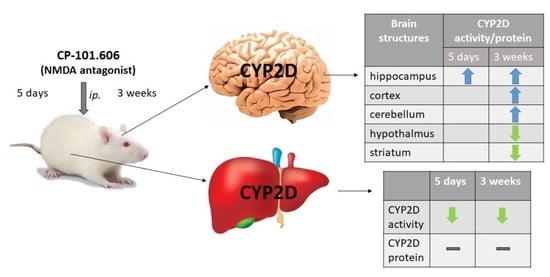
The Effect of the Selective N-methyl-D-aspartate (NMDA) Receptor GluN2B Subunit Antagonist CP-101,606 on Cytochrome P450 2D (CYP2D) Expression and Activity in the Rat Liver and Brain
Abstract
1. Introduction
2. Results
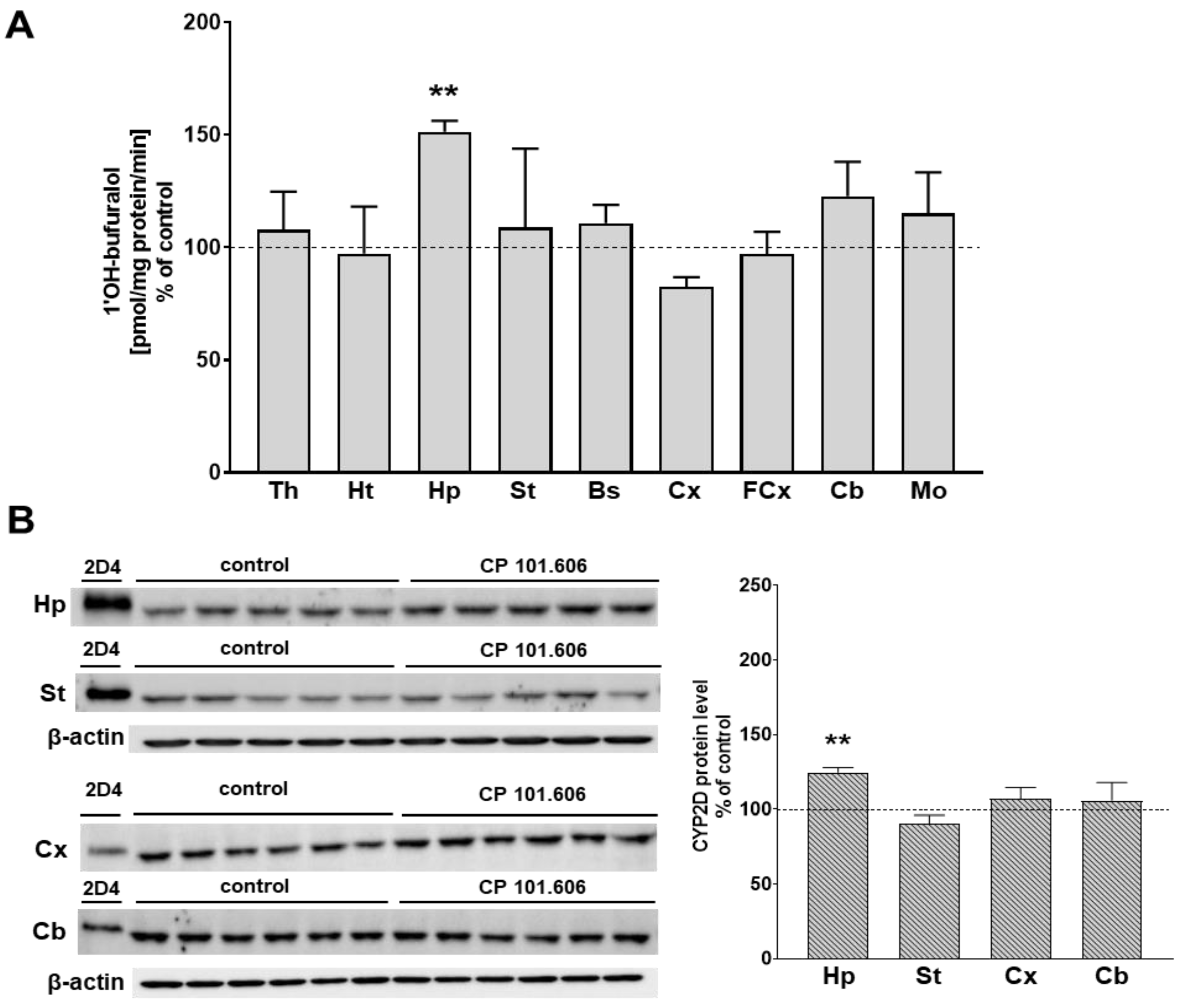
2.1. The Effect of CP-101,606 on the CYP2D Protein Level and Activity in Rat Brain Microsomes after 5-Day and 3-Week Treatment
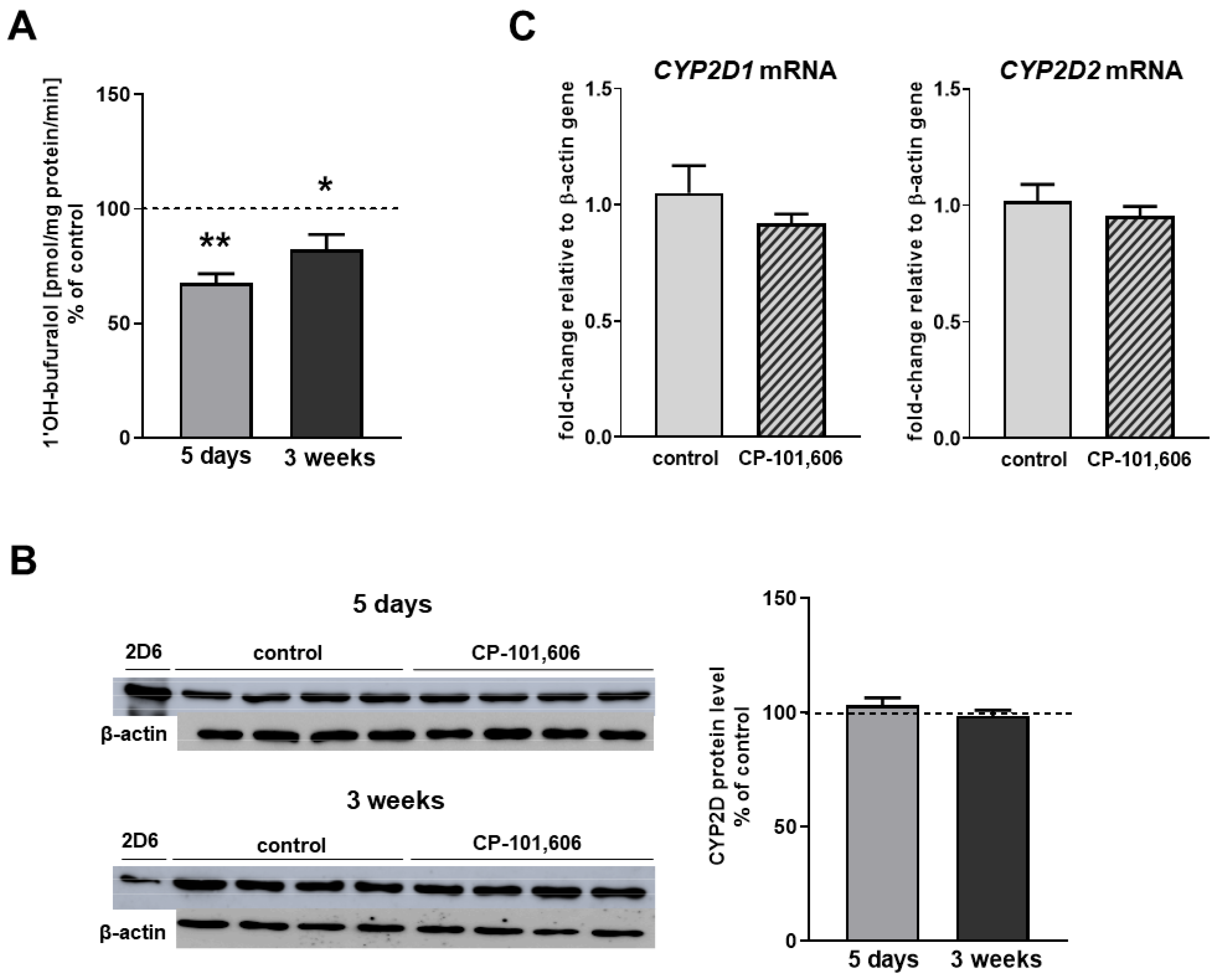
2.2. The Effect of CP-101,606 on the CYP2D Expression and Activity in Rat Liver Microsomes after 5-Day and 3-Week Treatment
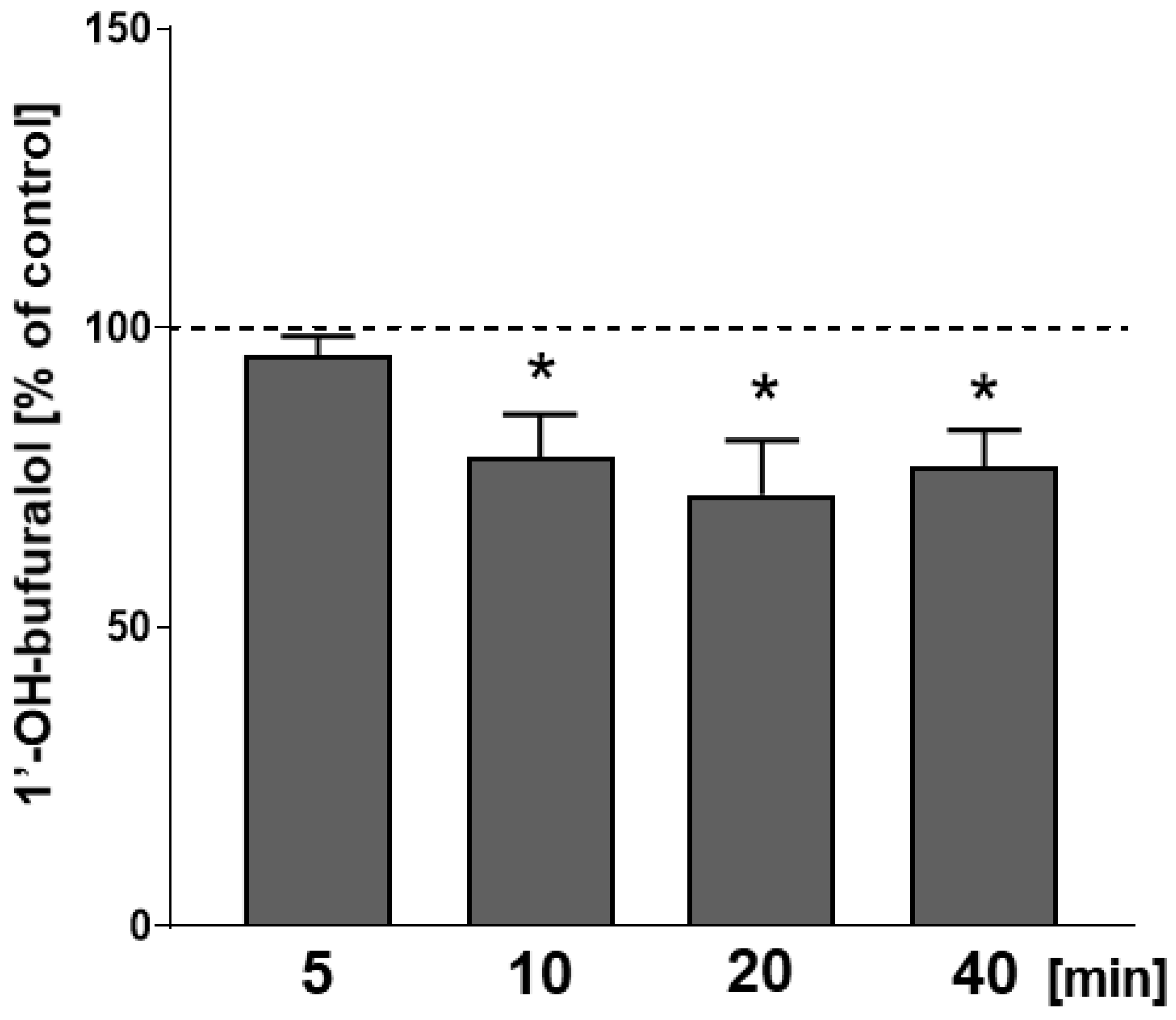
2.3. The Effect of CP-101,606 Added In Vitro to Liver Microsomes on the Activity of CYP2D
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. Animal Procedure and Microsome Preparation
4.4. Determination of Cytochrome P450 Enzyme Activities in Brain and Liver Microsomes
4.5. Analysis of CYP2D Proteins in Brain and Liver Microsomes
4.6. Analysis of the Expression of Genes Encoding CYP2D Isoforms in the Liver
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toselli, F.; Dodd, P.R.; Gillam, E.M.J. Emerging Roles for Brain Drug-Metabolizing Cytochrome P450 Enzymes in Neuropsychiatric Conditions and Responses to Drugs. Drug Metab. Rev. 2016, 48, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Haduch, A.; Daniel, W.A. The Engagement of Brain Cytochrome P450 in the Metabolism of Endogenous Neuroactive Substrates: A Possible Role in Mental Disorders. Drug Metab. Rev. 2018, 50, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.A.; Bromek, E.; Danek, P.J.; Haduch, A. The Mechanisms of Interactions of Psychotropic Drugs with Liver and Brain Cytochrome P450 and Their Significance for Drug Effect and Drug-Drug Interactions. Biochem. Pharmacol. 2022, 199, 115006. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T.; Imaoka, S.; Funae, Y. Dopamine Formation from Tyramine by CYP2D6. Biochem. Biophys. Res. Commun. 1998, 249, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Bromek, E.; Haduch, A.; Daniel, W.A. The Ability of Cytochrome P450 2D Isoforms to Synthesize Dopamine in the Brain: An in Vitro Study. Eur. J. Pharmacol. 2010, 626, 171–178. [Google Scholar] [CrossRef]
- Bromek, E.; Haduch, A.; Gołembiowska, K.; Daniel, W.A. Cytochrome P450 Mediates Dopamine Formation in the Brain in Vivo. J. Neurochem. 2011, 118, 806–815. [Google Scholar] [CrossRef]
- Yu, A.-M.; Idle, J.R.; Byrd, L.G.; Krausz, K.W.; Küpfer, A.; Gonzalez, F.J. Regeneration of Serotonin from 5-Methoxytryptamine by Polymorphic Human CYP2D6. Pharmacogenetics 2003, 13, 173–181. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Sadakierska-Chudy, A.; Wójcikowski, J.; Daniel, W.A. The Catalytic Competence of Cytochrome P450 in the Synthesis of Serotonin from 5-Methoxytryptamine in the Brain: An in Vitro Study. Pharmacol. Res. 2013, 67, 53–59. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Kot, M.; Kamińska, K.; Gołembiowska, K.; Daniel, W.A. The Cytochrome P450 2D-Mediated Formation of Serotonin from 5-Methoxytryptamine in the Brain in Vivo: A Microdialysis Study. J. Neurochem. 2015, 133, 83–92. [Google Scholar] [CrossRef]
- Hiroi, T.; Kishimoto, W.; Chow, T.; Imaoka, S.; Igarashi, T.; Funae, Y. Progesterone Oxidation by Cytochrome P450 2D Isoforms in the Brain. Endocrinology 2001, 142, 3901–3908. [Google Scholar] [CrossRef]
- Kishimoto, W.; Hiroi, T.; Shiraishi, M.; Osada, M.; Imaoka, S.; Kominami, S.; Igarashi, T.; Funae, Y. Cytochrome P450 2D Catalyze Steroid 21-Hydroxylation in the Brain. Endocrinology 2004, 145, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the Brain. Drug Metab. Pharm. 2003, 18, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.O.; Strobel, H.W. Regulation of Expression of Cytochrome P-450 2D MRNA in Rat Brain with Steroid Hormones. Brain Res. 1997, 765, 67–73. [Google Scholar] [CrossRef]
- Mann, A.; Miksys, S.L.; Gaedigk, A.; Kish, S.J.; Mash, D.C.; Tyndale, R.F. The Neuroprotective Enzyme CYP2D6 Increases in the Brain with Age and Is Lower in Parkinson’s Disease Patients. Neurobiol. Aging 2012, 33, 2160–2171. [Google Scholar] [CrossRef]
- Haduch, A.; Rysz, M.; Papp, M.; Daniel, W.A. The Activity of Brain and Liver Cytochrome P450 2D (CYP2D) Is Differently Affected by Antidepressants in the Chronic Mild Stress (CMS) Model of Depression in the Rat. Biochem. Pharmacol. 2018, 156, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Konstandi, M.; Andriopoulou, C.E.; Cheng, J.; Gonzalez, F.J. Sex Steroid Hormones Differentially Regulate CYP2D in Female Wild-Type and CYP2D6-Humanized Mice. J. Endocrinol. 2020, 245, 301–314. [Google Scholar] [CrossRef]
- Haduch, A.; Pukło, R.; Alenina, N.; Nikiforuk, A.; Popik, P.; Bader, M.; Daniel, W.A. The Effect of Ageing and Cerebral Serotonin Deficit on the Activity of Cytochrome P450 2D (CYP2D) in the Brain and Liver of Male Rats. Neurochem. Int. 2020, 141, 104884. [Google Scholar] [CrossRef]
- Haduch, A.; Danek, P.J.; Kuban, W.; Pukło, R.; Alenina, N.; Gołębiowska, J.; Popik, P.; Bader, M.; Daniel, W.A. Cytochrome P450 2D (CYP2D) Enzyme Dysfunction Associated with Aging and Serotonin Deficiency in the Brain and Liver of Female Dark Agouti Rats. Neurochem. Int. 2022, 152, 105223. [Google Scholar] [CrossRef]
- Pan, X.; Ning, M.; Jeong, H. Transcriptional Regulation of CYP2D6 Expression. Drug Metab. Dispos. 2017, 45, 42–48. [Google Scholar] [CrossRef]
- Kuban, W.; Daniel, W.A. Cytochrome P450 Expression and Regulation in the Brain. Drug Metab. Rev. 2021, 53, 1–29. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Na, S.; Wu, J.; Yang, Z.; Xie, X.; Wan, Y.; Li, K.; Yue, J. The Involvement of PPARs in the Selective Regulation of Brain CYP2D by Growth Hormone. Neuroscience 2018, 379, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, U.J.; Ho, K.K.Y. Modulation of Growth Hormone Action by Sex Steroids. Clin. Endocrinol. (Oxf) 2006, 65, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, M.; Wang, X.; Ouyang, X.; Wan, Y.; Dong, G.; Yang, Z.; Yang, J.; Yue, J. Sex Hormones Regulate Cerebral Drug Metabolism via Brain MiRNAs: Down-Regulation of Brain CYP2D by Androgens Reduces the Analgesic Effects of Tramadol. Br. J. Pharmacol. 2015, 172, 4639–4654. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Tyndale, R.F. Cytochrome P450-Mediated Drug Metabolism in the Brain. J. Psychiatry Neurosci. 2013, 38, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.; Wyss, A.; Kainu, T.; Backlund, M.; Köhler, C.; Pelto-Huikko, M.; Gustafsson, J.A.; Warner, M. Cytochrome P4502D4 in the Brain: Specific Neuronal Regulation by Clozapine and Toluene. Mol. Pharmacol. 1996, 50, 342–350. [Google Scholar] [PubMed]
- Yue, J.; Miksys, S.; Hoffmann, E.; Tyndale, R.F. Chronic Nicotine Treatment Induces Rat CYP2D in the Brain but Not in the Liver: An Investigation of Induction and Time Course. J. Psychiatry Neurosci. 2008, 33, 54–63. [Google Scholar]
- Danek, P.J.; Daniel, W.A. Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms. Cells 2021, 10, 3472. [Google Scholar] [CrossRef]
- McMillan, D.M.; Tyndale, R.F. CYP-Mediated Drug Metabolism in the Brain Impacts Drug Response. Pharmacol. Ther. 2018, 184, 189–200. [Google Scholar] [CrossRef]
- Bertilsson, L.; Alm, C.; De Las Carreras, C.; Widen, J.; Edman, G.; Schalling, D. Debrisoquine Hydroxylation Polymorphism and Personality. Lancet 1989, 1, 555. [Google Scholar] [CrossRef]
- Llerena, A.; Edman, G.; Cobaleda, J.; Benítez, J.; Schalling, D.; Bertilsson, L. Relationship between Personality and Debrisoquine Hydroxylation Capacity. Suggestion of an Endogenous Neuroactive Substrate or Product of the Cytochrome P4502D6. Acta Psychiatr. Scand. 1993, 87, 23–28. [Google Scholar] [CrossRef]
- González, I.; Peñas-Lledó, E.M.; Pérez, B.; Dorado, P.; Alvarez, M.; LLerena, A. Relation between CYP2D6 Phenotype and Genotype and Personality in Healthy Volunteers. Pharmacogenomics 2008, 9, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Peñas-Lledó, E.M.; Llerena, A. CYP2D6 Variation, Behaviour and Psychopathology: Implications for Pharmacogenomics-Guided Clinical Trials. Br. J. Clin. Pharmacol. 2014, 77, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.C.; Esslinger, C.; Tost, H.; Bilek, E.; Kirsch, P.; Ohmle, B.; Viviani, R.; Walter, H.; Rietschel, M.; Meyer-Lindenberg, A. Genetic Variation in CYP2D6 Impacts Neural Activation during Cognitive Tasks in Humans. Neuroimage 2012, 59, 2818–2823. [Google Scholar] [CrossRef]
- Viviani, R.; Messina, I.; Bosch, J.E.; Dommes, L.; Paul, A.; Schneider, K.L.; Scholl, C.; Stingl, J.C. Effects of Genetic Variability of CYP2D6 on Neural Substrates of Sustained Attention during On-Task Activity. Transl. Psychiatry 2020, 10, 338. [Google Scholar] [CrossRef]
- Cheng, J.; Zhen, Y.; Miksys, S.; Beyoğlu, D.; Krausz, K.W.; Tyndale, R.F.; Yu, A.; Idle, J.R.; Gonzalez, F.J. Potential Role of CYP2D6 in the Central Nervous System. Xenobiotica 2013, 43, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Gjota-Ergin, S.; Gökçek-Saraç, Ç.; Adalı, O.; Jakubowska-Doğru, E. Relationship between the Hippocampal Expression of Selected Cytochrome P450 Isoforms and the Animal Performance in the Hippocampus-Dependent Learning Task. Neurosci. Lett. 2018, 673, 104–110. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Daniel, W.A. The Effect of Psychotropic Drugs on Cytochrome P450 2D (CYP2D) in Rat Brain. Eur. J. Pharmacol. 2011, 651, 51–58. [Google Scholar] [CrossRef]
- Danek, P.J.; Bromek, E.; Haduch, A.; Daniel, W.A. Chronic Treatment with Asenapine Affects Cytochrome P450 2D (CYP2D) in Rat Brain and Liver. Pharmacological Aspects. Neurochem. Int. 2021, 151, 105209. [Google Scholar] [CrossRef]
- Danek, P.J.; Daniel, W.A. The Atypical Antipsychotic Lurasidone Affects Brain, but Not Liver Cytochrome P450 2D (CYP2D) Activity. A Comparison with Other Novel Neuroleptics and Significance for Drug Treatment of Schi-Zophrenia. Cells 2022, 20, 3513. [Google Scholar] [CrossRef]
- Hales, C.A.; Bartlett, J.M.; Arban, R.; Hengerer, B.; Robinson, E.S.J. Role of the Medial Prefrontal Cortex in the Effects of Rapid Acting Antidepressants on Decision-Making Biases in Rodents. Neuropsychopharmacology 2020, 45, 2278–2288. [Google Scholar] [CrossRef]
- Chazot, P.L.; Lawrence, S.; Thompson, C.L. Studies on the Subtype Selectivity of CP-101,606: Evidence for Two Classes of NR2B-Selective NMDA Receptor Antagonists. Neuropharmacology 2002, 42, 319–324. [Google Scholar] [CrossRef]
- Preskorn, S.H.; Baker, B.; Kolluri, S.; Menniti, F.S.; Krams, M.; Landen, J.W. An Innovative Design to Establish Proof of Concept of the Antidepressant Effects of the NR2B Subunit Selective N-Methyl-D-Aspartate Antagonist, CP-101,606, in Patients with Treatment-Refractory Major Depressive Disorder. J. Clin. Psychopharmacol. 2008, 28, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Gass, N.; Becker, R.; Sack, M.; Schwarz, A.J.; Reinwald, J.; Cosa-Linan, A.; Zheng, L.; von Hohenberg, C.C.; Inta, D.; Meyer-Lindenberg, A.; et al. Antagonism at the NR2B Subunit of NMDA Receptors Induces Increased Connectivity of the Prefrontal and Subcortical Regions Regulating Reward Behavior. Psychopharmacology 2018, 235, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Bromek, E.; Haduch, A.; Rysz, M.; Jastrzębska, J.; Pukło, R.; Wójcikowska, O.; Danek, P.J.; Daniel, W.A. The Selective NMDA Receptor GluN2B Subunit Antagonist CP-101,606 with Antidepressant Properties Modulates Cytochrome P450 Expression in the Liver. Pharmaceutics 2021, 13, 1643. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Cui, D.; Potchoiba, M.J.; Butler, T. Metabolism, Distribution and Excretion of a Selective N-Methyl-D-Aspartate Receptor Antagonist, Traxoprodil, in Rats and Dogs. Drug Metab. Dispos. 2007, 35, 1350–1364. [Google Scholar] [CrossRef]
- Hiroi, T.; Imaoka, S.; Chow, T.; Funae, Y. Tissue Distributions of CYP2D1, 2D2, 2D3 and 2D4 MRNA in Rats Detected by RT-PCR. Biochim. Biophys. Acta 1998, 1380, 305–312. [Google Scholar] [CrossRef]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications; Cambridge University Press: Cambridge, UK, 2013; ISBN 978-1-139-83259-5. [Google Scholar]
- Adell, A. Brain NMDA Receptors in Schizophrenia and Depression. Biomolecules 2020, 10, 947. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2007. [Google Scholar]
- Daniel, W.A.; Haduch, A.; Wójcikowski, J. Inhibition and Possible Induction of Rat CYP2D after Short- and Long-Term Treatment with Antidepressants. J. Pharm. Pharmacol. 2002, 54, 1545–1552. [Google Scholar] [CrossRef]
- Hiroi, T.; Chow, T.; Imaoka, S.; Funae, Y. Catalytic Specificity of CYP2D Isoforms in Rat and Human. Drug Metab. Dispos. 2002, 30, 970–976. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Rysz, M.; Bromek, E.; Haduch, A.; Liskova, B.; Wójcikowski, J.; Daniel, W.A. The Reverse Role of the Hypothalamic Paraventricular (PVN) and Arcuate (ARC) Nuclei in the Central Serotonergic Regulation of the Liver Cytochrome P450 Isoform CYP2C11. Biochem. Pharmacol. 2016, 112, 82–89. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haduch, A.; Bromek, E.; Pukło, R.; Jastrzębska, J.; Danek, P.J.; Daniel, W.A. The Effect of the Selective N-methyl-D-aspartate (NMDA) Receptor GluN2B Subunit Antagonist CP-101,606 on Cytochrome P450 2D (CYP2D) Expression and Activity in the Rat Liver and Brain. Int. J. Mol. Sci. 2022, 23, 13746. https://doi.org/10.3390/ijms232213746
Haduch A, Bromek E, Pukło R, Jastrzębska J, Danek PJ, Daniel WA. The Effect of the Selective N-methyl-D-aspartate (NMDA) Receptor GluN2B Subunit Antagonist CP-101,606 on Cytochrome P450 2D (CYP2D) Expression and Activity in the Rat Liver and Brain. International Journal of Molecular Sciences. 2022; 23(22):13746. https://doi.org/10.3390/ijms232213746
Chicago/Turabian StyleHaduch, Anna, Ewa Bromek, Renata Pukło, Joanna Jastrzębska, Przemysław Jan Danek, and Władysława Anna Daniel. 2022. "The Effect of the Selective N-methyl-D-aspartate (NMDA) Receptor GluN2B Subunit Antagonist CP-101,606 on Cytochrome P450 2D (CYP2D) Expression and Activity in the Rat Liver and Brain" International Journal of Molecular Sciences 23, no. 22: 13746. https://doi.org/10.3390/ijms232213746
APA StyleHaduch, A., Bromek, E., Pukło, R., Jastrzębska, J., Danek, P. J., & Daniel, W. A. (2022). The Effect of the Selective N-methyl-D-aspartate (NMDA) Receptor GluN2B Subunit Antagonist CP-101,606 on Cytochrome P450 2D (CYP2D) Expression and Activity in the Rat Liver and Brain. International Journal of Molecular Sciences, 23(22), 13746. https://doi.org/10.3390/ijms232213746