In Vivo Glutathione S-Transferases Superfamily Proteome Analysis: An Insight into Aedes albopictus Mosquitoes upon Acute Xenobiotic Challenges
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Strains
2.2. Determination of Toxicity Parameters and Insecticide Treatment
2.3. Protein Determination and Enzyme Assays
2.4. Trypsin Digestion and Tandem Mass Tag (TMT) Labelling
2.5. Liquid Chromatography Mass Spectrometry
2.6. Analytical Statistics
2.7. Querying Domain and Interaction Information
2.8. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) and Protein-Protein Interaction (PPI) Analysis
3. Results
3.1. Identification of GST Isoforms
3.2. Protein-Protein Interaction (PPI) Network Analysis of GST Families
3.3. Effect of Acute Insecticide Treatment on GSTs Activities
3.4. Differential Analysis of the Peptide Abundance under Xenobiotic Challenge
4. Discussion
4.1. Effect of the GST Enzymatic Activities of Aedes albopictus Larvae under 24 h of Acute Insecticide Challenges
4.2. Proteomic Study of Aedes albopictus Larvae upon 24 h of Acute Insecticide Treatments
4.3. Identification of GST Enzyme Classes up to the Isoform and Their Response towards Acute Insecticide Treatments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ketterman, A.J.; Saiwasang, C.; Wongsantichon, J. Insect glutathione S-transferases. Drug Metab. Rev. 2011, 43, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione s-transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- Ranson, H.; Hemingway, J. Mosquito glutathione s-transferases. Methods Enzymol. 2005, 401, 226–241. [Google Scholar]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata Lugens. Biochem. J. 2001, 357, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione s-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance part I. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–520. [Google Scholar] [CrossRef]
- Ranson, H.; Collins, F.; Hemingway, J. The role of alternative mRNA splicing in generating heterogeneity within the Anopheles gambiae class I glutathione s-transferase family. Proc. Natl. Acad. Sci. USA 1998, 95, 14284–14289. [Google Scholar] [CrossRef] [PubMed]
- Che-Mendoza, A.; Penilla, R.P.; Rodriguez, D.A. Insecticide resistance and glutathione S-transferases in mosquitoes: A review. Afr. J. Biotechnol. 2009, 8, 1386–1397. [Google Scholar]
- Ranson, H.; Prapanthadara, L.A.; Hemingway, J. Cloning and characterization of two glutathione S-transferases from a DDT-resistant strain of Anopheles gambiae. Biochem. J. 1997, 324, 97–102. [Google Scholar] [CrossRef]
- Dou, W.; Wu, S.; Hassan, M.W.; Wang, J.J. Purification and biochemical characterization of glutathione S-transferases from three strains of Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae): Implication of insecticide resistance. Pestic. Biochem. Physiol. 2009, 94, 10–14. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Hemingway, J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem. Mol. Biol. 2000, 30, 1009–1015. [Google Scholar] [CrossRef]
- Lewis, A.D.; Hayes, J.D.; Wolf, C.R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: Intrinsic differences and cell cycle effects. Carcinogenesis 1988, 9, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Rossiter, L.; Ortelli, F.; Jensen, B.; Wang, X.; Roth, C.W.; Collins, F.H.; Hemingway, J. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles Gambiae. Biochem. J. 2001, 359, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Claudianos, C.; Ortelli, F.; Abgrall, C.; Hemingway, J.; Sharakhova, M.V.; Unger, M.F.; Collins, F.H.; Feyereisen, R. Evolution of supergene families associated with insecticide resistance. Science 2002, 298, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hawkes, N.; Meredith, J.; Eggleton, P.; Hemingway, J.; Ranson, H. Characterization of the promoters of Epsilon glutathione S-transferases in the mosquito Anopheles gambiae and their response to oxidative stress. Biochem. J. 2005, 387, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, S.N.; Alias, Z. Purification, expression and partial characterisation of glutathione S-transferases (GSTs) from three different strains of Aedes albopictus (Diptera: Culicidae). Trop. Biomed. 2016, 33, 335–347. [Google Scholar]
- Penilla, R.P.; Rodriguez, A.D.; Hemingway, J.; Torres, J.L.; Solis, F.; Rodriguez, M.H. Changes in glutathione s-transferase activity in DDT resistant natural Mexican populations of Anopheles albimanus under different insecticide resistance management strategies. Pestic. Biochem. Physiol. 2006, 86, 63–71. [Google Scholar] [CrossRef]
- Hemingway, J.; Hawkes, N.; Prapanthadara, L.A.; Jayawardenal, K.I.; Ranson, H. The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1695–1699. [Google Scholar] [CrossRef]
- Caminade, C.; Medlock, J.M.; Ducheyne, E.; McIntyre, K.M.; Leach, S.; Baylis, M.; Morse, A.P. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: Recent trends and future scenarios. J. R. Soc. Interface 2012, 9, 2708–2717. [Google Scholar] [CrossRef]
- Vanlerberghe, V.; Toledo, M.E.; Rodriguez, M.; Gomez, D.; Baly, A.; Benitez, J.R.; Van Der Stuyft, P. Community involvement in dengue vector control: Cluster randomised trial. Br. Med. J. 2009, 338, b1959. [Google Scholar] [CrossRef]
- Ab Hamid, N.; Noor, S.N.M.; Saadatian-Elahi, M.; Isa, N.R.; Rodzay, R.M.; Ruslan, B.M.; Omar, T.; Norsham, M.I.M.; Amanzuri, N.H.; Abd Khalil, N.; et al. Residual spray for the control of Aedes vectors in dengue outbreak residential areas. Adv. Entomol. 2019, 7, 105. [Google Scholar] [CrossRef]
- Wan-Norafikah, O.; Nazni, W.A.; Lee, H.L.; Zainol-Ariffin, P.; Sofian-Azirun, M. Susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to permethrin in Kuala Lumpur, Malaysia. Asian Biomed. 2013, 7, 51–62. [Google Scholar]
- Koou, S.Y.; Chong, C.S.; Vythilingam, I.; Ng, L.C.; Lee, C.Y. Pyrethroid resistance in Aedes aegypti larvae (Diptera: Culicidae) from Singapore. J. Med. Entomol. 2014, 51, 1–12. [Google Scholar] [CrossRef]
- Grigoraki, L.; Lagnel, J.; Kioulos, I.; Kampouraki, A.; Morou, E.; Labbé, P.; Weill, M.; Vontas, J. Transcriptome profiling and genetic study reveal amplified carboxylesterase genes implicated in temephos resistance, in the Asian tiger mosquito Aedes albopictus. PLoS Negl. Trop. Dis. 2015, 9, e0003771. [Google Scholar] [CrossRef] [PubMed]
- Sokhna, C.; Ndiath, M.O.; Rogier, C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin. Microbiol. Infect. 2013, 19, 902–907. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Ranson, H.; N’Guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef]
- World Health Organisation Instructions for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticides. WHO/VBC/81.807. 1981. Available online: https://apps.who.int/iris/bitstream/handle/10665/69615/WHO_VBC_81.807_eng.pdf (accessed on 8 March 2018).
- World Health Organization Guidelines for Laboratory and Field Testing of Mosquito Larvicides. WHO/CDS/WHOPES/GCDPP/2005.13. 2005. Available online: https://apps.who.int/iris/bitstream/handle/10665/69101/WHO_CDS_WHOPES_GCDPP_2005.13.pdf (accessed on 1 August 2018).
- IBM SPSS Statistics for Windows, Version 24.0; IBM Corp: Armonk, NY, USA, 2016.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- World Health Organisation Manual for Techniques to Detect Insecticide Resistance Mechanism (Field and Laboratory Manual). WHO/CDC/CPC/MAL/98.6. World Health Organization: Geneva, Switzerland, 1998. Available online: https://apps.who.int/iris/handle/10665/83780 (accessed on 3 May 2018).
- Hamzah, S.N.; Alias, Z. Developmental expression and oxidative stress induction of proteome of glutathione S-transferases in Aedes albopictus (Diptera: Culicidae). J. Asia-Pac. Entomol. 2016, 19, 869–875. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Marriel, N.B.; Tomé, H.V.V.; Guedes, R.C.N.; Martins, G.F. Deltamethrin-mediated survival, behaviour, and ooenocyte morphology of insecticide-susceptible and resistant yellow fever mosquitoes (Aedes aegypti). Acta Trop. 2016, 158, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Brogdon, W.G.; Janet, C.; McAllister, J.C.; Vulule, J. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J. Am. Mosq. Control Assoc. 1997, 13, 233–237. [Google Scholar] [PubMed]
- Poupardin, R.; Reynaud, S.; Strode, C.; Ranson, H.; Vontas, J.; David, J.P. Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: Impact on larval tolerance to chemical insecticides. Insect Biochem. Mol. Biol. 2008, 38, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, A.L.; Field, L.M.; Williams, M.S. Molecular Biology of Insecticide Resistance; Academic Press: London, UK, 1992. [Google Scholar]
- Yan, H.; Jia, H.; Gao, X.; Xu, B. Identification, genomic organization, and oxidative stress response of a sigma class glutathione s-transferase gene (AccGSTS1) in the honey bee, Apis Cerana Cerana. Cell Stress Chaperones 2013, 18, 415–462. [Google Scholar] [CrossRef]
- Wang, X.; Martínez, M.A.; Dai, M.; Chen, D.; Ares, I.; Romero, A.; Castellano, V.; Martínez, M.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ. Res. 2016, 149, 86–104. [Google Scholar] [CrossRef]
- Ullah, S.; Li, Z.; Hasan, Z.; Khan, S.U.; Fahad, S. Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicol. Environ. Saf. 2018, 161, 270–280. [Google Scholar] [CrossRef]
- Panini, M.; Manicardi, G.C.; Moores, G.D.; Mazzoni, E. An overview of the main pathways of metabolic resistance in insects. Invertebr. Surviv. J. 2016, 13, 326–335. [Google Scholar]
- Montella, I.R.; Martins, A.J.; Viana-Medeiros, P.F.; Lima, J.B.P.; Braga, I.A.; Valle, D. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am. J. Trop. Med. Hyg. 2007, 77, 467–477. [Google Scholar] [CrossRef]
- Jagadeshwaran, U.; Vijayan, V.A. Biochemical characterization of deltamethrin resistance in a laboratory-selected strain of Aedes aegypti. Parasitol. Res. 2009, 104, 1431–1438. [Google Scholar] [CrossRef]
- Amelia-Yap, Z.H.; Chen, C.D.; Sofian-Azirun, M.; Low, V.L. Pyrethroid resistance in the dengue vector Aedes aegypti in the Southeast Asia: Present situation in the prospects for management. Parasites Vectors 2018, 11, 332. [Google Scholar] [CrossRef]
- Jeffrey, S.S.; Lønning, P.E.; Hillner, B.E. Genomics-based prognosis and therapeutic prediction in breast cancer. J. Natl. Compr. Cancer Netw. 2005, 3, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, N.; Gakhar, S.K. Mosquito proteomics: Present and future prospective. Res. Biotechnol. 2014, 5, 25–33. [Google Scholar]
- Rosilawati, R.; Nabila, R.; Siti Futri Farahininajua, F.; Nazni, W.A.; Lee, H.L. A preliminary proteomic study of permethrin resistant and susceptible Aedes aegypti (L.). Trop. Biomed. 2019, 36, 855–865. [Google Scholar] [PubMed]
- Müller, P.; Warr, E.; Stevenson, B.J.; Pignatelli, P.M.; Morgan, J.C.; Steven, A.; Yawson, A.E.; Mitchell, S.N.; Ranson, H.; Hemingway, J.; et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008, 4, e1000286. [Google Scholar] [CrossRef]
- Ngoagouni, C.; Kamgang, B.; Brengues, C.; Yahouedo, G.; Paupy, C.; Nakouné, E.; Kazanji, M.; Chandre, F. Susceptibility profile and metabolic mechanisms involved in Aedes aegypti and Aedes albopictus resistant to DDT and deltamethrin in the Central African Republic. Parasites Vectors 2016, 9, 599. [Google Scholar] [CrossRef]
- Paiva, M.H.; Lovin, D.D.; Mori, A.; Melo-Santos, M.A.; Severson, D.W.; Ayres, C.F. Identification of a major quantitative trait locus determining resistance to the organophosphate temephos in the dengue vector mosquito Aedes Aegypti. Genomics 2016, 107, 40–48. [Google Scholar] [CrossRef]
- Hamzah, S.N.; Farouk, S.; Alias, Z. Isoenzymes of Aedes albopictus (Diptera: Culicidae) Glutathione S-transferases: Isolation and expression after acute insecticide treatment. Pestic. Biochem. Physiol. 2019, 153, 116–121. [Google Scholar] [CrossRef]
- Ito, T.; Tashiro, K.; Muta, S.; Ozawa, R.; Chiba, T.; Nishizawa, M.; Sakaki, Y. Toward a protein-protein interaction map of the budding yeast: A comprehensive system to examine two-hybrid interactions in all possible combinations between the yeast proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 1143–1147. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, J.; Sun, W.; Huo, Y.; Zhang, L.; Hao, P.; Zhuang, M. A proximity-tagging system to identify membrane protein-protein interactions. Nat. Methods 2018, 15, 715–722. [Google Scholar] [CrossRef]
- Xin, S.; Zhang, W. Construction and analysis of the protein–protein interaction network for the olfactory system of the silkworm Bombyx mori. Arch. Insect Biochem. Physiol. 2020, 105, e21737. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W. Protein-protein interaction network analysis of insecticide resistance molecular mechanism in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2019, 100, e21523. [Google Scholar] [CrossRef] [PubMed]
- Lumjuan, N.; Stevenson, B.J.; Prapanthadara, L.A.; Somboon, P.; Brophy, P.M.; Loftus, B.J.; Severson, D.W.; Ranson, H. The Aedes aegypti glutathione s-transferase family. Insect Biochem. Mol. Biol. 2007, 37, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Wongsantichon, J.; Robinson, R.C.; Ketterman, A.J. Epsilon glutathione transferases possess a unique class-conserved subunit interface motif that directly interacts with glutathione in the active site. Biosci. Rep. 2015, 35, e00272. [Google Scholar] [CrossRef] [PubMed]
- Alias, Z.; Clark, A. Studies on the glutathione s-transferase proteome of adult Drosophila melanogaster: Responsiveness to chemical challenge. Proteomics 2007, 7, 2618–3628. [Google Scholar] [CrossRef] [PubMed]
- Nardini, L.; Christian, R.N.; Coetzer, N.; Koekemoer, L.L. DDT and pyrethroid resistance in Anopheles arabiensis from South Africa. Parasites Vectors 2013, 6, 229–238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blackburn, A.C.; Matthaei, K.I.; Lim, C.; Taylor, M.C.; Cappello, J.Y.; Hayes, J.D.; Anders, M.W.; Board, P.G. Deficiency of glutathione transferase zeta causes oxidative stress and activation of antioxidant response pathways. Mol. Pharmacol. 2005, 69, 650–657. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V.; et al. Identification, characterization, and crystal structure of the Omega class glutathione s-transferases. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef]
- Wildenburg, G.; Liebau, E.; Henkle-Dührsen, K. Onchocerca volvulus: Ultrastructural localization of two glutathione s-transferases. Exp. Parasitol. 1998, 88, 34–42. [Google Scholar] [CrossRef]
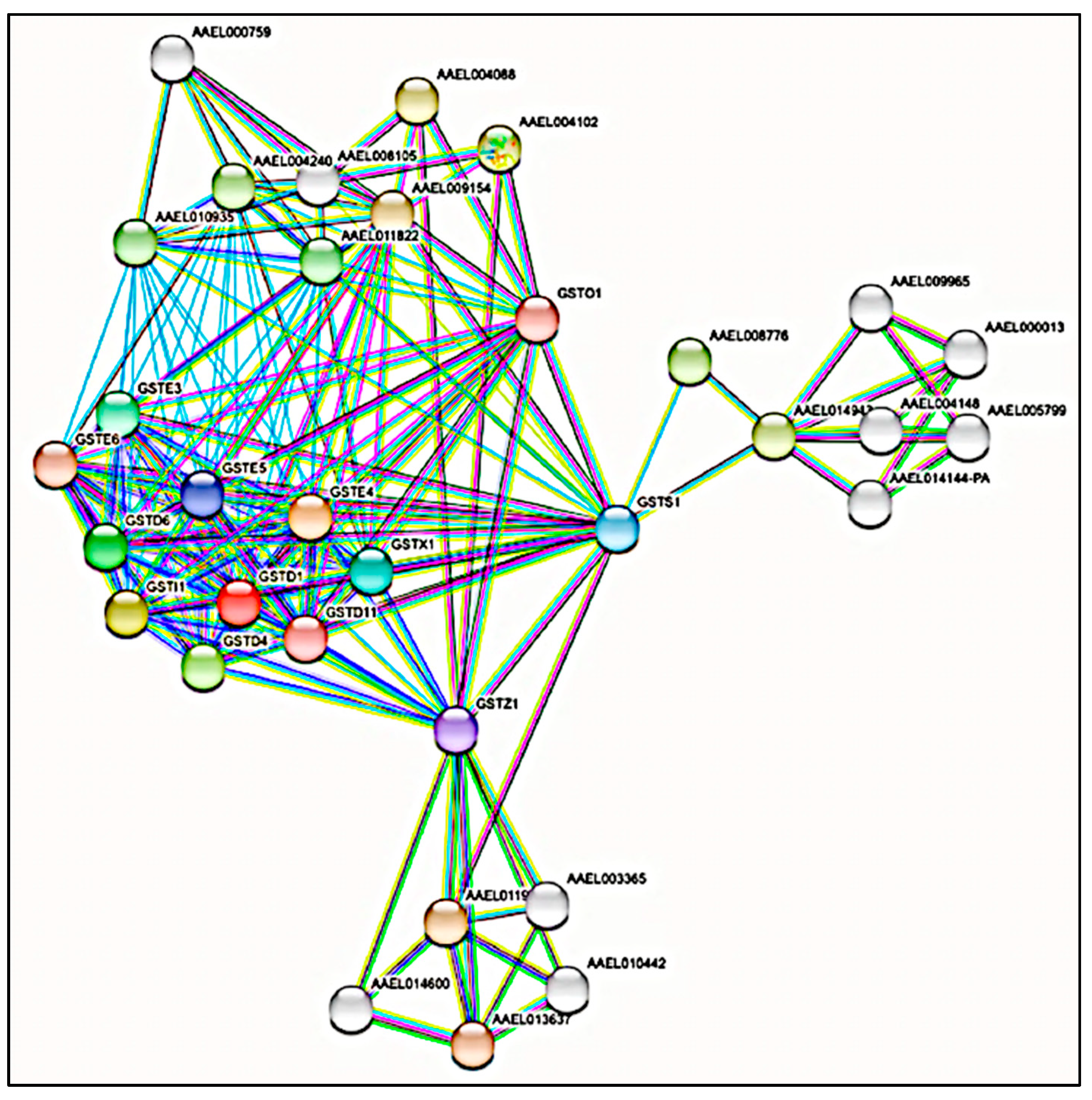
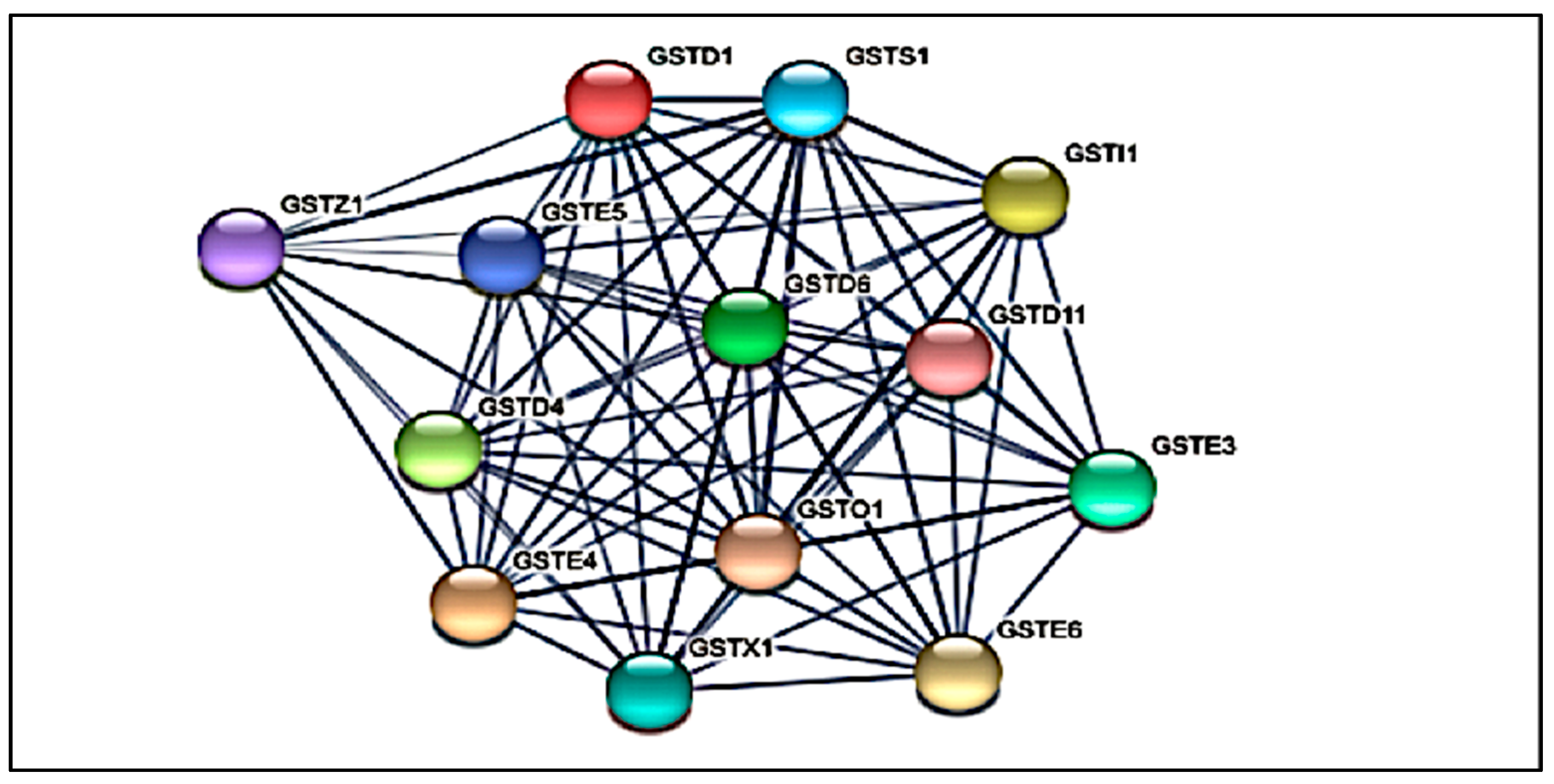
| Accession | Identified Protein | Class | MW (kDa) | pI Value | Peptide No. | AA Coverage | Proposed Isoform Identification |
|---|---|---|---|---|---|---|---|
| J9HXZ8 | AAEL001061-PC GN = GSTD1 | Delta1 | 23.845 | 5.96 | 8 | 211 | GSTD1-1 |
| J9HHL7 | AAEL001061-PB | Delta1 | 23.778 | 6.07 | 7 | 209 | GSTD1-2 |
| Q17MA9 | AAEL001061-PA GN = GSTD1 | Delta1 | 24.755 | 5.44 | 2 | 219 | GSTD1-3 |
| A0A1S4EXN6 | Glutathione S-transferase GN = 5568355 | Delta1 | 24.538 | 5.9 | 2 | 216 | GSTD1-4 |
| Q6PTY1 | Glutathione S-transferase OX = 7159 | Delta4 | 23.146 | 7.94 | 2 | 205 | GSTD4-1 |
| A0A0N8ES64 | Glutathione S-transferase GSTD4 OX = 7159 | Delta4 | 24.855 | 6.14 | 1 | 218 | GSTD4-2 |
| Q17MB8 | AAEL001054-PA GN = GSTD4 | Delta4 | 24.085 | 6.14 | 1 | 211 | GSTD4-3 |
| Q16SH6 | AAEL010591-PA GN = GSTD6 | Delta6 | 28.215 | 5.74 | 1 | 249 | GSTD6-1 |
| A0A023ENG1 | Glutathione S-transferase e2 OX = 7160 | Delta6 | 28.148 | 5.54 | 1 | 249 | GSTD6-2 |
| Q16SH7 | AAEL010582-PA GN = GSTD11 | Delta11 | 25.879 | 5.95 | 3 | 222 | GSTD11-1 |
| Q170C6 | AAEL007947-PA GN = GSTE3 | Epsilon3 | 24.824 | 6.54 | 1 | 222 | GSTE3-1 |
| Q5PY78 | AAEL007962-PA GN = GSTe4 | Epsilon4 | 25.03 | 7.11 | 3 | 224 | GSTE4-1 |
| A0A0P6IV26 | Putative glutathione S-transferase e4 | Epsilon4 | 27.264 | 6.81 | 2 | 244 | GSTE4-2 |
| Q170C9 | AAEL007964-PA GN = GSTE5 | Epsilon5 | 24.635 | 5.2 | 1 | 221 | GSTE5-1 |
| Q170C7 | AAEL007946-PA GN = GSTE6 | Epsilon6 | 24.738 | 5.97 | 2 | 220 | GSTE6-1 |
| Q16P53 | AAEL011752-PA GN = GSTI1 | Iota1 | 26.177 | 6.15 | 1 | 231 | GSTI1-1 |
| J9E9C0 | AAEL017085-PA GN = GSTO1 | Omega1 | 28.589 | 7.03 | 6 | 248 | GSTO1-1 |
| A0A1S4G560 | Glutathione S-transferase GN = 23687505 | Omega1 | 29.618 | 8.51 | 6 | 257 | GSTO1-2 |
| A0A0P6J0T5 | Glutathione S-transferase OX = 7159 | Omega1 | 29.572 | 8.51 | 6 | 257 | GSTO1-3 |
| Q1HQK1 | Glutathione S-transferaseOX = 7159 | Omega1 | 28.608 | 7.42 | 6 | 248 | GSTO1-4 |
| Q16P79 | AAEL011741-PA GN = GSTS1 | Sigma1 | 23.249 | 5.2 | 9 | 203 | GSTS1-1 |
| Q16P80 | AAEL011741-PB GN = GSTS1 | Sigma1 | 23.216 | 5.17 | 2 | 203 | GSTS1-2 |
| Q0C791 | AAEL000092-PA GN = GSTX1 | GSTX1 | 24.817 | 5.78 | 4 | 218 | GSTX1-1 |
| Q16NL9 | AAEL011934-PA GN = GSTZ1 | Zeta1 | 26.39 | 6.92 | 1 | 233 | GSTZ1-1 |
| Input Protein | Information | |
|---|---|---|
| GSTD1 | Glutathione s-transferase 1 isoform ×1; Glutathione S-transferase (GSTD1); Glutathione transferase (219 aa) | |
| GSTE4 | Glutathione S-transferase e4; Glutathione transferase (224 aa) | |
| GSTI1 | Glutathione S-transferase 1; Belongs to the GST superfamily (231 aa) | |
| GSTD4 | Glutathione S-transferase 1; AAEL001054-PA; Glutathione transferase (211 aa) | |
| GSTD6 | Glutathione S-transferase 1; Glutathione transferase; Belongs to the GST superfamily (249 aa) | |
| GSTE3 | Glutathione S-transferase 1; Belongs to the GST superfamily (222 aa) | |
| GSTX1 | Glutathione S-transferase d4; Glutathione transferase (218 aa) | |
| GSTS1 | Prostaglandin-H2 D-isomerase/glutathione transferase; Glutathione transferase (203 aa) | |
| GSTE5 | Glutathione S-transferase 1; Belongs to the GST superfamily (221 aa) | |
| GSTZ1 | Probable maleylacetoacetate isomerase 2 isoform x1; Belongs to the GST superfamily (233 aa) | |
| GSTD11 | Glutathione S-transferase 1-1; Belongs to the GST superfamily (222 aa) | |
| GSTE6 | Glutathione S-transferase 1; Glutathione transferase (220 aa) | |
| GSTO1 | Pyrimidodiazepine synthase; Belongs to the GST superfamily (248 aa) | |
| Predicted Functional Partner | ||
| Accession | Information | Score |
| AAEL013637 | Homogentisate 1,2-dioxygenase | 0.999 |
| AAEL011973 | Fumarylacetoacetate hydrolase | 0.998 |
| AAEL009154 | Glutathione synthetase isoform x1; Glutathione synthetase | 0.968 |
| AAEL004088 | Aldose reductase isoform x1; Aldo-keto reductase | 0.958 |
| AAEL004102 | Aldehyde reductase; Aldo-keto reductase | 0.958 |
| AAEL013637 | Homogentisate 1,2-dioxygenase | 0.999 |
| AAEL011973 | Fumarylacetoacetate hydrolase | 0.998 |
| AAEL009154 | Glutathione synthetase isoform x1; Glutathione synthetase | 0.968 |
| AAEL004088 | Aldose reductase isoform x1; Aldo-keto reductase | 0.958 |
| AAEL004102 | Aldehyde reductase; Aldo-keto reductase | 0.958 |
| Biological Process (GO) | |||
|---|---|---|---|
| GO-Term | Pathway Description | Count in Network | p-Values |
| GO:0000413 | Protein peptidyl-prolyl isomerization | 3 of 29 | 0.0134 |
| GO:0006457 | Protein folding | 8 of 134 | 2.39 × 10−6 |
| GO:0006518 | Peptide metabolic process | 21 of 471 | 1.25 × 10−17 |
| GO:0006559 | L-phenylalanine catabolic process | 3 of 5 | 0.00028 |
| GO:0006570 | Tyrosine metabolic process | 4 of 13 | 4.58 × 10−5 |
| GO:0006572 | Tyrosine catabolic process | 3 of 3 | 0.00014 |
| GO:0006749 | Glutathione metabolic process | 20 of 43 | 1.91 × 10−34 |
| GO:0006750 | Glutathione biosynthetic process | 3 of 4 | 0.00020 |
| GO:0006751 | Glutathione catabolic process | 4 of 12 | 3.76 × 10−5 |
| GO:0006807 | Nitrogen compound metabolic process | 29 of 4303 | 2.34 × 10−5 |
| GO:0008152 | Metabolic process | 34 of 5833 | 1.22 × 10−5 |
| GO:0009074 | Aromatic amino acid family catabolic process | 5 of 12 | 5.50 × 10−7 |
| GO:0009987 | Cellular process | 39 of 8701 | 0.00012 |
| GO:0034605 | Cellular response to heat | 4 of 39 | 0.00016 |
| GO:0034641 | Cellular nitrogen compound metabolic process | 23 of 2098 | 0.0011 |
| GO:0034605 | Cellular response to heat | 4 of 39 | 4.25 × 10−7 |
| GO:0043043 | Peptide biosynthetic process | 7 of 366 | 0.0093 |
| GO:0043171 | Peptide catabolic process | 5 of 54 | 0.00017 |
| GO:0044237 | Cellular metabolic process | 31 of 4455 | 2.26 × 10−6 |
| GO:0044248 | Cellular catabolic process | 10 of 885 | 0.0164 |
| GO:0050821 | Protein stabilization | 4 of 47 | 0.0020 |
| GO:0071704 | Organic substance metabolic process | 29 of 5131 | 0.00063 |
| GO:0071722 | Detoxification of arsenic-containing substance | 2 of 2 | 0.0072 |
| GO:0098754 | Detoxification | 4 of 71 | 0.0080 |
| GO:1901564 | Organonitrogen compound metabolic process | 28 of 3183 | 2.23 × 10−7 |
| GO:1901565 | Organonitrogen compound catabolic process | 10 of 597 | 0.00081 |
| GO:1901606 | Alpha-amino acid catabolic process | 5 of 60 | 0.00024 |
| Molecular Function (GO) | |||
| GO:0000048 | Peptidyltransferase activity | 4 of 10 | 1.57 × 10−5 |
| GO:0003755 | Peptidyl-prolyl cis-trans isomerase activity | 3 of 30 | 0.0089 |
| GO:0003824 | Catalytic activity | 33 of 4721 | 2.22 × 10−7 |
| GO:0003868 | 4-hydroxyphenylpyruvate dioxygenase activity | 2 of 2 | 0.0049 |
| GO:0004032 | alditol:NADP+ 1-oxidoreductase activity | 2 of 11 | 0.0373 |
| GO:0004364 | Glutathione transferase activity | 13 of 30 | 4.08 × 10−21 |
| GO:0008144 | Drug binding | 3 of 16 | 0.0027 |
| GO:0016018 | Cyclosporin a binding | 2 of 9 | 0.0283 |
| GO:0016702 | Oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen | 3 of 16 | 0.0027 |
| GO:0016740 | Transferase activity | 17 of 1385 | 1.57 × 10−5 |
| GO:0016823 | Hydrolase activity, acting on acid carbon-carbon bonds, in ketonic substances | 2 of 2 | 0.0049 |
| GO:0016848 | Carbon-halide lyase activity | 4.96 × 10−6 | |
| GO:0016853 | Isomerase activity | 6 of 108 | 0.00012 |
| GO:0016859 | Cis-trans isomerase activity | 4 of 32 | 0.00044 |
| GO:0031072 | Heat shock protein binding | 3 of 42 | 0.0199 |
| GO:0033218 | Amide binding | 5 of 138 | 0.0049 |
| GO:0036374 | Glutathione hydrolase activity | 4 of 10 | 1.57 × 10−5 |
| GO:0042277 | Peptide binding | 4 of 105 | 0.0180 |
| GO:0050220 | Prostaglandin-E synthase activity | 2 of 3 | 0.0069 |
| GO:0051879 | Hsp90 protein binding | 2 of 10 | 0.0327 |
| KEGG Pathway | |||
|---|---|---|---|
| Pathway ID | Pathway Description | Count in Network | p-Values |
| aag00051 | Fructose and mannose metabolism | 2 of 23 | 0.0260 |
| aag00052 | Galactose metabolism | 2 of 28 | 0.0343 |
| aag00270 | Cysteine and methionine metabolism | 3 of 27 | 0.0011 |
| aag00350 | Tyrosine metabolism | 5 of 27 | 3.81 × 10−7 |
| aag00430 | Taurine and hypotaurine metabolism | 4 of 7 | 3.02 × 10−7 |
| aag00480 | Glutathione metabolism | 21 of 74 | 2.66 × 10−34 |
| aag00590 | Arachidonic acid metabolism | 8 of 17 | 4.27 × 10−14 |
| aag00790 | Folate biosynthesis | 3 of 33 | 0.0017 |
| aag00980 | Metabolism of xenobiotics by cytochrome P450 | 11 of 53 | 3.76 × 10−16 |
| aag00982 | Drug metabolism—cytochrome P450 | 11 of 52 | 3.76 × 10−16 |
| aag00983 | Drug metabolism—other enzymes | 10 of 80 | 7.93 × 10−13 |
| aag01100 | Metabolic pathways | 30 of 1039 | 5.51 × 10−24 |
| Insecticide | Concentration (mg/L) | Mortality (%) | LC50 (mg/L) | 95% Confidence Limit (mg/L) | Slope ± SD | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Malathion | 0.024 | 0 | ||||
| 0.053 | 15 | |||||
| 0.072 | 30 | |||||
| 0.115 | 60 | 0.099 | 0.090 | 0.108 | 3.692 ± 0.228 | |
| 0.25 | 94 | |||||
| 0.40 | 100 | |||||
| Permethrin | 0.002 | 0 | ||||
| 0.006 | 10 | |||||
| 0.018 | 40 | 0.023 | 0.019 | 0.027 | 3.482 + 0.223 | |
| 0.034 | 65 | |||||
| 0.120 | 95 | |||||
| 0.203 | 100 | |||||
| Treatment | Treatment Duration | Total Protein Contents (mg) | PF | Total Activity (μmol/min) | TAF | Specific Activity (μmol/min/mg) | SAF |
|---|---|---|---|---|---|---|---|
| Permethrin | Control 24 h | 3.9 × 10−3 ± 0.001 5.3 × 10−3 ± 0.006 * | 1.00 1.36 | 7.59 × 10−3 ± 0.01 13.97 × 10−3 ± 0.01 * | 1.00 1.84 | 1.947 ± 0.08 2.635 ± 0.08 * | 1.00 1.35 |
| Malathion | Control 24 h | 3.9 × 10−3 ± 0.001 4.3 × 10−3 ± 0.002 * | 1.00 1.10 | 7.59 × 10−3 ± 0.04 10.75 × 10−3 ± 0.02* | 1.00 1.41 | 1.947 ± 0.08 2.502 ± 0.05 * | 1.00 1.29 |
| Peptide Abundance | Fold-Change | |||||
|---|---|---|---|---|---|---|
| No | Proposed Identification | Control | Permethrin | Malathion | Permethrin | Malathion |
| 1 | GSTD1-1 | 155.8 | 180.2 | 155.3 | 1.16 | 1.00 |
| 2 | GSTD1-2 | 48.5 | 107.3 | 102.4 | 2.21 | 2.11 |
| 3 | GSTD1-3 | 27.6 | 62.5 | 53.1 | 2.26 | 1.92 |
| 4 | GSTD1-4 | 27.6 | 62.5 | 53.1 | 2.26 | 1.92 |
| 5 | GSTD4-1 | 162.8 | 176.5 | 188.8 | 1.08 | 1.16 |
| 6 | GSTD4-2 | 78.5 | 95.8 | 90.1 | 1.22 | 1.15 |
| 7 | GSTD4-3 | 78.5 | 95.8 | 90.1 | 1.22 | 1.15 |
| 8 | GSTD6-1 | 173.3 | 174 | 252.6 | 1.00 | 1.46 |
| 9 | GSTD6-2 | 173.3 | 174 | 252.6 | 1.00 | 1.46 |
| 10 | GSTD11-1 | 147.3 | 126 | 131.4 | 0.86 | 0.89 |
| 11 | GSTE3-1 | 148 | 192 | 227.2 | 1.30 | 1.54 |
| 12 | GSTE4-1 | 198.3 | 217.7 | 183.9 | 1.10 | 0.93 |
| 13 | GSTE4-2 | 143.1 | 230.1 | 226.7 | 1.61 | 1.58 |
| 14 | GSTE5-1 | 175.6 | 116.1 | 191.8 | 0.66 | 1.09 |
| 15 | GSTE6-1 | 175.6 | 116.1 | 191.8 | 0.66 | 1.09 |
| 16 | GSTI1-1 | 198.9 | 224.4 | 176.7 | 1.13 | 0.89 |
| 17 | GSTO1-1 | 47.6 | 47 | 53.8 | 1.00 | 1.13 |
| 18 | GSTO1-2 | 47.6 | 47 | 53.8 | 1.00 | 1.13 |
| 19 | GSTO1-3 | 47.6 | 47 | 53.8 | 1.00 | 1.13 |
| 20 | GSTO1-4 | 47.6 | 47 | 53.8 | 1.00 | 1.13 |
| 21 | GSTS1-1 | 165.5 | 149.4 | 139 | 0.90 | 0.84 |
| 22 | GSTS1-2 | 166.4 | 136.9 | 124.6 | 0.82 | 0.75 |
| 23 | GSTX1-1 | 87.8 | 109.4 | 110.9 | 1.25 | 1.26 |
| 24 | GSTZ1-1 | 16.4 | 20.1 | 19.4 | 1.23 | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzah, S.N.; Avicor, S.W.; Alias, Z.; Razak, S.A.; Bakhori, S.K.M.; Hsieh, T.C.; Syanizam, N.N.; Farouk, S.A. In Vivo Glutathione S-Transferases Superfamily Proteome Analysis: An Insight into Aedes albopictus Mosquitoes upon Acute Xenobiotic Challenges. Insects 2022, 13, 1028. https://doi.org/10.3390/insects13111028
Hamzah SN, Avicor SW, Alias Z, Razak SA, Bakhori SKM, Hsieh TC, Syanizam NN, Farouk SA. In Vivo Glutathione S-Transferases Superfamily Proteome Analysis: An Insight into Aedes albopictus Mosquitoes upon Acute Xenobiotic Challenges. Insects. 2022; 13(11):1028. https://doi.org/10.3390/insects13111028
Chicago/Turabian StyleHamzah, Siti Nasuha, Silas Wintuma Avicor, Zazali Alias, Sarah Abdul Razak, Siti Khadijah Mohd Bakhori, Ting Chuan Hsieh, Nurin Nazifa Syanizam, and Salinah Abdul Farouk. 2022. "In Vivo Glutathione S-Transferases Superfamily Proteome Analysis: An Insight into Aedes albopictus Mosquitoes upon Acute Xenobiotic Challenges" Insects 13, no. 11: 1028. https://doi.org/10.3390/insects13111028
APA StyleHamzah, S. N., Avicor, S. W., Alias, Z., Razak, S. A., Bakhori, S. K. M., Hsieh, T. C., Syanizam, N. N., & Farouk, S. A. (2022). In Vivo Glutathione S-Transferases Superfamily Proteome Analysis: An Insight into Aedes albopictus Mosquitoes upon Acute Xenobiotic Challenges. Insects, 13(11), 1028. https://doi.org/10.3390/insects13111028






