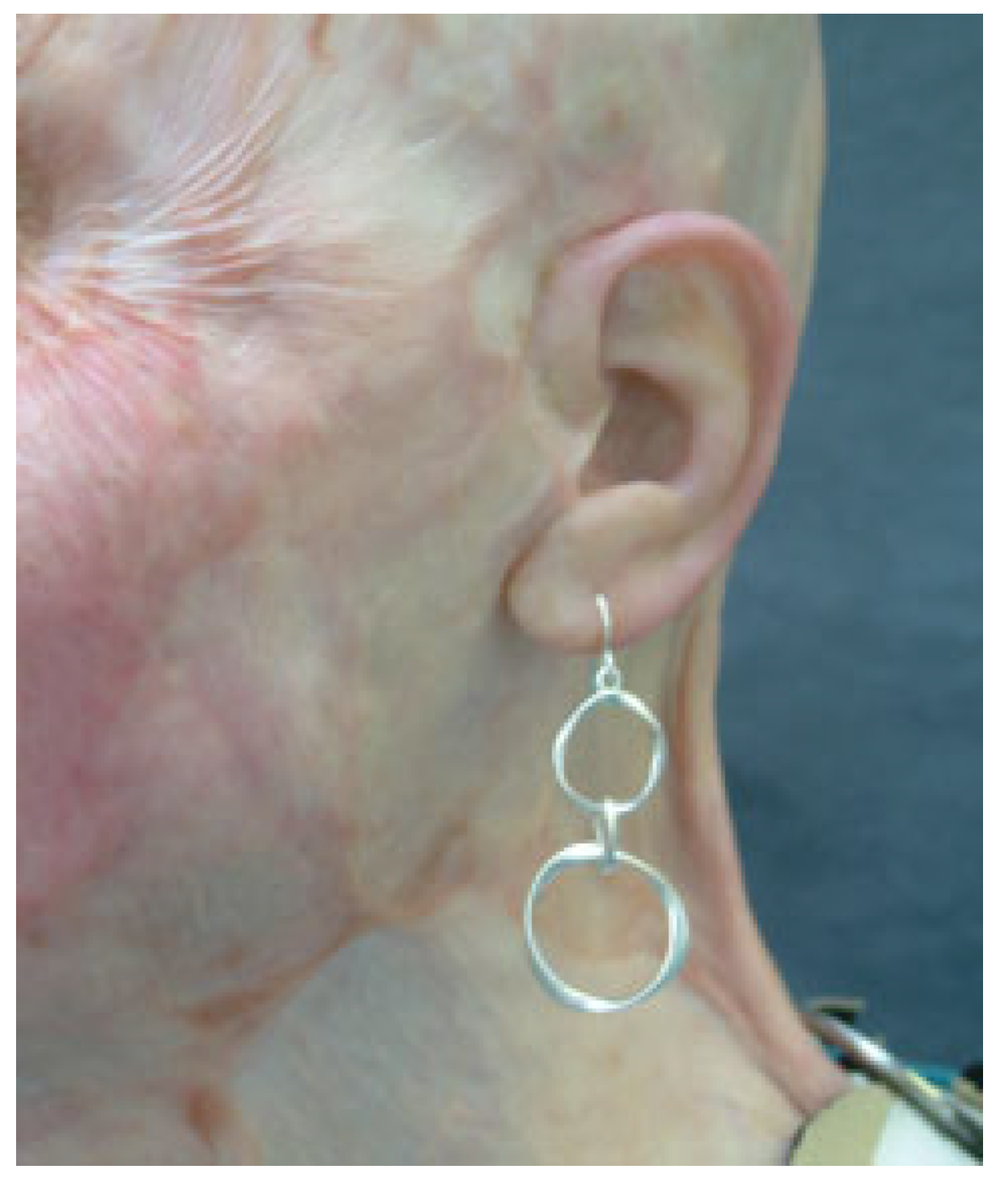
Auricular Prostheses
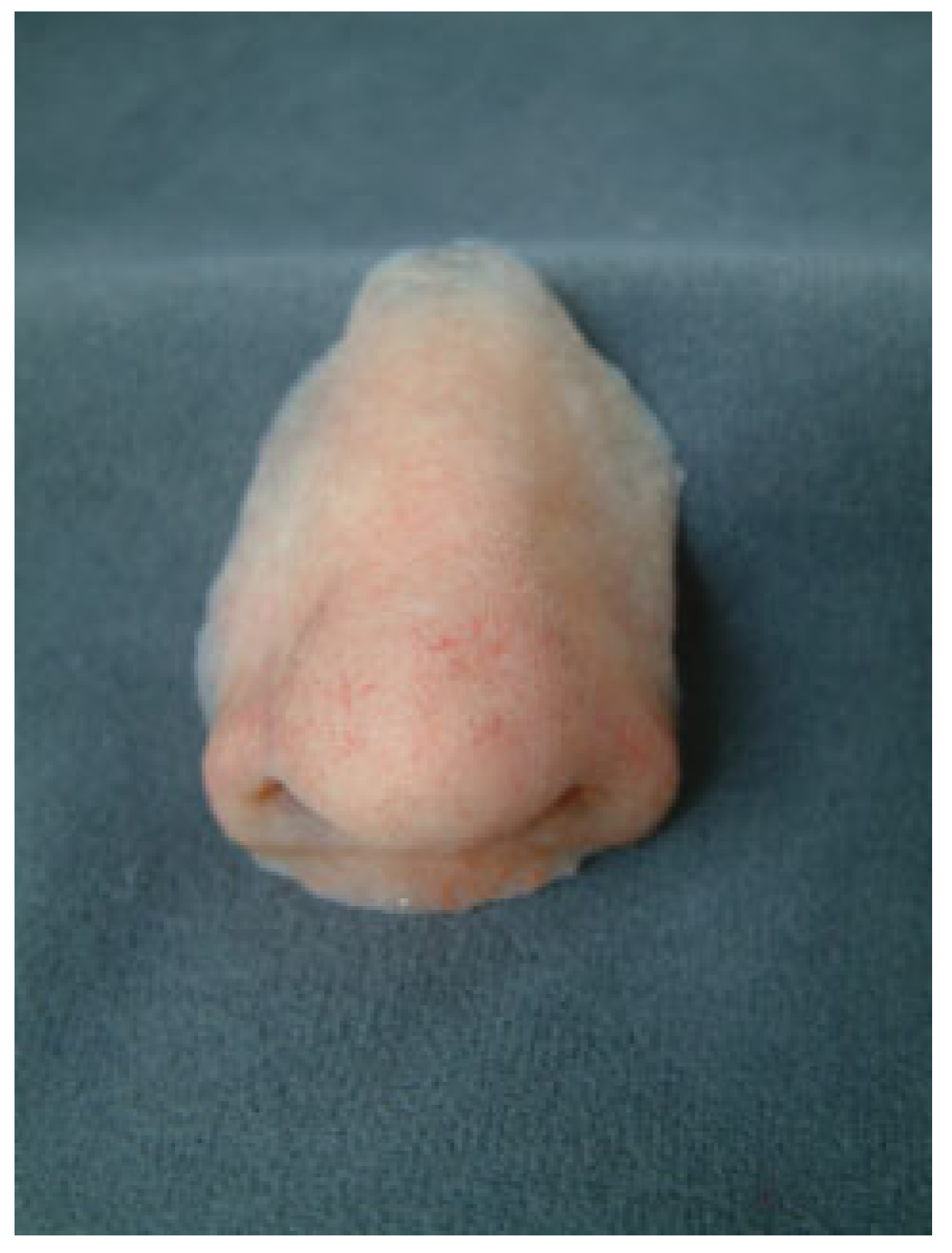
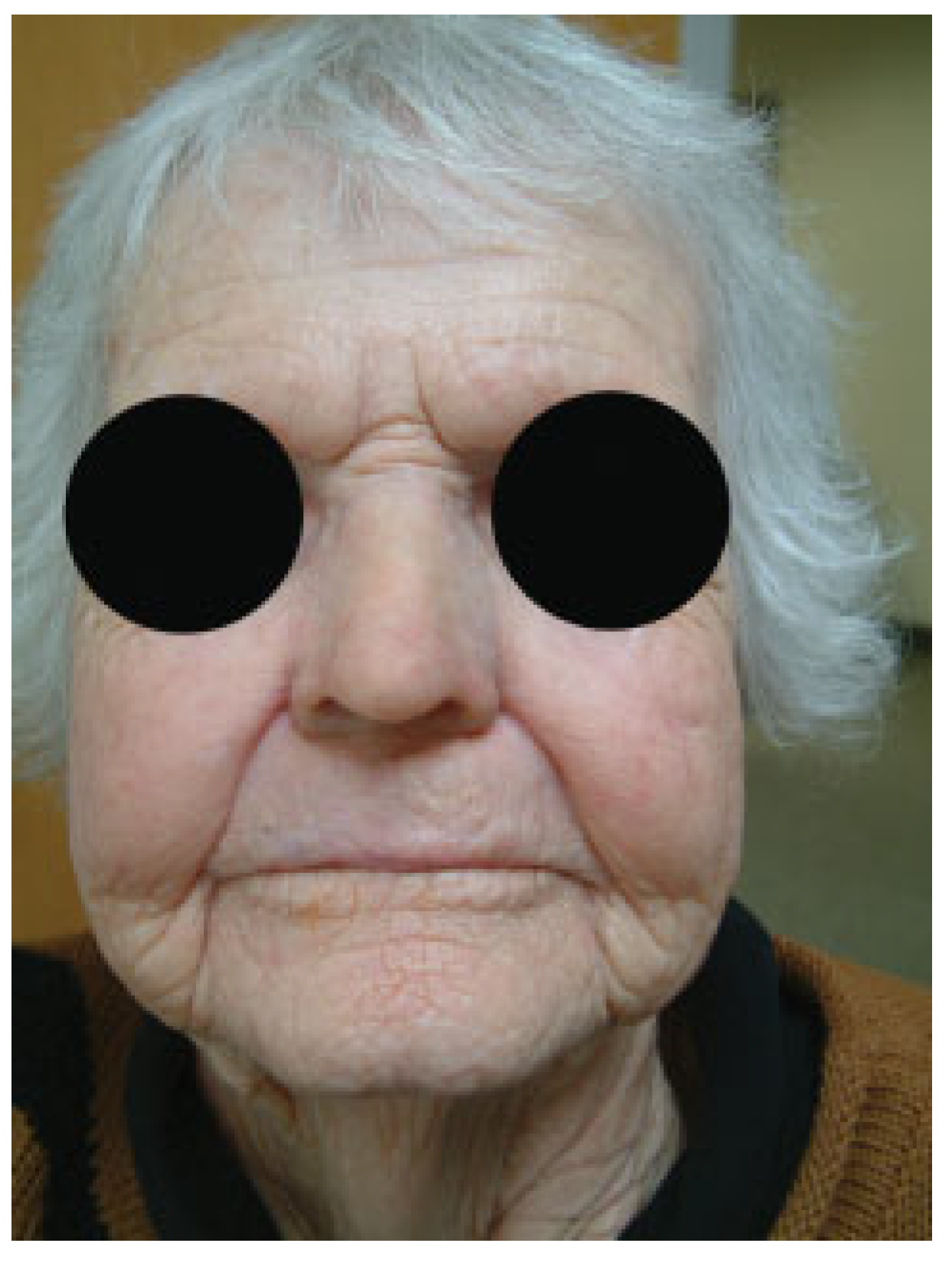
Nasal Prostheses
Maxillary and Midface Prostheses
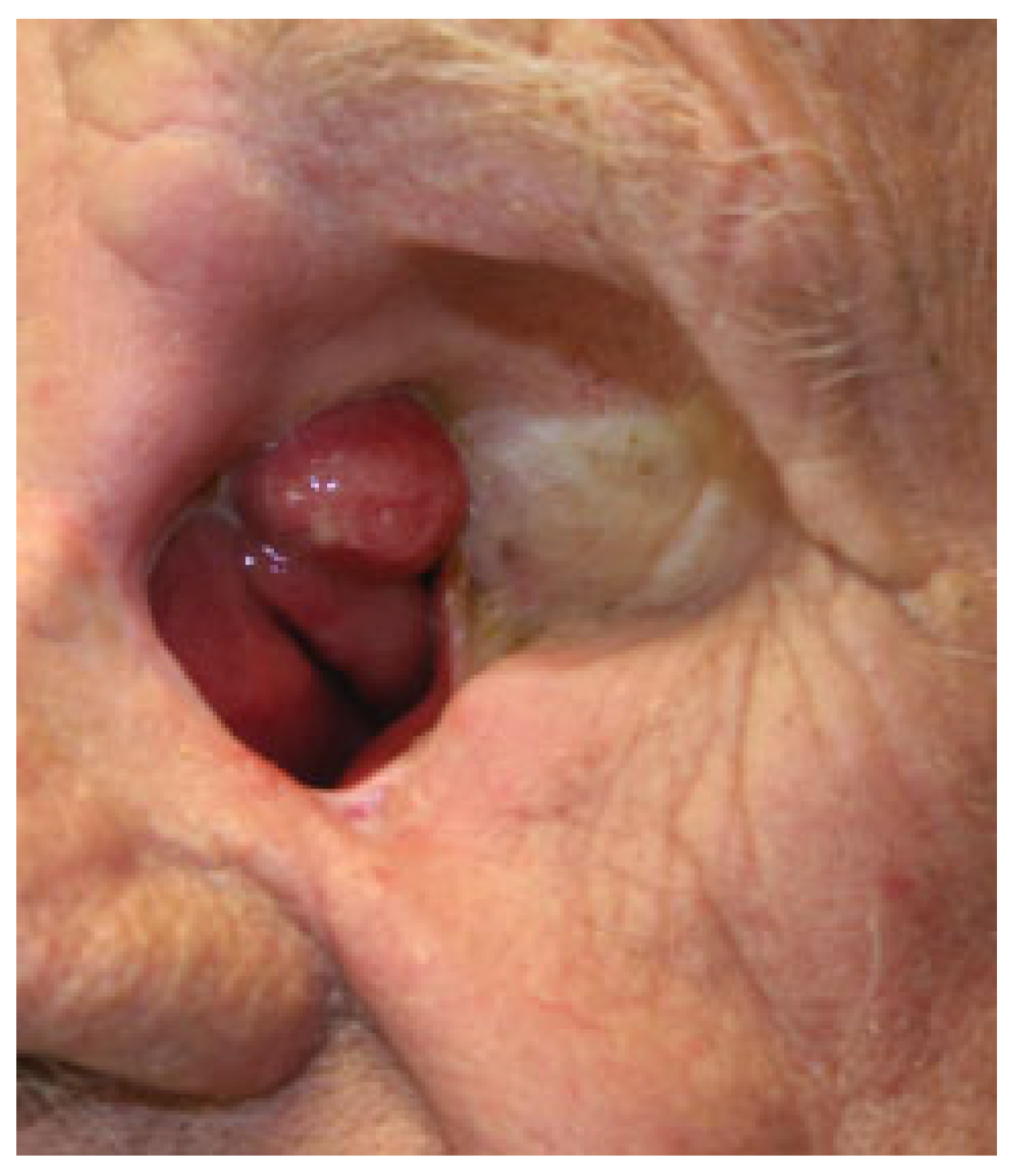
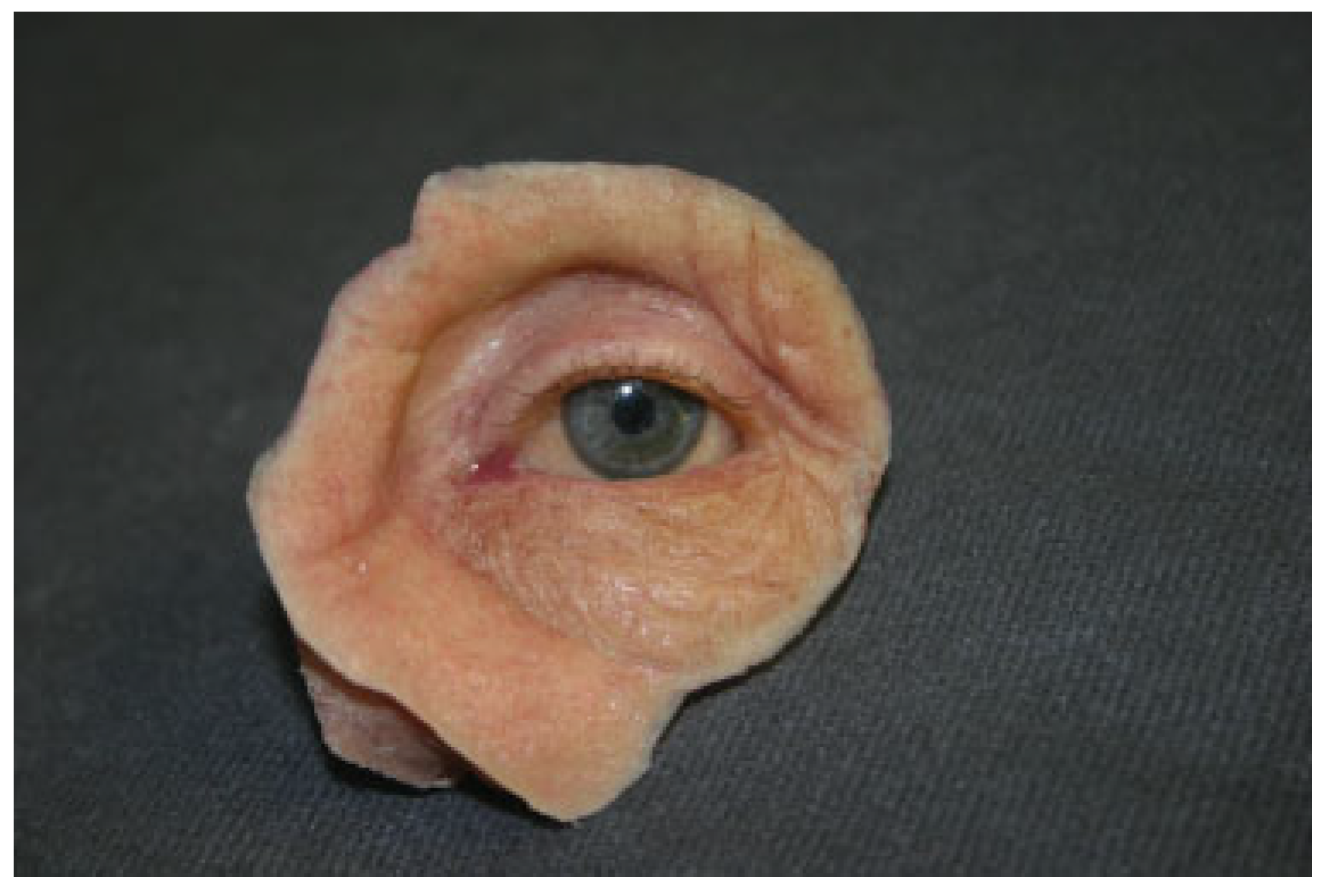
Orbital Prostheses
Conclusions
References
- Gibson, M.K.; Forastiere, A.A. Multidisciplinary approaches in the management of advanced head and neck tumors: State of the art. Curr Opin Oncol 2004, 16, 220–224. [Google Scholar]
- Thawley, S.E.; Batsakis, J.G.; Lindberg, R.D.; et al. Comprehensive Management of Head and Neck Tumors; Elsevier: St Louis, MO, USA, 1998; pp. 526–527. [Google Scholar]
- Harrison, D.F. Total rhinectomy–a worthwhile operation? J Laryngol Otol 1982, 96, 1113–1123. [Google Scholar] [PubMed]
- Bhandari, S. Prosthetic considerations in oral and maxillofacial trauma. J Postgrad Med Educ Res 2014, 48, 87–90. [Google Scholar]
- Salinas, T.J. Prosthetic rehabilitation of defects of the head and neck. Semin Plast Surg 2010, 24, 299–308. [Google Scholar] [CrossRef]
- Thaller, S.; Bradley, J.P.; Garri, J.I. Craniofacial Surgery; CRC Press: New York, NY, USA, 2007; p. 334. [Google Scholar]
- Lemon, J.C.; Chambers, M.S.; Wesley, P.J.; Reece, G.P.; Martin, J.W. Rehabilitation of a midface defect with reconstructive surgery and facial prosthetics: A case report. Int J Oral Maxillofac Implant 1996, 11, 101–105. [Google Scholar]
- Parr, G.R.; Goldman, B.M.; Rahn, A.O. Maxillofacial prosthetic principles in the surgical planning for facial defects. J Prosthet Dent 1981, 46, 323–329. [Google Scholar]
- Oh, H.K.; Chambers, M.S.; Martin, J.W.; Lim, H.J.; Park, H.J. Osteoradionecrosis of the mandible: Treatment outcomes and factors influencing the progress of osteoradionecrosis. J Oral Maxillofac Surg 2009, 67, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.P.; Wiens, R.L. Psychological management of the maxillofacial prosthetic patient. In Clinical Maxillofacial Prosthetics; Taylor, T.D., Ed.; Quintessence Publishing: Batavia, IL, USA, 2000; pp. 1–14. [Google Scholar]
- Raghoebar, G.M.; van Oort, R.P.; Roodenburg, J.L.; Reintsema, H.; Dikkers, F.G. Fixation of auricular prostheses by osseointegrated implants. J Invest Surg 1994, 7, 283–290. [Google Scholar]
- de Bree, R.; Leemans, C.R. Recent advances in surgery for head and neck cancer. Curr Opin Oncol 2010, 22, 186–193. [Google Scholar]
- Moreno, M.A.; Skoracki, R.J.; Hanna, E.Y.; Hanasono, M.M. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck 2010, 32, 860–868. [Google Scholar]
- Roumanas, E.D.; Freymiller, E.G.; Chang, T.L.; Aghaloo, T.; Beumer, J., III. Implant-retained prostheses for facial defects: An up to 14-year follow-up report on the survival rates of implants at UCLA. Int J Prosthodont 2002, 15, 325–332. [Google Scholar]
- Guttal, S.S.; Patil, N.P.; Shetye, A.D. Prosthetic rehabilitation of a midfacial defect resulting from lethal midline granuloma–a clinical report. J Oral Rehabil 2006, 33, 863–867. [Google Scholar] [CrossRef]
- Markt, J.C.; Lemon, J.C. Extraoral maxillofacial prosthetic rehabilitation at the M. D. Anderson Cancer Center: A survey of patient attitudes and opinions. J Prosthet Dent 2001, 85, 608–613. [Google Scholar] [CrossRef]
- Eleni, P.N.; Krokida, M.; Polyzois, G.; Gettleman, L.; Bisharat, G.I. Effects of outdoor weathering on facial prosthetic elastomers. Odontology 2011, 99, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.; Raghoebar, G.M.; van Oort, R.P.; Vissink, A. Fate of implantretained craniofacial prostheses: Life span and aftercare. Int J Oral Maxillofac Implant 2008, 23, 89–98. [Google Scholar]
- Feng, Z.; Dong, Y.; Zhao, Y.; et al. Computer-assisted technique for the design and manufacture of realistic facial prostheses. Br J Oral Maxillofac Surg 2010, 48, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Verdonck, H.W.; Poukens, J.; Overveld, H.V.; Riediger, D. Computerassisted maxillofacial prosthodontics: A new treatment protocol. Int J Prosthodont 2003, 16, 326–328. [Google Scholar]
- Wu, G.; Zhou, B.; Bi, Y.; Zhao, Y. Selective laser sintering technology for customized fabrication of facial prostheses. J Prosthet Dent 2008, 100, 56–60. [Google Scholar] [CrossRef]
- Jiao, T.; Zhang, F.; Huang, X.; Wang, C. Design and fabrication of auricular prostheses by CAD/CAM system. Int J Prosthodont 2004, 17, 460–463. [Google Scholar]
- Subburaj, K.; Nair, C.; Rajesh, S.; Meshram, S.M.; Ravi, B. Rapid development of auricular prosthesis using CAD and rapid prototyping technologies. Int J Oral Maxillofac Surg 2007, 36, 938–943. [Google Scholar] [CrossRef]
- Federspil, P.A. Implant-retained craniofacial prostheses for facial defects. GMS Curr Top Otorhinolaryngol Head Neck Surg 2009, 8, Doc03. [Google Scholar] [PubMed]
- Giot, J.P.; Labbé, D.; Soubeyrand, E.; et al. Prosthetic reconstruction of the auricle: Indications, techniques, and results. Semin Plast Surg 2011, 25, 265–272. [Google Scholar]
- Montgomery, P.C.; Kiat-Amnuay, S. Survey of currently used materials for fabrication of extraoral maxillofacial prostheses in North America, Europe, Asia, and Australia. J Prosthodont 2010, 19, 482–490. [Google Scholar] [PubMed]
- Kiat-amnuay, S.; Jacob, R.F.; Chambers, M.S.; et al. Clinical trial of chlorinated polyethylene for facial prosthetics. Int J Prosthodont 2010, 23, 263–270. [Google Scholar]
- Kiat-Amnuay, S.; Gettleman, L.; Goldsmith, L.J. Effect of multi-adhesive layering on retention of extraoral maxillofacial silicone prostheses in vivo. J Prosthet Dent 2004, 92, 294–298. [Google Scholar]
- Wolfaardt, J.; Gehl, G.; Farmand, M.; Wilkes, G. Indications and methods of care for aspects of extraoral osseointegration. Int J Oral Maxillofac Surg 2003, 32, 124–131. [Google Scholar]
- Granström, G. Craniofacial osseointegration. Oral Dis 2007, 13, 261–269. [Google Scholar] [PubMed]
- Alvi, R.; McPhail, J.; Hancock, K. Closed-field titanium magnets for the retention of complex craniofacial prostheses. Br J Plast Surg 2002, 55, 668–670. [Google Scholar]
- Sencimen, M.; Bal, H.E.; Demiroğullari, M.; Kocaoglu, M.; Dogan, N. Auricular epithesis retained by an attachment system (2 case reports). Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008, 105, e28–e34. [Google Scholar]
- Dahl, J.E.; Polyzois, G.L. Irritation test of tissue adhesives for facial prostheses. J Prosthet Dent 2000, 84, 453–457. [Google Scholar]
- Huband, M. Prosthetic rehabilitation. Dermatol Clin 2011, 29, 325–330. [Google Scholar] [PubMed]
- Wondergem, M.; Lieben, G.; Bouman, S.; van den Brekel, M.W.; Lohuis, P.J. Patients’ satisfaction with facial prostheses. Br J Oral Maxillofac Surg 2016, 54, 394–399. [Google Scholar]
- Tjellström, A.; Lindström, J.; Hallén, O.; Albrektsson, T.; Brånemark, P.I. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol 1981, 2, 304–310. [Google Scholar] [PubMed]
- Albrektsson, T. Dental and maxillofacial implantology. In Principles of Osseointegration; Hobkirk, J.A., Watson, K., Eds.; Mosby-Wolfe: London, UK, 1995; pp. 9–19. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001, 10 (Suppl. S2), S96–S101. [Google Scholar]
- Frost, H.M. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res 1989, 248, 283–293. [Google Scholar]
- Ihde, S.; Kopp, S.; Gundlach, K.; Konstantinović, V.S. Effects of radiation therapy on craniofacial and dental implants: A review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009, 107, 56–65. [Google Scholar]
- Voigt, A.; Christ, S.; Klein, M. Experimental analysis of retention forces of different magnetic devices for bone-anchored auricular facial prostheses. Int J Oral Maxillofac Surg 2008, 37, 664–668. [Google Scholar]
- de Sousa, A.A.; Mattos, B.S. Magnetic retention and bar-clip attachment for implant-retained auricular prostheses: A comparative analysis. Int J Prosthodont 2008, 21, 233–236. [Google Scholar]
- Ariani, N.; Visser, A.; van Oort, R.P.; et al. Current state of craniofacial prosthetic rehabilitation. Int J Prosthodont 2013, 26, 57–67. [Google Scholar]
- Art as Applied to Medicine. The Facial Prosthetics Clinic of Johns Hopkins University Web Site. Available online: http://hopkinsmedi-cine.org/medart/Prosthetics.htm (accessed on 2 December 2016).
- Mardani, M.A.; Aminian, G.; Tabatabaian, F.; Arazpour, M.; Hutchins, S.W.; Head, J.S. A novel technique for fabricating an ear prosthesis in a patient with congenital ear deformity. Prosthet Orthot Int 2013, 37, 340–343. [Google Scholar]
- Cox, A.; Sabbagh, W.; Gault, D. Costal cartilage or conchal cartilage for aesthetic and structural reconstruction of lower pole ear defects. Aesthet Surg J 2012, 32, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Firmin, F. State-of-the-art autogenous ear reconstruction in cases of microtia. Adv Otorhinolaryngol 2010, 68, 25–52. [Google Scholar]
- Firmin, F.; Sanger, C.; O’Toole, G. Ear reconstruction following severe complications of otoplasty. J Plast Reconstr Aesthet Surg 2008, 61 (Suppl. S1), S13–S20. [Google Scholar] [CrossRef]
- Mevio, E.; Facca, L.; Schettini, S.; Mullace, M. Bone-anchored titanium implants in patients with auricular defects: Three years and 27 patients’ experience. Int J Otolaryngol 2016, 2016, 9872048. [Google Scholar]
- Arora, V.; Sahoo, N.K.; Gopi, A.; Saini, D.K. Implant-retained auricular prostheses: A clinical challenge. Int J Oral Maxillofac Surg 2016, 45, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.D. Clinical Maxillofacial Prosthetics; Quintessence Publishing Co., Inc.: Chicago, IL, USA, 2000; pp. 233–276. [Google Scholar]
- Zhao, Y.; Wang, Y.; Zhuang, H.; et al. Clinical evaluation of three total ear reconstruction methods. J Plast Reconstr Aesthet Surg 2009, 62, 1550–1554. [Google Scholar] [PubMed]
- Lemon, J.C.; Kiat-amnuay, S.; Gettleman, L.; Martin, J.W.; Chambers, M.S. Facial prosthetic rehabilitation: Preprosthetic surgical techniques and biomaterials. Curr Opin Otolaryngol Head Neck Surg 2005, 13, 255–262. [Google Scholar]
- Eriksson, E.; Brånemark, P.I. Osseointegration from the perspective of the plastic surgeon. Plast Reconstr Surg 1994, 93, 626–637. [Google Scholar] [CrossRef]
- Black, B. Use of titanium in repair of external auditory canal defects. Otol Neurotol 2009, 30, 930–935. [Google Scholar] [CrossRef]
- Walsh, W.E.; Dougherty, B.; Reisberg, D.J.; et al. The importance of auricular prostheses for speech recognition. Arch Facial Plast Surg 2008, 10, 321–328. [Google Scholar]
- Wright, R.F.; Zemnick, C.; Wazen, J.J.; Asher, E. Osseointegrated implants and auricular defects: A case series study. J Prosthodont 2008, 17, 468–475. [Google Scholar] [PubMed]
- Subaşı, M.G.; Alnıaçık, G.; Kalaycı, A.; Akman, S.; Durmuş, E. Prosthetic rehabilitation of partial ear defect: 2 case reports. J Indian Prosthodont Soc 2014, 14 (Suppl. S1), 196–201. [Google Scholar]
- Badie-Modiri, B.; Kaplanski, P. Extra-oral implants: Principal areas of implantation [in French]. Rev Stomatol Chir Maxillofac 2001, 102, 229–233. [Google Scholar] [PubMed]
- Correa, B.J.; Weathers, W.M.; Wolfswinkel, E.M.; Thornton, J.F. The forehead flap: The gold standard of nasal soft tissue reconstruction. Semin Plast Surg 2013, 27, 96–103. [Google Scholar]
- Menick, F.J. A 10-year experience in nasal reconstruction with the three-stage forehead flap. Plast Reconstr Surg 2002, 109, 1839—1855, Discussion 1856—1861. [Google Scholar]
- Ahmed, B.; Butt, A.M.; Hussain, M.; Amin, M.; Yazdanie, N. Rehabilitation of nose using silicone based maxillofacial prosthesis. J Coll Physicians Surg Pak 2010, 20, 65–67. [Google Scholar] [PubMed]
- Thornton, J.F.; Griffin, J.R.; Constantine, F.C. Nasal reconstruction: An overview and nuances. Semin Plast Surg 2008, 22, 257–268. [Google Scholar]
- Bourget, A.; Chang, J.T.; Wu, D.B.; Chang, C.J.; Wei, F.C. Free flap reconstruction in the head and neck region following radiotherapy: A cohort study identifying negative outcome predictors. Plast Reconstr Surg 2011, 127, 1901–1908. [Google Scholar]
- Thankappan, K. Microvascular free tissue transfer after prior radiotherapy in head and neck reconstruction—A review. Surg Oncol 2010, 19, 227–234. [Google Scholar]
- Klug, C.; Berzaczy, D.; Reinbacher, H.; et al. Influence of previous radiotherapy on free tissue transfer in the head and neck region: Evaluation of 455 cases. Laryngoscope 2006, 116, 1162–1167. [Google Scholar]
- Ciocca, L.; Maremonti, P.; Bianchi, B.; Scotti, R. Maxillofacial rehabilitation after rhinectomy using two different treatment options: Clinical reports. J Oral Rehabil 2007, 34, 311–315. [Google Scholar] [PubMed]
- Seçilmiş, A.; Oztürk, A.N. Nasal prosthesis rehabilitation after partialrhinectomy: A clinical report. Eur J Dent 2007, 1, 115–118. [Google Scholar]
- Dimitroulis, G. Nasal implants following nasectomy. Int J Oral Maxillofac Surg 2007, 36, 447–449. [Google Scholar]
- Scott, N.; Kittur, M.A.; Evans, P.L.; Dovgalski, L.; Hodder, S.C. The use of zygomatic implants for the retention of nasal prosthesis following rhinectomy: The Morriston experience. Int J Oral Maxillofac Surg 2016, 45, 1044–1048. [Google Scholar]
- Brånemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lindström, J.; Rockler, B. An experimental and clinical study of osseointegrated implants penetrating the nasal cavity and maxillary sinus. J Oral Maxillofac Surg 1984, 42, 497–505. [Google Scholar] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Survival and complications of Zygomatic Implants: An updated systematic review. J Oral Maxillofac Surg 2016, 74, 1949–1964. [Google Scholar] [PubMed]
- Futran, N.D.; Mendez, E. Developments in reconstruction of midface and maxilla. Lancet Oncol 2006, 7, 249–258. [Google Scholar]
- Har-El, G.; Nathan, C.O.; Day, T.A.; Nguyen, S.A. Rehabilitation of patients with head and neck cancer: Prosthetic rehabilitation: Intraoral and extraoral prostheses. In Head and Neck Surgery; Thieme Medical Publishers: New York, NY, USA, 2013. [Google Scholar]
- Okay, D.J.; Genden, E.; Buchbinder, D.; Urken, M. Prosthodontic guidelines for surgical reconstruction of the maxilla: A classification system of defects. J Prosthet Dent 2001, 86, 352–363. [Google Scholar]
- Iyer, S.; Thankappan, K. Maxillary reconstruction: Current concepts and controversies. Indian J Plast Surg 2014, 47, 8–19. [Google Scholar]
- Nekora-Azak, A.; Evlioglu, G.; Ozdemir-Karataş, M.; Keskin, H. Use of biofunctional prosthetic system following partial maxillary resection: A clinical report. J Oral Rehabil 2005, 32, 693–695. [Google Scholar]
- Etienne, O.M.; Taddei, C.A. Use of bar-clip attachments to enhance the retention of a maxillofacial prosthetic obturator: A clinical report. J Oral Rehabil 2004, 31, 618–621. [Google Scholar] [CrossRef]
- Shipman, B. Evaluation of occlusal force in patients with obturator defects. J Prosthet Dent 1987, 57, 81–84. [Google Scholar] [CrossRef]
- Watson, R.M.; Gray, B.J. Assessing effective obturation. J Prosthet Dent 1985, 54, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Parr, G.R.; Tharp, G.E.; Rahn, A.O. Prosthodontic principles in the framework design of maxillary obturator prostheses. 1989. J Prosthet Dent 2005, 93, 405–411. [Google Scholar] [CrossRef]
- Brown, J.S.; Rogers, S.N.; McNally, D.N.; Boyle, M. A modified classification for the maxillectomy defect. Head Neck 2000, 22, 17–26. [Google Scholar] [CrossRef]
- Murat, S.; Gurbuz, A.; Isayev, A.; Dokmez, B.; Cetin, U. Enhanced retention of a maxillofacial prosthetic obturator using precision attachments: Two case reports. Eur J Dent 2012, 6, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Aramany, M.A. Basic principles of obturator design for partially edentulous patients. Part II: Design principles. J Prosthet Dent 2001, 86, 562–568. [Google Scholar] [PubMed]
- Cordeiro, P.G.; Santamaria, E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg 2000, 105, 2331—2346, Discussion 2347—2348. [Google Scholar] [CrossRef]
- McBain, H.B.; Ezra, D.G.; Rose, G.E.; Newman, S.P.; Appearance Research Collaboration (ARC). The psychosocial impact of living with an ocular prosthesis. Orbit 2014, 33, 39–44. [Google Scholar] [CrossRef]
- Baino, F.; Potestio, I. Orbital implants: State-of-the-art review with emphasis on biomaterials and recent advances. Mater Sci Eng C 2016, 69, 1410–1428. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ramesh, C. Reproduction of custom-made eye prosthesis manoeuvre: A case report. J Dent Oral Hyg 2009, 1, 59–63. [Google Scholar]
- Pruthi, G.; Jain, V.; Rajendiran, S.; Jha, R. Prosthetic rehabilitation after orbital exenteration: A case series. Indian J Ophthalmol 2014, 62, 629–632. [Google Scholar] [PubMed]
- Parr, G.R.; Goldman, B.M.; Rahn, A.O. Surgical considerations in the prosthetic treatment of ocular and orbital defects. J Prosthet Dent 1983, 49, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Begum, Z.; Kola, M.Z.; Joshi, P. Analysis of the properties of commercially available silicone elastomers for maxillofacial prostheses. J Dent 2011, 31, 67–74. [Google Scholar]
- Cafiero-Chin, M.; Marques, C.; Danz, H. Ocular prosthesis: Indications to management. Canad J Optometr 2015, 77, 24–33. [Google Scholar] [CrossRef]
- Sami, D.; Young, S.; Petersen, R. Perspective on orbital enucleation implants. Surv Ophthalmol 2007, 52, 244–265. [Google Scholar] [CrossRef]
- Hughes, M.O. A pictorial anatomy of the human eye/anophthalmic socket: A review for ocularists. J Ophthal Prosthet 2007, 12, 51–63. [Google Scholar]
- Gunaseelaraj, R.; Karthikeyan, S.; Kumar, M.N.; Balamurugan, T.; Jagadeeshwaran, A.R. Custom-made ocular prosthesis. J Pharm Bioallied Sci 2012, 4 (Suppl. S2), S177–S179. [Google Scholar]
- Toljanic, J.A.; Eckert, S.E.; Roumanas, E.; et al. Osseointegrated craniofacial implants in the rehabilitation of orbital defects: An update of a retrospective experience in the United States. J Prosthet Dent 2005, 94, 177–182. [Google Scholar]

© 2017 by the author. The Author(s) 2017.
Share and Cite
Klimczak, J.; Helman, S.; Kadakia, S.; Sawhney, R.; Abraham, M.; Vest, A.K.; Ducic, Y. Prosthetics in Facial Reconstruction. Craniomaxillofac. Trauma Reconstr. 2018, 11, 6-14. https://doi.org/10.1055/s-0037-1603459
Klimczak J, Helman S, Kadakia S, Sawhney R, Abraham M, Vest AK, Ducic Y. Prosthetics in Facial Reconstruction. Craniomaxillofacial Trauma & Reconstruction. 2018; 11(1):6-14. https://doi.org/10.1055/s-0037-1603459
Chicago/Turabian StyleKlimczak, Jaclyn, Samuel Helman, Sameep Kadakia, Raja Sawhney, Manoj Abraham, Allison K. Vest, and Yadranko Ducic. 2018. "Prosthetics in Facial Reconstruction" Craniomaxillofacial Trauma & Reconstruction 11, no. 1: 6-14. https://doi.org/10.1055/s-0037-1603459
APA StyleKlimczak, J., Helman, S., Kadakia, S., Sawhney, R., Abraham, M., Vest, A. K., & Ducic, Y. (2018). Prosthetics in Facial Reconstruction. Craniomaxillofacial Trauma & Reconstruction, 11(1), 6-14. https://doi.org/10.1055/s-0037-1603459


