Trauma in the craniomaxillofacial region requires surgical reconstruction to reestablish the aesthetic and functional binomial. Such reconstructions vary in the degree of complexity and, therefore, frequently require multiple uses of combined resources.
Regarding the desirable physical–biological properties and optimum handling of biomaterials, the following should be mentioned: (1) biocompatibility, (2) atoxicity, (3) chemical and organic stability, (4) low infection rate, (5) allowing bone growth or neoformation, (6) having natural resistance which allows adjacent reconstructions of the sinus cavities in the craniomaxillofacial segment, (7) low cost, (8) ease of handling [
1,
2,
3,
4], and (9) viability for prototyping, according to the authors.
Castor Oil
The castor bean—
Ricinus communis, class
Dicotyledoneae, order
Geraniales, family
Euphorbiaceae—represents a particular source for obtaining compounds that supply ricinoleic acid (12-hydroxyoleic) with a high degree of chemical purity, with the extracted oil composed of 81 to 96% ricinoleic acid triglycerides and considered to be a natural polyol [
2].
The castor polymer has received Food and Drug Administration (FDA) approval in the United States. The first medical reports concerning the use of polyurethanes date back to 1959, when Mandarino and Salvatore implemented a rigid polyurethane sponge to fix the bone in situ [
7].
The castor bean has great mechanical features, which ensures the supply of polyols and prepolymers from the large-scale production of fatty acids [
5,
8]. Furthermore, castor oil can be considered a natural polyol because it contains three radical hydroxyls capable of being used in the synthesis of polymers [
5,
8].
The polymer derived from castor oil has a molecular formula that is compatible with living tissue and shows favorable characteristics for processing, formulation flexibility, temperature curve versatility, and exothermic peak control in the liquid–gel transition. It also has excellent structural properties, does not release vapors and toxic radicals when implanted, and is low cost [
5,
8].
There are articles reporting cases in which the castor polymer was implanted into defective bone, with good reports of its clinical and biological response. Presently, the polyurethane derived from castor oil shows a molecular formula with favorable characteristics for processability, formulation flexibility, no emission of toxic fumes, good adhesion power, no release of toxic radicals when implanted, and low cost [
9,
10].
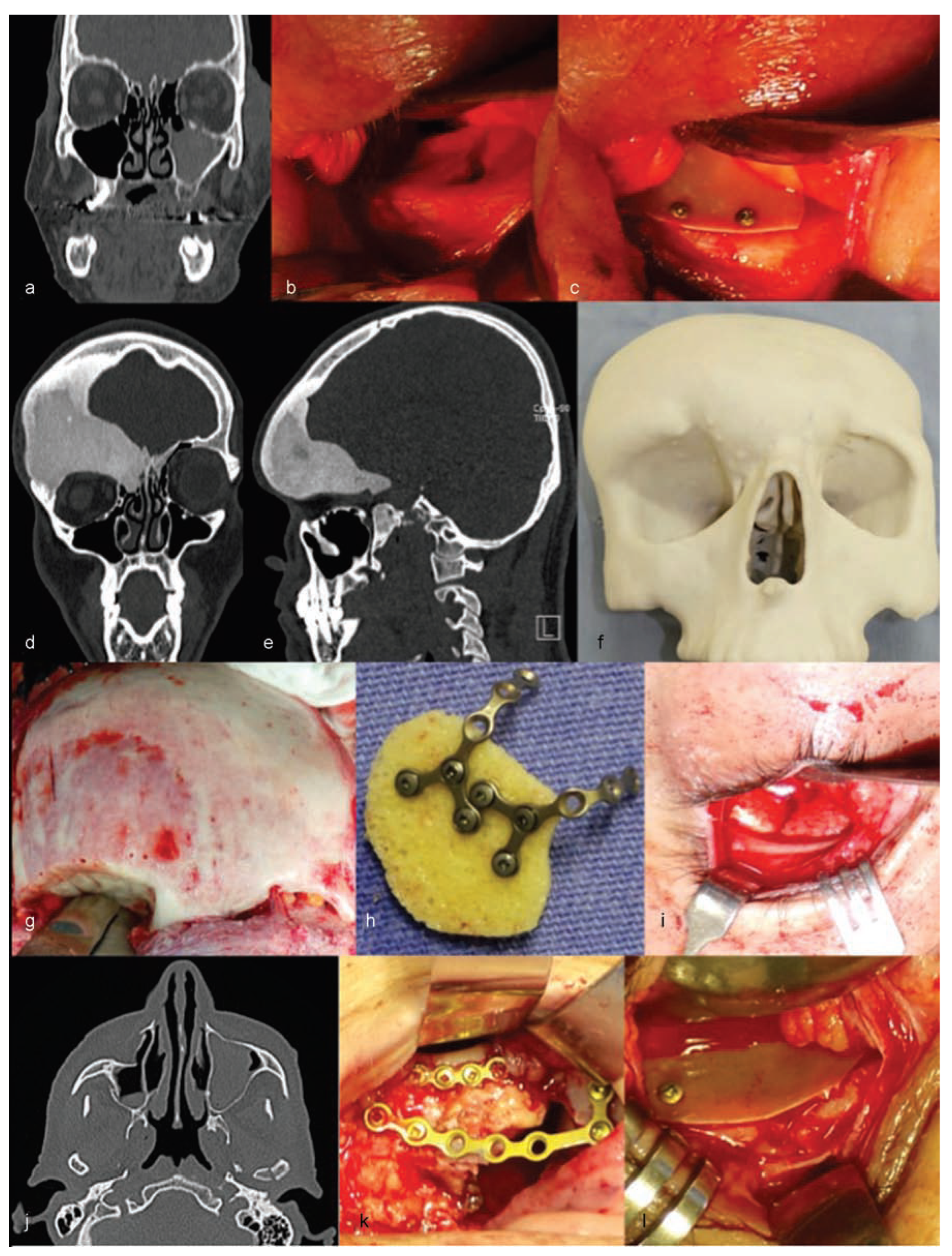
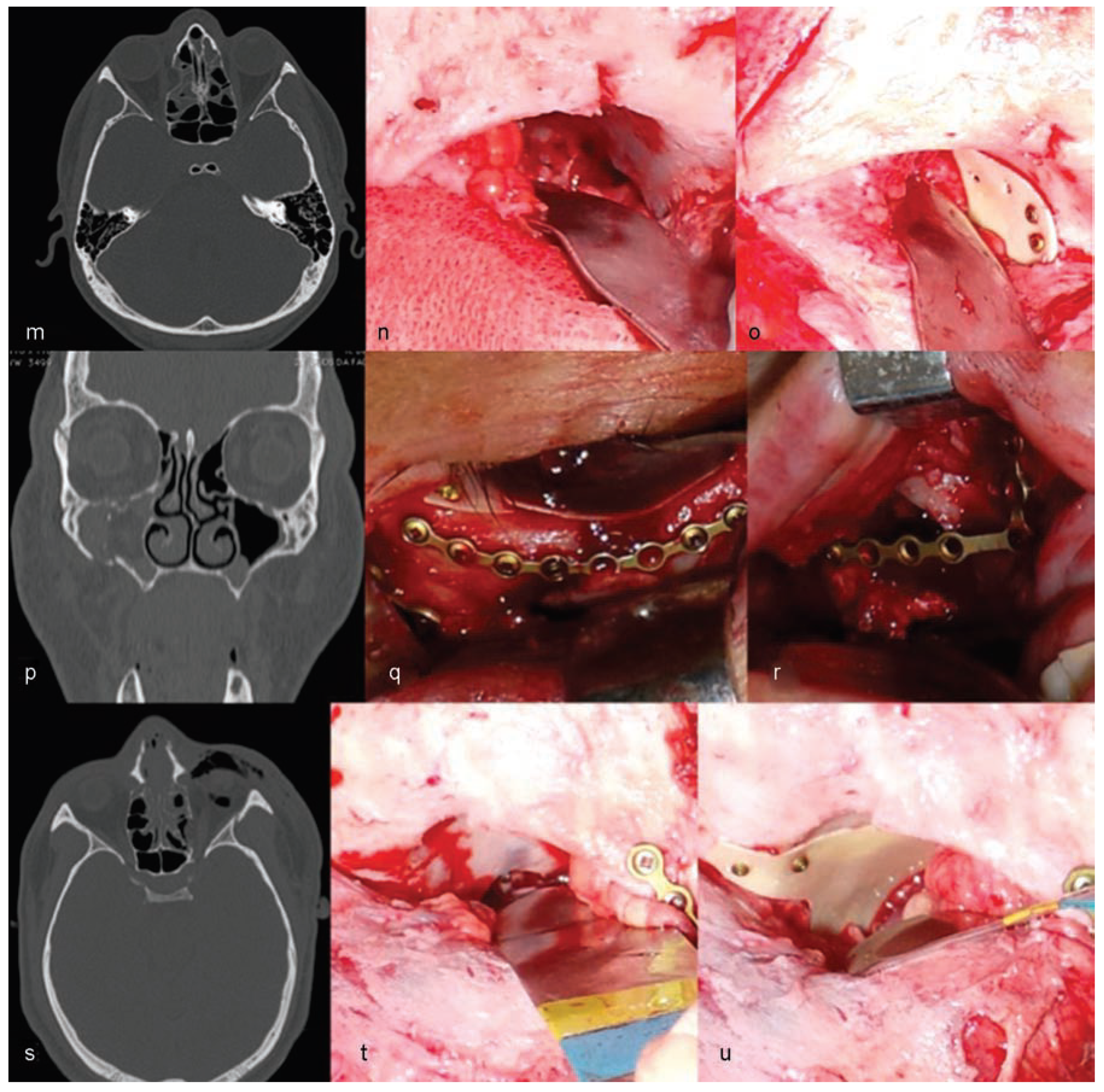
The authors are experienced with the use of castor oil polymer prostheses in several cases of trauma and surgery for camouflage deformities (
Figure 1 and
Figure 2).
This study aims to report a clinical case in which reconstruction in the temporoparietal region was performed, using a castor polymer custom prosthesis.
Case Report
A 26-year-old male patient was the victim of a motorcycle accident. In July 2012, this patient presented with sequelae of traumatic brain injury (TBI) and defect of the cranial vault caused by decompressive craniectomy performed when initial care was provided, and also with superior orbital fissure syndrome.
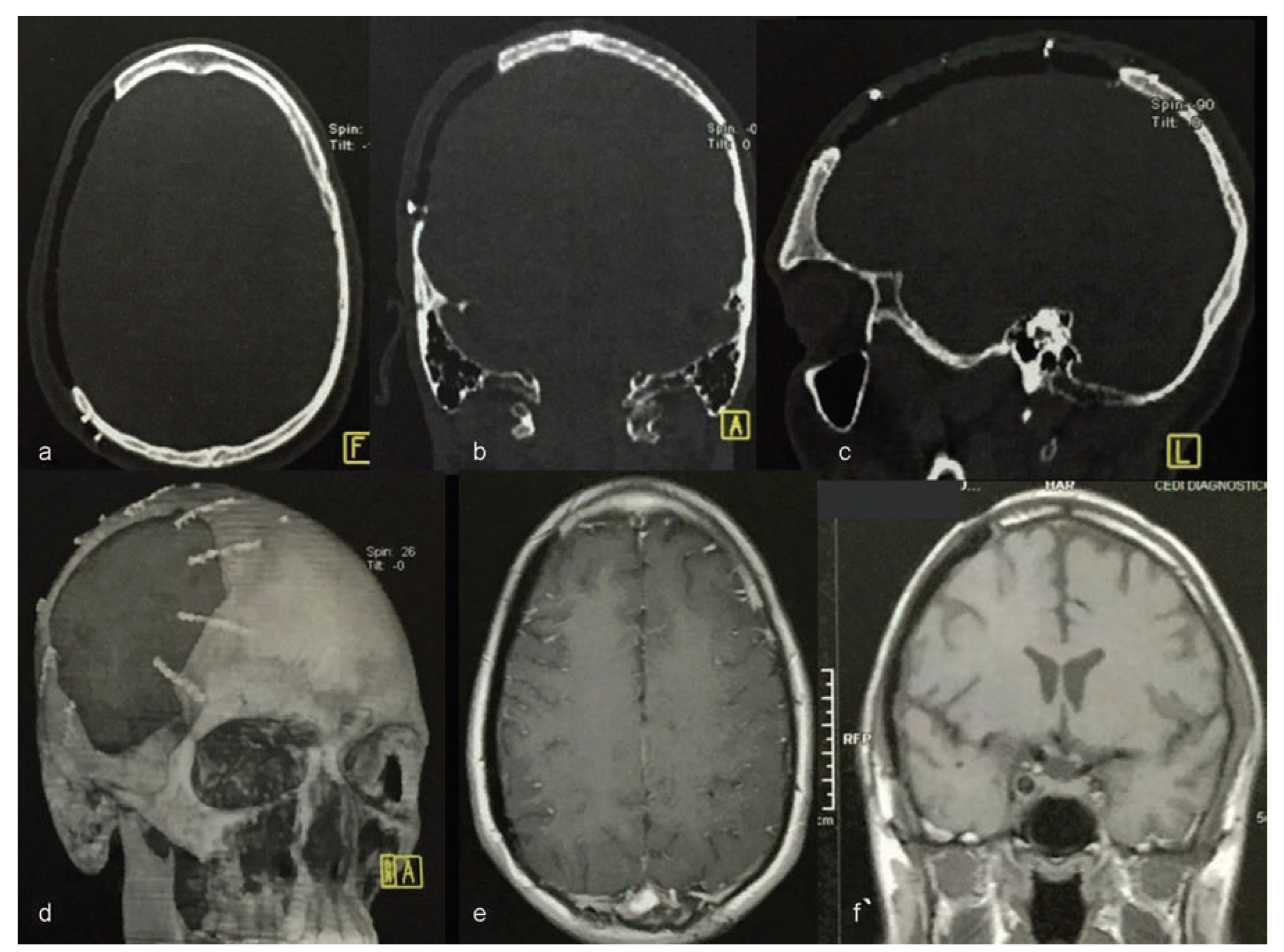
Computerized tomography (CT) was conducted using a 4-channel helical CT scanner Medical Toshiba Asteion 4-slice CT Scanner (Toshiba America Medical Systems, Inc., Tustin, CA). Axial, coronal, and sagittal sections and three-dimensional (3D) reconstruction were conducted (
Figure 3).
The CT scan enabled the construction of the prototype prosthesis using the castor bean polymer. This was done to protect the brain and to rebuild the general anatomical contours of the skull.
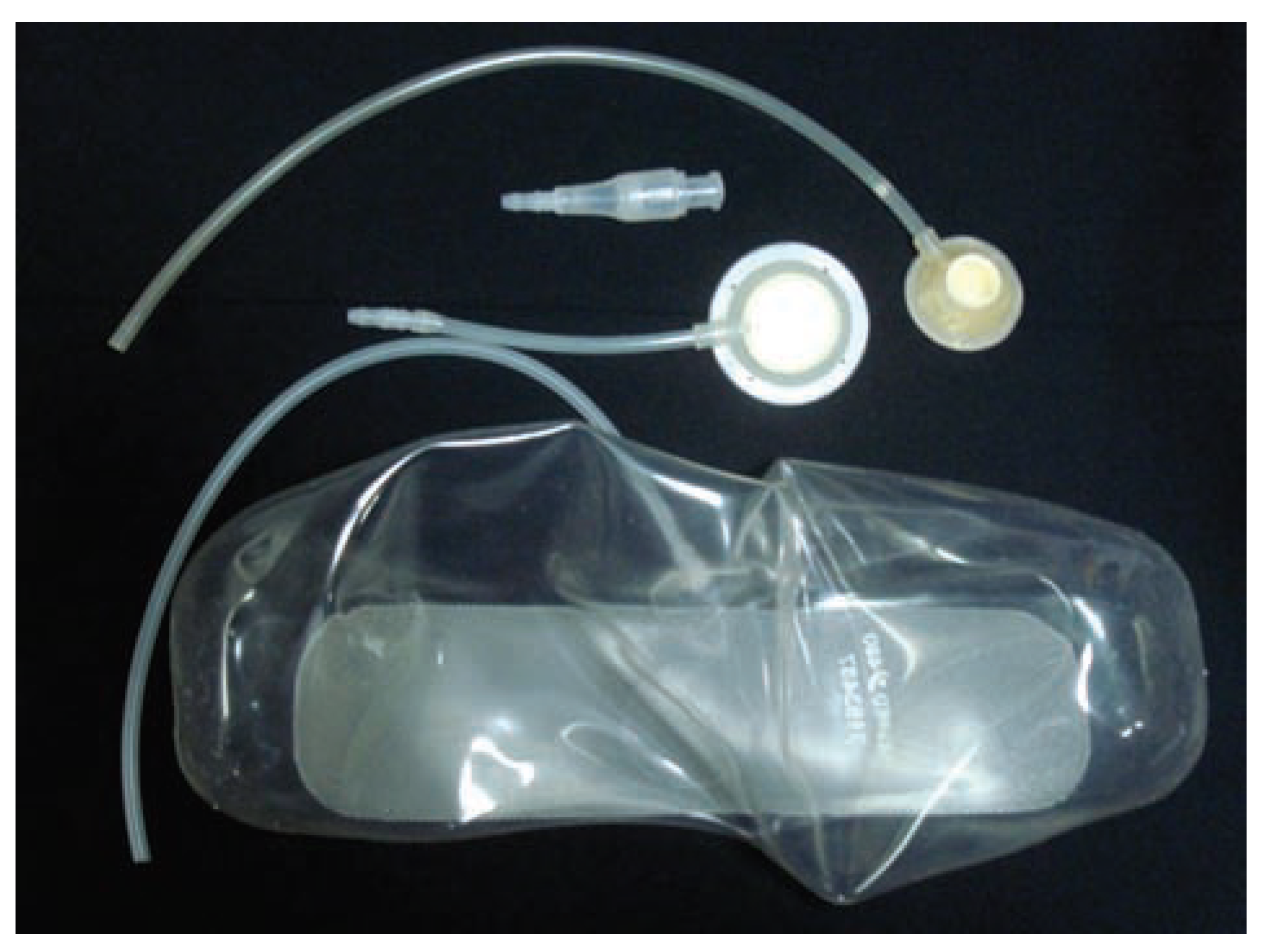
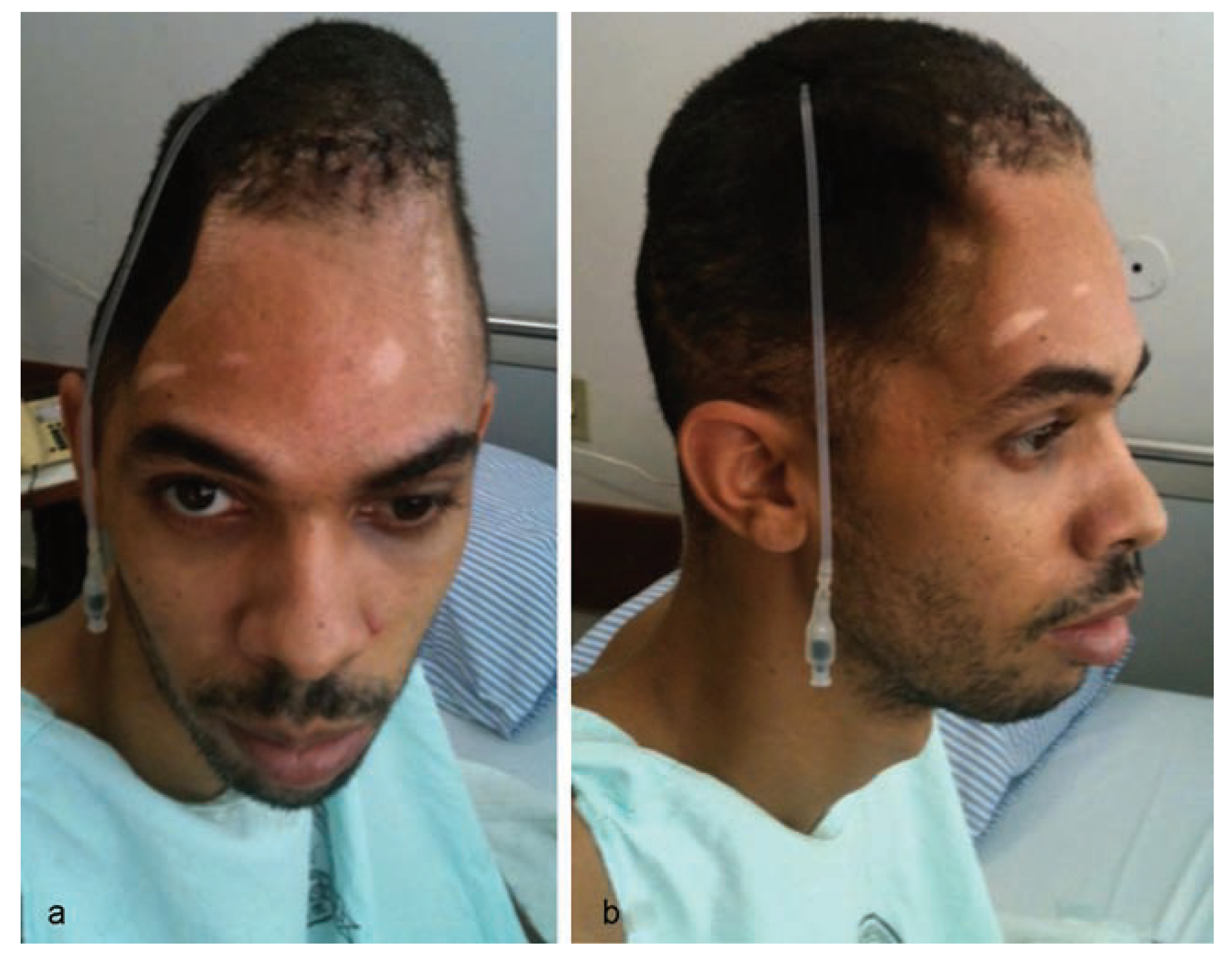
Considering the extensive bone loss and soft-tissue retraction, the installation of a standard type of implantable silicone device for tissue expansion was planned (
Figure 4). The device was longitudinally curved and had 480 mL volumetric capacity, with a semi-implanted tissue expander (SILIMED, Comércio de Produtos Médico-Hospitalares, LTDA, Rio de Janeiro, Brazil).
The first surgery was performed using two access paths: an anterior path located 2.0 cm from the hairline and a posterior path near the area of alopecia. Both accesses were intended for tunneling, which allowed the introduction of the expander (
Figure 5). Following installation of the device, during performance of the same surgery, the first injection of 24 mL saline solution was given to activate the implanted expander.
Owing to the low extensibility of the scalp, it was decided to inject 5% of the total volumetric capacity of the expander each day to allow slow and gradual expansion of the tissues. A 480 mL expander was inflated to 360 mL over a period of 28 days (
Figure 6).
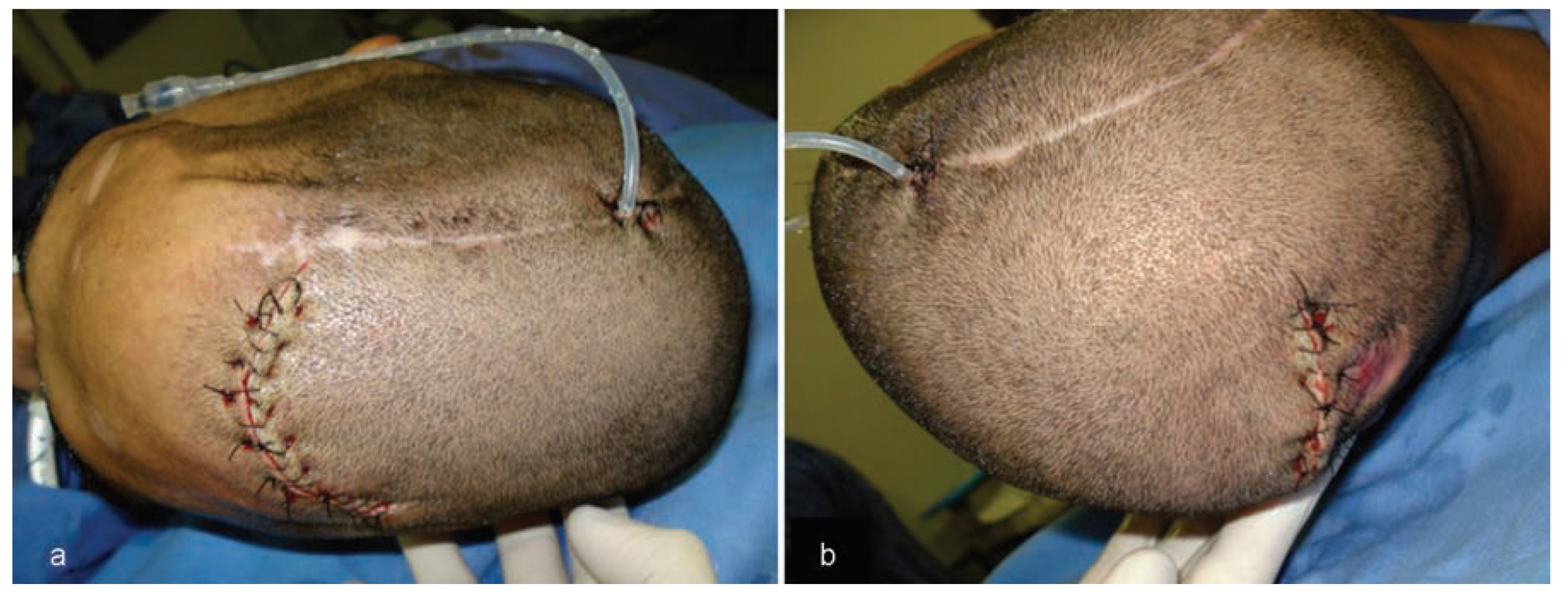
The second surgery allowed extraction of the expander, thus providing an extension of the scalp that permitted closure of the wound with no tension (
Figure 7).
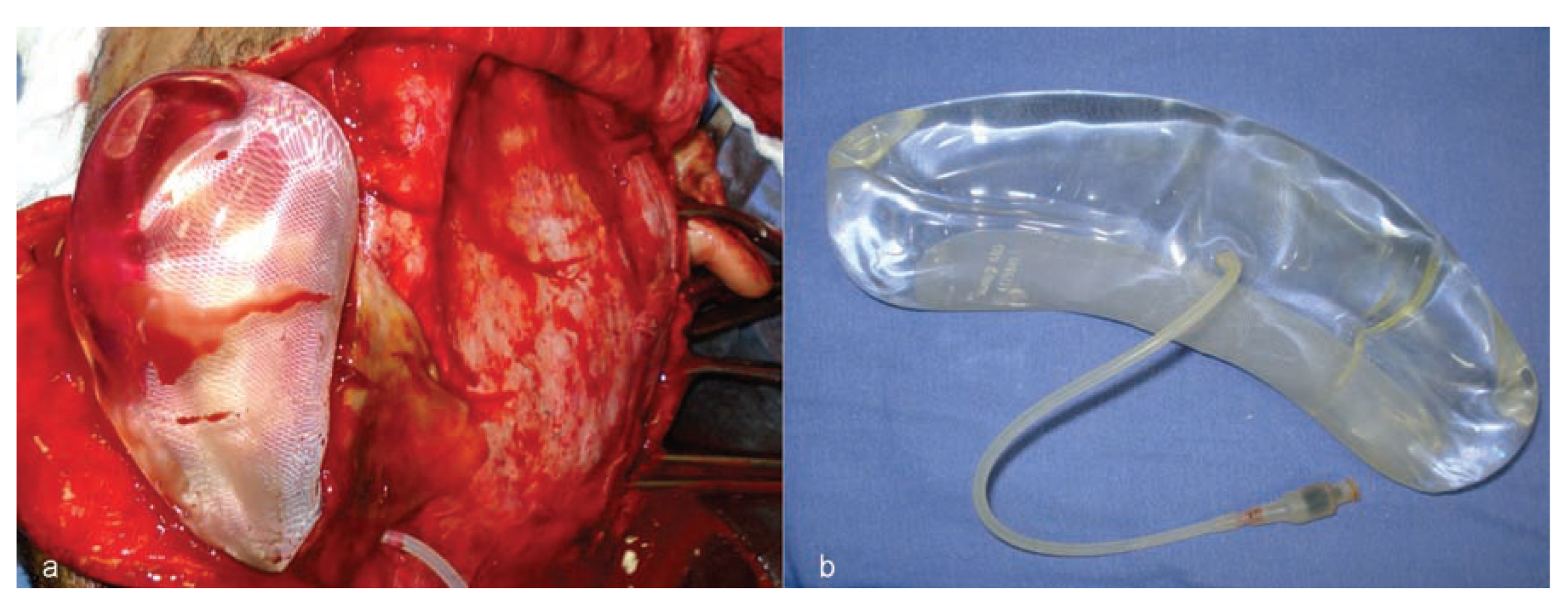
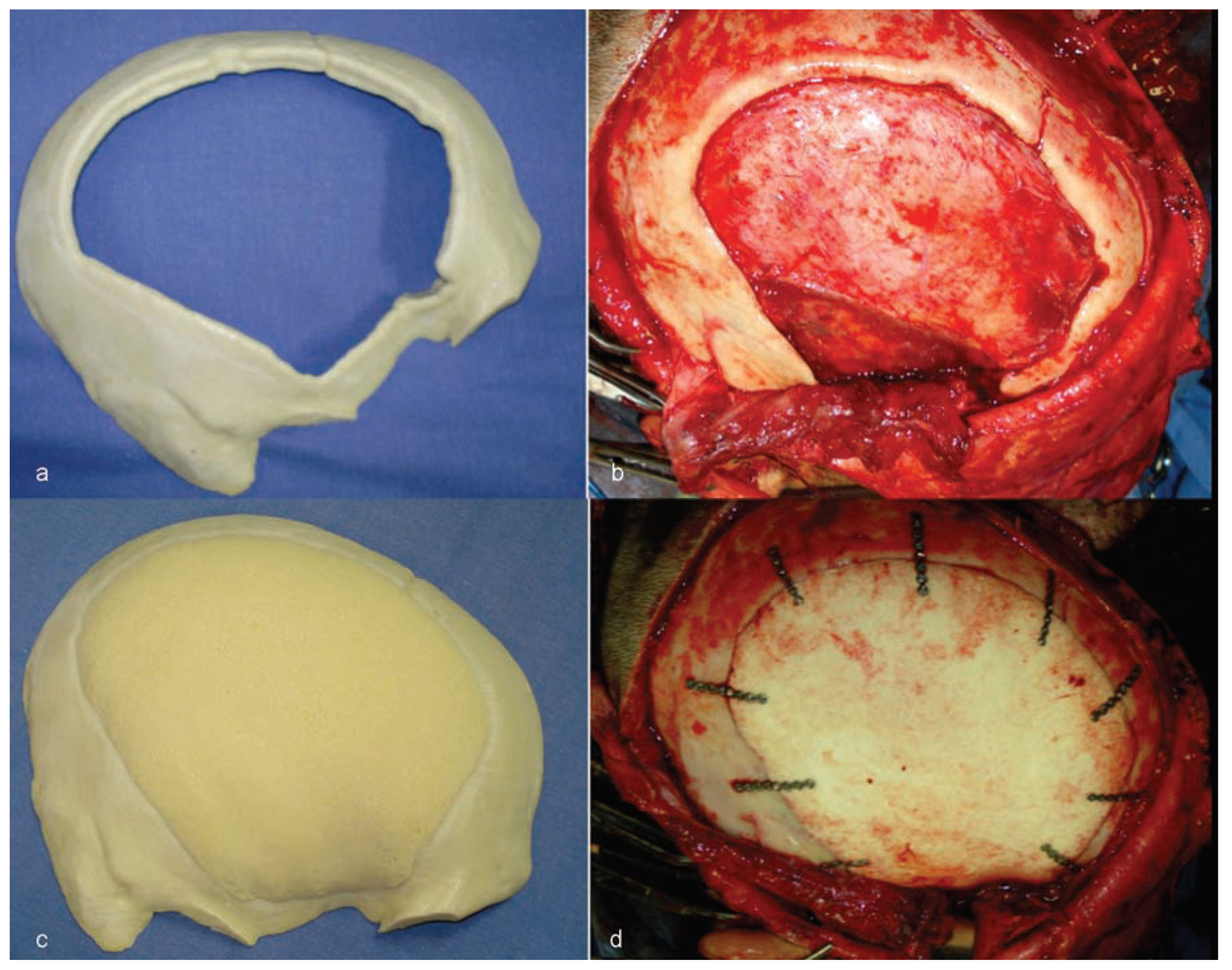
The custom castor oil polymer prosthesis (Poliquil Araraquara Polímeros Químicos, LTDA, Araraquara, Brazil) had the following specifications: dimensions of 125 × 110 × 05 mm; reference no. 3.000.02; registered with the Agência Nacional de Vigilância Sanitária do Brasil (ANVISA; National Agency for Sanitation Oversight of Brazil)—no. 1.03.056.9.0013; lot no. 053.381.001; made and sterilized on November 28, 2012; and with an expiration date of November 2015 (
Figure 8).
Following adaptation of the prosthesis to the area of bone loss, nine titanium (Ti) microplates (KLS Martin, Tuttlingen, Germany; System 1.2 mm) were used to ensure the attachment of the plates to the skull (
Figure 9). In the next step, a pericranial flap taken from the adjacent area, in which the expander was implanted and fixed with resorbable Vicryl 3–0 suture, was used. This was followed by the installation of a drainage vacuum, and then the edges of the surgical wound were closed using Vicryl 2–0 sutures, and the synthesis of the skin was completed using Stapler SW35 mechanical sutures. The exit for the drainage vacuum was attached to the skin with Nylon 3–0 thread. The covering used was a compression bandage with a crepe dressing.
The patient recovered well and the drain was removed on the second postoperative day.
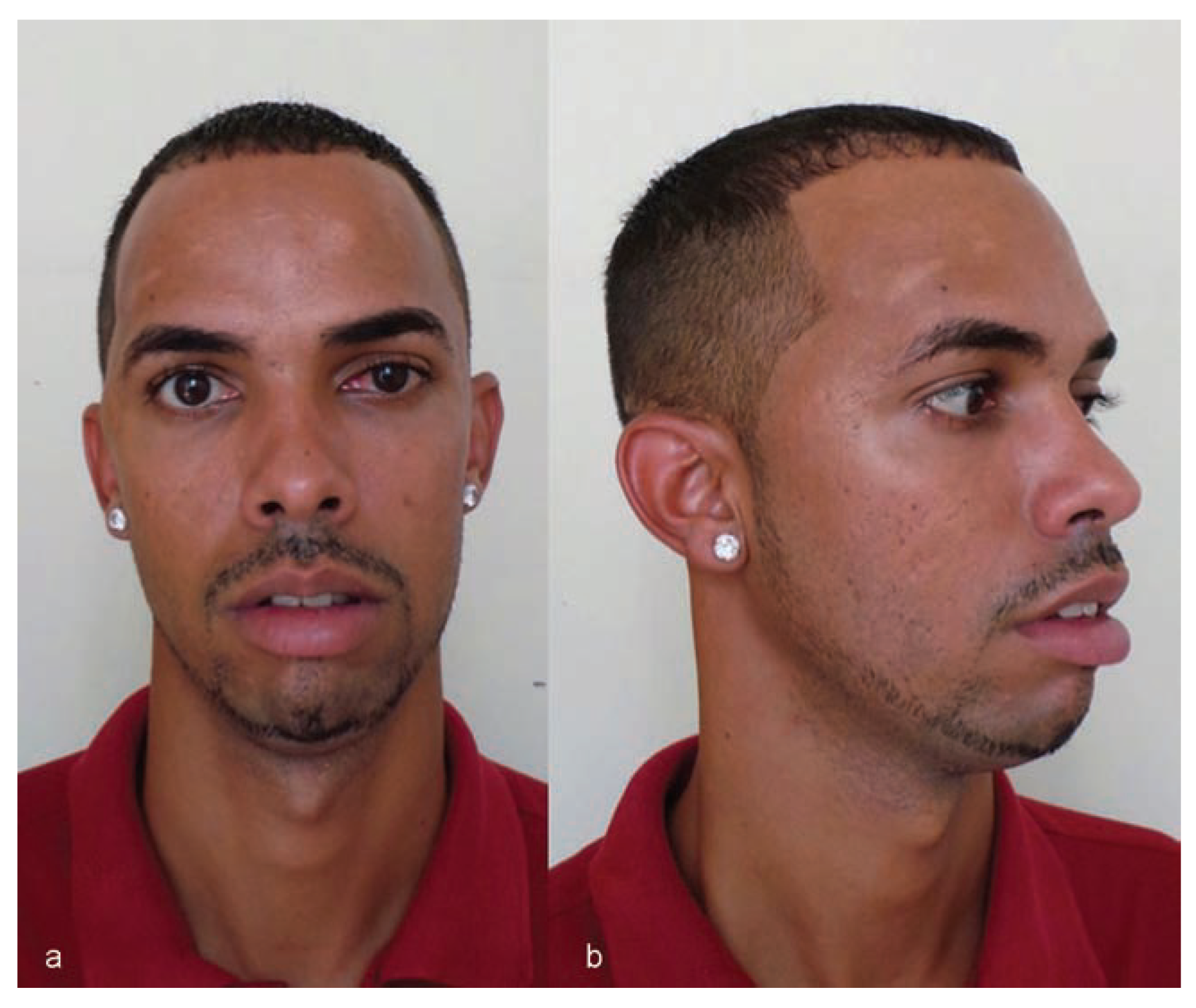
Twenty-eight months after the procedure, the patient was well, with appropriate skull contour reporting no complaints and was very happy with the result (
Figure 10 and
Figure 11).
Discussion
Patients with craniofacial trauma who present with increased intracranial pressure (ICP) are sometimes treated with decompressive craniectomy, allowing the brain to expand and an improvement in ICP, generating conditions that are more favorable for the central nervous system [
11].
When, for various reasons, the skullcap segment is not preserved, a secondary cranioplasty will often be necessary [
11] for restoring brain tissue protection using intact soft and hard tissues, or through prosthetic resources, in addition to restoring local aesthetics [
11].
Regarding the local soft tissue, the following must be evaluated: (1) volume of the area to be reconstructed; (2) local vascularity conditions and local tissue aspect; (3) amount of tissue and eventual retractions; (4) location of previous incisions and presence of traumatic scars; (5) need for covering the calvarial patch during cranioplasty; (6) history of irradiation or infection; and (7) in the case of surgical retreatment, history of previous expansion techniques [
11].
In this study, due to the extensive bone loss and scar retraction, tissue expansion was performed to enable adequate prosthetic coverage of temporoparietal region.
Regarding the reconstruction of craniofacial defects, the choice of material should aim at restoring the thickness and diameter of the lost bone tissue, as well as keeping it stable and biocompatible [
11].
Various materials are available for the reconstruction of craniofacial defects, ranging from the autogenous bone grafts to titanium meshes, osteoinductive and osteoconductive cements, and other biomaterials [
11,
12]. All options have advantages and disadvantages, and no single ideal material for this has yet been found [
11].
The literature presents several options of polymers for the reconstruction of cranial defects, which present desirable characteristics for reconstructions such as the following: (1) they have suitable resistance for the reconstruction of extensive total defects; (2) they are radiolucent but not ferromagnetic, allowing postoperative control with CT scans and nuclear magnetic resonance imaging (NMRI)/MRI. On the other hand, the polymers can be related to postoperative infections [
10].
There is a continuous search for the ideal material for craniofacial reconstructions. Currently, all eyes are directed toward the use of the castor oil polymer as an option for these reconstructions. Among the factors that influence the expectations for the use of this material, the following stand out: (1) easily obtainable; (2) low cost [
1,
2,
5,
6]; (3) does not release toxic radicals [
13]; (4) no inflammatory reaction [
14]; (5) reduced risk of infection; (6) bactericidal effect [
1,
14]; (7) biocompatible [
1,
5,
6]; and (8) osteoconductive [
1,
5,
6,
14].
As the castor oil polymer is still a very recent option, few publications are found in the literature with respect to its use in craniofacial reconstructions in customized prosthesis.
Nunes [
13] conducted a study comparing the use of titanium plates and screws and castor oil polymer (Poliquil/OsteomedRio Claro, SP, Brazil). According to the author, in cases where castor oil polymer–derived materials were used, fracture healing as well as local bone neoformation, in addition to normalizing the inflammatory process, occurred after 60 days. According to the author, both materials have similar results, which justified the use of the castor oil polymer for facial fractures.
Kubrusly et al. [
7] wanted to compare the tissue reaction between the titanium and the castor oil polymer found in the peritoneal region of animals. The authors observed a chronic inflammation related to the use of both materials; however, there was no structural damage macroscopic and/or microscopic with the use of the castor oil polymer. When submitted to the guinea pig’s organism temperature, the polyurethanes showed a thermal stability of 210 °C, which proved that these polymers will not suffer thermolysis when left in room temperature.
Dias et al. [
1] evaluated the biocompatibility of the castor oil polymer used in defects in the nasal dorsum of monkeys (
Cebus apella monkeys). The result is favorable, as no inflammatory reaction was observed, and there was local bone formation. When the castor oil polymer was used in the skullcaps of rabbits, Laureano Filho et al. [
6] also obtained encouraging results, as, according to the authors, this material is related to osteoconduction and the absence of infection and inflammation.
Leite and Ramalho [
15] compared the use of castor oil polymer and demineralized bovine matrix in the mandibular bone defects of rats. The use of both materials was related to bone formation and bone conduction. These authors considered the castor oil polymer as an alternative for the reconstruction of bone defects.
Gurgel et al. [
16] presented two clinical cases in which they use a castor oil polymer membrane for the reconstruction of orbital defects. According to the authors, the material was suitable and did not present any postoperative complications within 24 months.
The use of castor oil polymer helps in the integration of the adjacent tissues and the interaction with nontoxic bone tissue [
15]. In addition, this polymer undergoes resorption and replacement by bone tissue free of pus and inflammation. However, the duration of this reaction cannot be defined, as the castor oil polymer exhibits with different sized molecules [
15].
Based on the earlier-mentioned studies on the castor oil polymer, the authors expose a clinic case of the extensive reconstruction of the defect in the temporoparietal region.
This article is in accordance with the fundamentals of the several studies [
1,
6,
15,
16] that conclude for a successful reconstruction of the temporoparietal defect. In a follow-up evaluation, 1 year after the surgery, there was no extrusion of the prosthesis with prosthetic–bone interface, and also there was no infection.
Insaurralde et al. [
17] made a comparison between two biomaterials (inorganic bovine bone and castor oil polymer) and a regular bone tissue (mineralized and demineralized) to evaluate the traction resistance and the bone elastic modulus of the newly formed bone after grafting.
The specimens were subjected to microtensile, and the authors observed an increased strength of the mineralized bone and a higher resistance of the castor oil polymer on the inorganic bone. The higher elasticity modulus of the mineralized bone is similar between the bovine bone group and the castor oil polymer.
Despite the fact that the use of castor oil polymer needs to be more explored and studied in order to be a certified reconstructive material option for the craniomaxillofacial region, this article concords with the earlier studies concludes to satisfactory results in the use of this material.