Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exlusion Criteria
2.3. Study Design
2.4. Polymorphism Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaddox, L.M.; Morford, L.A.; Nibali, L. Periodontal health and disease: The contribution of genetics. Periodontol. 2000 2021, 85, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 4, 268–288. [Google Scholar] [CrossRef]
- Ebersole 3rd, J.L.; Dawson, D.; Emecen-Huja, P.; Nagarajan, R.; Howard, K.; Grady, M.E.; Thompson, K.; Peyyala, R.; Al-Attar, A.; Lethbridge, K.; et al. The periodontal war: Microbes and immunity. Periodontol. 2000 2017, 75, 52–115. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Offenbacher, S.; Schroeder, H.E.; Seymour, G.J.; Kornman, K.S. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol. 2000 1997, 14, 216–248. [Google Scholar] [CrossRef]
- Garlande, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Baidaa, T.A.; Maha, S. Assessment of salivary interleukin-1 receptor antagonist and obesity measures of patients with chronić periodontitis in comparison to healthy control. Int. J. Med. Res. Health Sci. 2017, 6, 173–178. [Google Scholar]
- Kornman, K.S.; Crane, A.; Wang, H.Y.; di Giovine, F.S.; Newman, M.G.; Pirk, F.W.; Wilson, T.G., Jr.; Higginbottom, F.L.; Duff, G.W. The interleukin–1 genotype as a severity gfactor in adult periodontal disease. J. Clin. Periodontol. 1997, 24, 72–77. [Google Scholar] [CrossRef]
- Da Silva, F.R.P.; Pessoa, L.D.S.; Shin, J., II; Alves, E.H.P.; Koga, R.S.; Smith, C.V.; Vasconcelos, D.F.P.V.; De Cuhna Pereira, A.C.T. Polymorphisms in the interleukin genes and chronic periodontitis: A field synopsis and revaluation by Bayesian approaches. Cytokines 2021, 138, 155361. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, J.B.; Zacarias, J.M.V.; Tsuneto, P.Y.; Souza, V.H.; Silva, C.O.E.; Visentainer, J.E.L.; Sell, A.M. Influence of inflammasome NLRP3, and IL1B and IL2 gene polymorphisms in periodontitis susceptibility. PLoS ONE 2020, 15, e0227905. [Google Scholar] [CrossRef]
- Liu, X.; Li, H. A systematic review and meta-anlysis on multiple cytokine gene polymorphisms in the pathogenesis of periodontits. Front. Immunol. 2022, 12, 713198. [Google Scholar] [CrossRef]
- Da Silva, F.R.P.; Galeno, J.G.; Bentes Leal, A.L.A.; Koga, R.S.; Batista, N.Y.; da Conceição Furtado, S.; Vasconcelos, D.F.P.; Carvalho, M.D.; Barcellos, J.F.M. Non-significant association between—330 T/G polymorphism in interleukin-2 gene and chronic periodontitis: Findings from a meta-analysis. BMC Oral Health 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shirodaria, S.; Smith, J.; McKay, I.J.; Kennett, C.N.; Hughes, F.J. Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1α protein in gingival crevicular fluid of teeth with severe periodontal disease. J. Dent. Res. 2000, 79, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Hulkonen, J.; Laippala, P.; Hurne, M. A rare allele combination of the interleukin-1 gene complex is associated with high interleukin-1b plasma levels in healthy individuals. Eur. Cytukine Netw. 2000, 11, 251–255. [Google Scholar]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), 149–161. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38–46. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposal for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Dominguez-Perez, R.A.; Loyola-Rodriguez, J.P.; Abud-Mendoza, C.; Alpuche-Solis, A.G.; Ayala-Herrera, J.L.; Martínez-Martínez, R.E. Association of cytokines polymorphisms with chronic peridontitis and rheumatoid arthritis in a Mexican population. Acta Odontol. Scand. 2017, 75, 243–248. [Google Scholar] [CrossRef]
- da Silva, F.R.P.; Vasconcelos, A.C.; de Carvalho Franca, L.F.; Di Lenardo, D.; Silva Nascimento, H.M.; Pereira Vasconcelos, D.F. Association between the rs1143634 polymorphism in interleukin-1B and chronic periodontitis: Results from a meta-analysis composed by 54 case/control studies. Gene 2018, 668, 97–106. [Google Scholar] [CrossRef]
- Huang, W.; He, B.Y.; Shao, J.; Jia, X.W.; Yuan, Y.D. Interleukin-1β rs1143627 polymorphism with susceptibility to periodontal disease. Oncotarget 2017, 8, 31406–31414. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyake, Y.; Hanioka, T.; Arakawa, M. Relationship between IL1 gene polymorphisms and periodontal disease in Japanese women. DNA Cell Biol. 2014, 33, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Trevilatto, P.C.; de Souza Pardo, A.P.; Scarel-Caminaga, R.M.; de Brito, R.B., Jr.; Alvim-Pereira, F.; Alvim-Pereira, C.C.; Probst, C.M.; Garlet, G.P.; Sallum, A.W.; Line, S.R.P. Association of IL1 gene polymorphisms with chronic periodontitis in Brazilians. Arch. Oral Biol. 2011, 56, 54–62. [Google Scholar] [CrossRef]
- Wu, X.; Offenbacher, S.; López, N.J.; Chen, W.; Wang, H.Y.; Rogus, J.; Zhou, J.; Beck, S.; Jiang, X.; Bao, L.; et al. Association of interleukin-1 gene variations with moderate to severe chronic periodontitis in multiple ethnicities. J. Periodontal. Res. 2015, 50, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Karimbux, N.Y.; Saraiya, V.M.; Elangovan, S.; Allareddy, V.; Kinnunen, T.; Kornamn, K.S.; Duff, G.W. Interleukin-1 gene polymorphisms and chronic periodontitis in adult whites: A systematic review and meta- analysis. J. Periodontol. 2012, 83, 1407–1419. [Google Scholar] [CrossRef]
- Sakellari, D.; Koukoudetsos, S.; Arsenakis, M.; Konstantinidis, A. Prevalence of Il-1A and IL-1B polymorphisms in a Greek population. J. Clin. Periodontol. 2003, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, R.G.; Westerman, B.; Hamlet, S.M.; Palmer, J.E.; Faddy, M.J.; Lang, N.P.; Seymourt, G.J. A longitudinal study of interleukin-1 gene polymorphisms and periodontal disease in a general adult population. J. Clin. Periodonytol. 2001, 28, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.J.; Wang, H.-Y.; Knobelman, C.; Newman, M.G.; di Giovine, F.S.; Timms, J.; Duff, G.W.; Kornman, K.S. Interleukin-1 genetic association with periodontitis in clinical practice. J. Periodontol. 2000, 71, 156–163. [Google Scholar] [CrossRef]
- Zeng, X.T.; Liu, D.Y.; Kwong, J.S.; Leng, W.D.; Xia, L.Y.; Mao, M. Meta-analysis of association between interleukin-1β C-511T polymorphism and chronic periodontitis susceptibility. J. Periodontol. 2015, 86, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, D.; Bazzato, M.F.; Ferlin, A.; Pengo, M.; Frigo, A.C.; Favero, G.; Foresta, C.; Stellini, E. Role of familiarity versus interleukin-1 genes cluster polymorphisms in chronic periodontitis. Gene 2014, 535, 286–289. [Google Scholar] [CrossRef]
- Armingohar, Z.; Jorgensen, J.J.; Kristoffersen, A.K.; Schenck, K.; Dembic, Z. Polymorphisms in the interleukin-1 gene locus and chronic periodontitis in patients with atherosclerotic and aortic aneurysmal vascular diseases. Scand. J. Immunol. 2014, 79, 338–345. [Google Scholar] [CrossRef]
- Mohammadi, H.; Roochi, M.M.; Sadeghi, M.; Garajei, A.; Heidar, H.; Meybodi, A.A.; Dallband, M.; Mostafavi, S.; Mostafavi, M.; Salehi, M.; et al. Association between Interleukin-1 Polymorphisms and Susceptibility to Dental Peri-Implant Disease: A Meta-Analysis. Pathogens 2021, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Dominici, R.; Cattaneo, M.; Malferrari, G.; Archi, D.; Mariani, C.; Grimaldi, L.; Biunno, I. Cloning and functional analysis of the allelic polymorphism in the transcription regulatory region of interleukin-1α. Immunogenetics 2002, 54, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Anusaksathien, O.; Sukboon, A.; Sitthiphong, P.; Teanpaisan, R. Distribution of interleukin-b+3954 and IL-1a−889 genetic variations in a Thai population group. J. Periodontol. 2003, 74, 1796–1802. [Google Scholar] [CrossRef]
- Boukortt, K.N.; Saidi-Ouahrani, N.; Boukerzaza, B.; Ouhaibi-Djellouli, H.; Hachmaoui, K.; Benaissa, F.Z.; Taleb, L.; Drabla-Ouahrani, H.; Deba, T.; Ouledhamou, S.A.; et al. Association analysis of the IL-1 gene cluster polymorphisms with aggressive and chronic periodontitis in the Algerian population. Arch. Oral Biol. 2015, 60, 1463–1470. [Google Scholar] [CrossRef]
- da Silva, F.R.; Guimaraes-Vasconcelos, A.C.; de-Carvalho-Franca, L.F.; di-Lenardo, D.; Rodrigues, L.S.; Barreto-do-Nascimento, M.L.; Vasconcelos, D.F. Relationship between -889 C/T polymorphism in interleukin-1A gene and risk of chronic periodontitis: Evidence from a meta-analysis with new published findings. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Slotwinska, S.M. The Interleukin-1 receptor antagonist (IL-1-Ra) and soluble tumor necrosis factor receptor I (sTNF RI) in periodontal disease. Open J. Immunol. 2013, 3, 10–16. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Nikolic, N.; Jacimovic, J.; Miletic, M.; Andric, M.; Milasin, J.; Aminoshariae, A.; Azarpazhooh, A. Tumor Necrosis Factor Alpha-308 G/A Single-Nucleoide Polymorphism and Apical Periodontitis: An Updated Systematic Review and Meta-analysis. J. Endod. 2021, 47, 1061–1069. [Google Scholar] [CrossRef]
- Cui, L.; Sun, Y.P.; Li, D.G.; Wang, S.H.; Shao, D. Transforming growth factor-β1 rs1800469 polymorphism and periodontitis risk: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 15569–15574. [Google Scholar]
- Huang, J.; Ding, C.; Chen, X.; He, R.; Chen, N. Association of TGF-β1-509C/T, +869T/C, and +915G/C polymorphisms with periodontitis susceptibility. Oral Dis. 2015, 21, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Walther, K.A.; Gonzales, J.R.; Gröger, S.; Ehmke, B.; Kaner, D.; Lorenz, K.; Eickholz, P.; Kocher, T.; Kim, T.S.; Schlagenhauf, U.; et al. The Role of Polymorphisms at the Interleukin-1, Interleukin-4, GATA-3 and Cyclooxygenase-2 Genes in Non-Surgical Periodontal Therapy. Int. J. Mol. Sci. 2022, 23, 7266. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, L.; Sun, Y.; Li, L.; Xu, Y. Interleukin-1 receptor antagonist polymorphism (Rs2234663) and periodontitis susceptibility: A meta-analysis. Arch. Oral Biol. 2012, 57, 585–593. [Google Scholar] [CrossRef]
- Lu, J.; Ren, B.; Wang, L.; Li, M.; Liu, Y. Preparation and evaluation of IL-1ra-loaded dexttran/PLGA nicrospheres for inhibiting periodontal inflammation in vitro. Inflammation 2020, 43, 168–178. [Google Scholar] [CrossRef]
- Hoffmann, S.C.; Stanley, E.M.; Cox, E.D.; DiMercurio, B.S.; Koziol, D.E.; Harlan, D.M.; Kirk, A.D.; Blair, P.J. Ethnicity greatly influences cytokine gene polymorphism distribution. Am. J. Transplant. 2002, 2, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kobyashi, T.; Ito, S.; Kuroda, T.; Yamamoto, K.; Sugita, N.; Narita, I.; Sumida, T.; Gejyo, F.; Yoshie, H. The interleukin-1 and Fcγ receptor gene polymorphisms in Japanese patients with rheumatoid arthritis and periodontitis. J. Periodontol. 2007, 12, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Isaza-Guzmán, D.M.; Hernández-Viana, M.; Bonilla-León, D.M.; Hurtado-Cadavid, M.C.; Tobón-Arroyave, S.I. Determination of NLRP3 (rs4612666) and IL-1B (rs1143634) genetic polymorphisms in periodontally diseased and healthy subjects. Arch. Oral Biol. 2016, 65, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.S.; Pacheco, R.B.; Fischer, R.G.; Macedo, J.M. Interaction of IL1B and IL1RN polymorphisms, smoking habit, gender, and ethnicity with aggressive and chronic periodontitis susceptibility. Contemp. Clin. Dent. 2016, 7, 349–356. [Google Scholar] [PubMed]
- Papapanou, P.N.; Neiderud, A.M.; Sandros, J.; Dahlen, G. Interleukin-1 gene polymorphisms and periodontal status. A case control study. J. Clin. Periodontol. 2001, 28, 389–396. [Google Scholar] [CrossRef]
- Meisel, P.; Siegemund, A.; Dombrowa, S.; Sawaf, H.; Fanghaenel, J.; Kocher, T. Smoking and polymorphisms of interleukin-1 gene cluster (IL-1alpha, IL-1beta, and IL-1RN) in patients with periodontal disease. J. Periodontol. 2002, 73, 27–32. [Google Scholar] [CrossRef]
| Variable | Tests | Controls | p |
|---|---|---|---|
| Age mean ± SD (years) | 36.31 ± 8.27 | 35.53 ± 6.88 | 0.431 |
| Sex F/M n (%) | 26/24 (52/48) | 19/16 (54.2/45.8) | 0.425 |
| Number of teeth | 22.66 ± 1.53 | 23.12 ± 1.10 | 0.406 |
| PPD (mm) | 4.32 ± 1.68 | 1.45 ± 0.66 | <0.001 |
| CAL (mm) | 3.68 ± 1.87 | 0.083 ± 0.22 | <0.001 |
| FMPI (%) | 20.51 ± 6.57 | 6.59 ± 5.69 | <0.001 |
| FMBOP (%) | 38.82 ± 19.02 | 15.25 ± 11.65 | <0.001 |
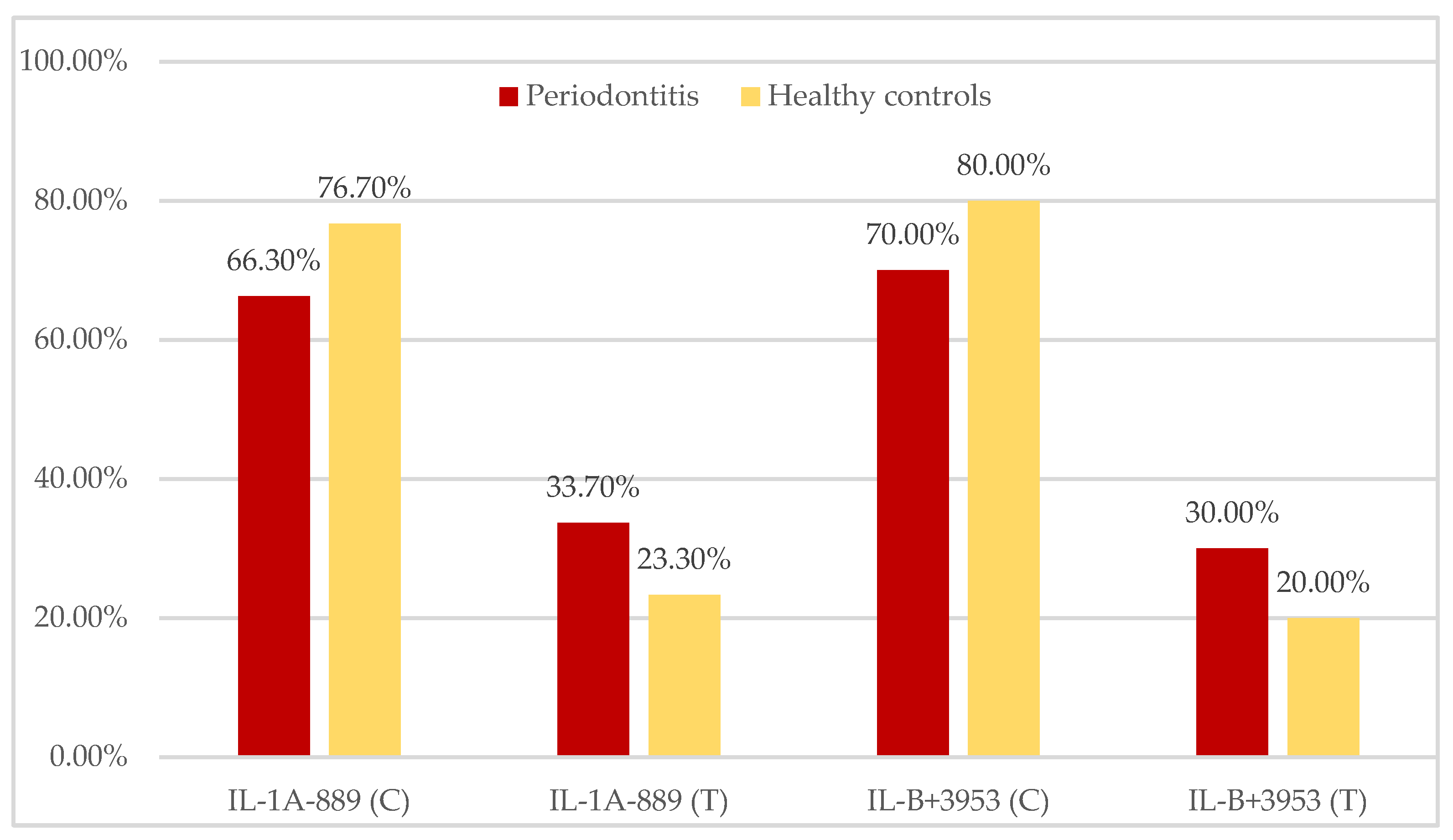
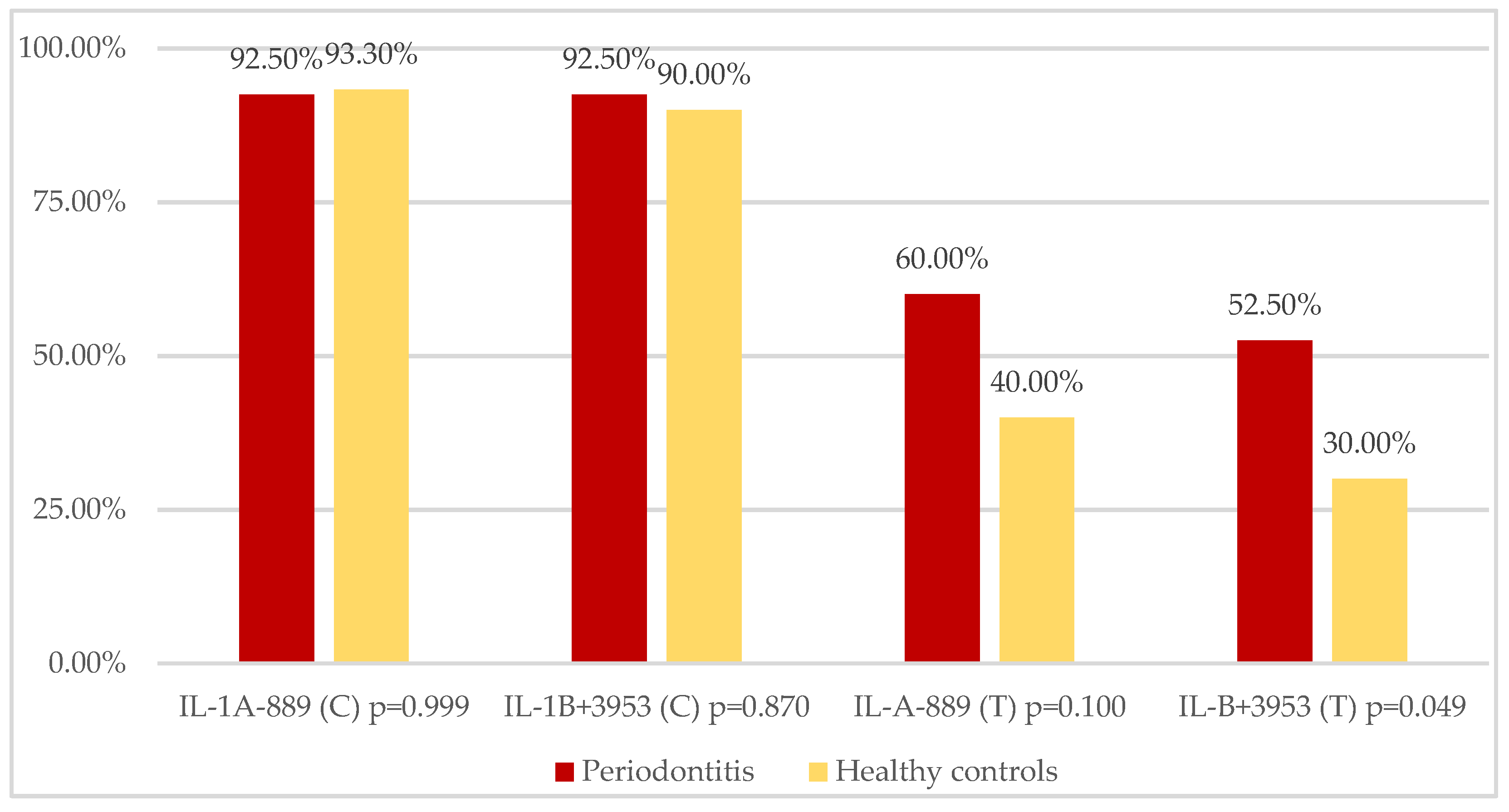
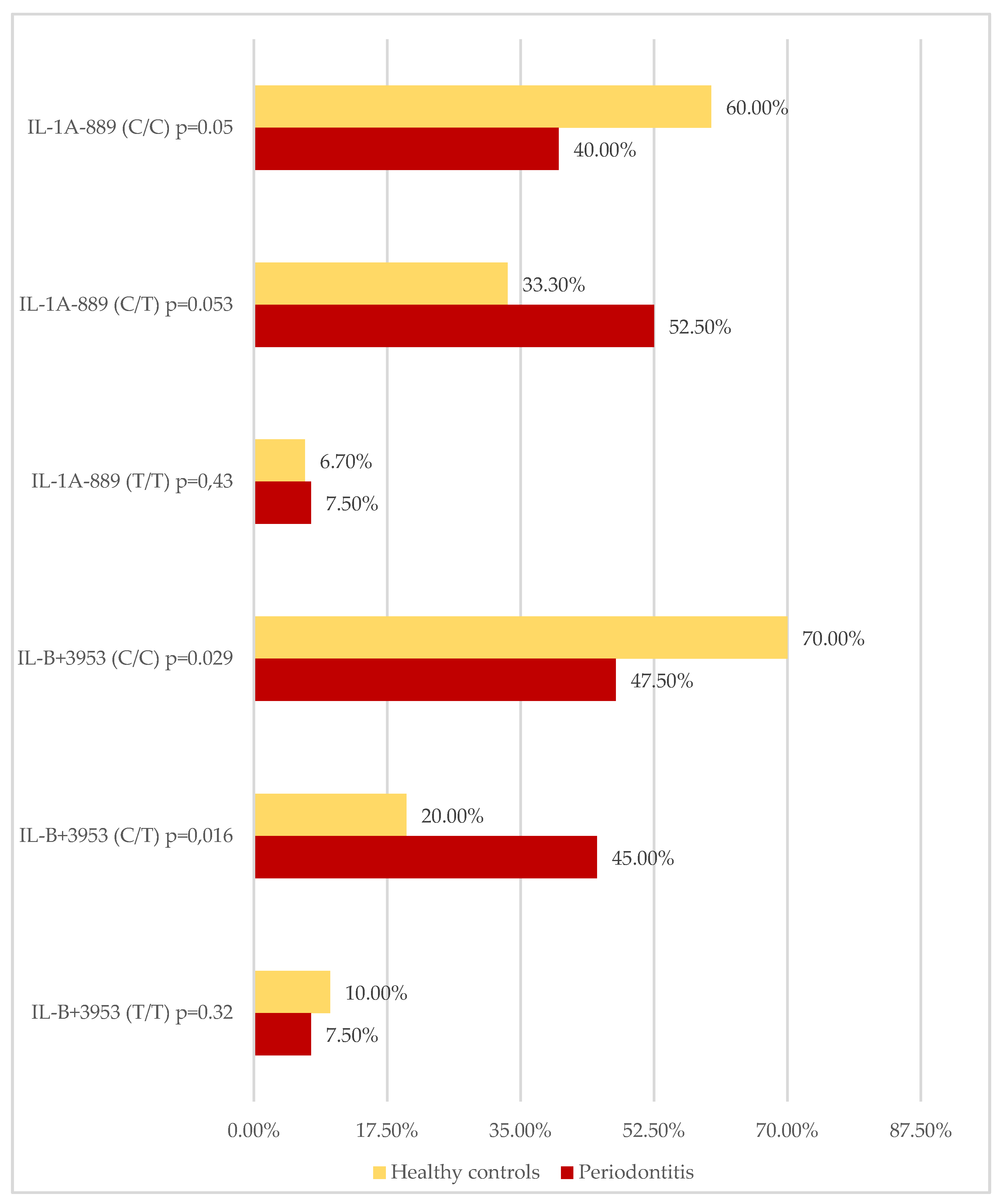
| IL-1A CC/CT/TT n (%) | 16/40 (40.0%) 21/40 (52.5%) 3/40 (7.5%) | 18/30 (60.0%) 10/30 (33.3%) 2/30 (6.7%) | 0.051 0.053 0.431 |
| IL-1B CC/CT/TT n (%) | 19/40 (47.5%) 18/40 (45.0%) 3/40 (7.5%) | 21/30 (70.0%) 6/30 (20.0%) 3/30 (10.0%) | 0.029 0.016 0.322 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodzikowska, A.; Górski, B.; Bogusławska-Kapała, A. Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population. Int. J. Environ. Res. Public Health 2022, 19, 14687. https://doi.org/10.3390/ijerph192214687
Brodzikowska A, Górski B, Bogusławska-Kapała A. Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population. International Journal of Environmental Research and Public Health. 2022; 19(22):14687. https://doi.org/10.3390/ijerph192214687
Chicago/Turabian StyleBrodzikowska, Aniela, Bartłomiej Górski, and Agnieszka Bogusławska-Kapała. 2022. "Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population" International Journal of Environmental Research and Public Health 19, no. 22: 14687. https://doi.org/10.3390/ijerph192214687
APA StyleBrodzikowska, A., Górski, B., & Bogusławska-Kapała, A. (2022). Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population. International Journal of Environmental Research and Public Health, 19(22), 14687. https://doi.org/10.3390/ijerph192214687







