Therapeutic Effects of Heterotrigona itama (Stingless Bee) Bee Bread in Improving Hepatic Lipid Metabolism through the Activation of the Keap1/Nrf2 Signaling Pathway in an Obese Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bee Bread Collection and Preparation
2.2. High-Performance Liquid Chromatography (HPLC) Detection and Quantification of Polyphenolic Compounds
2.3. Animals and Diet
2.4. Experimental Design
2.5. Measurements of Obesity Parameters and Nutritional Composition
2.6. Blood and Tissue Collection
2.7. Determination of Serum Glucose, Insulin and HOMA-IR
2.8. Evaluations of Lipid Profiles
2.9. Liver Biochemical Analyses
2.10. RNA Extraction and RT-qPCR Analysis
2.11. Immunohistochemical Detections of Keap1 and Nrf2 Expressions
2.12. Liver Histopathological Examination
2.13. Statistical Analysis
3. Results
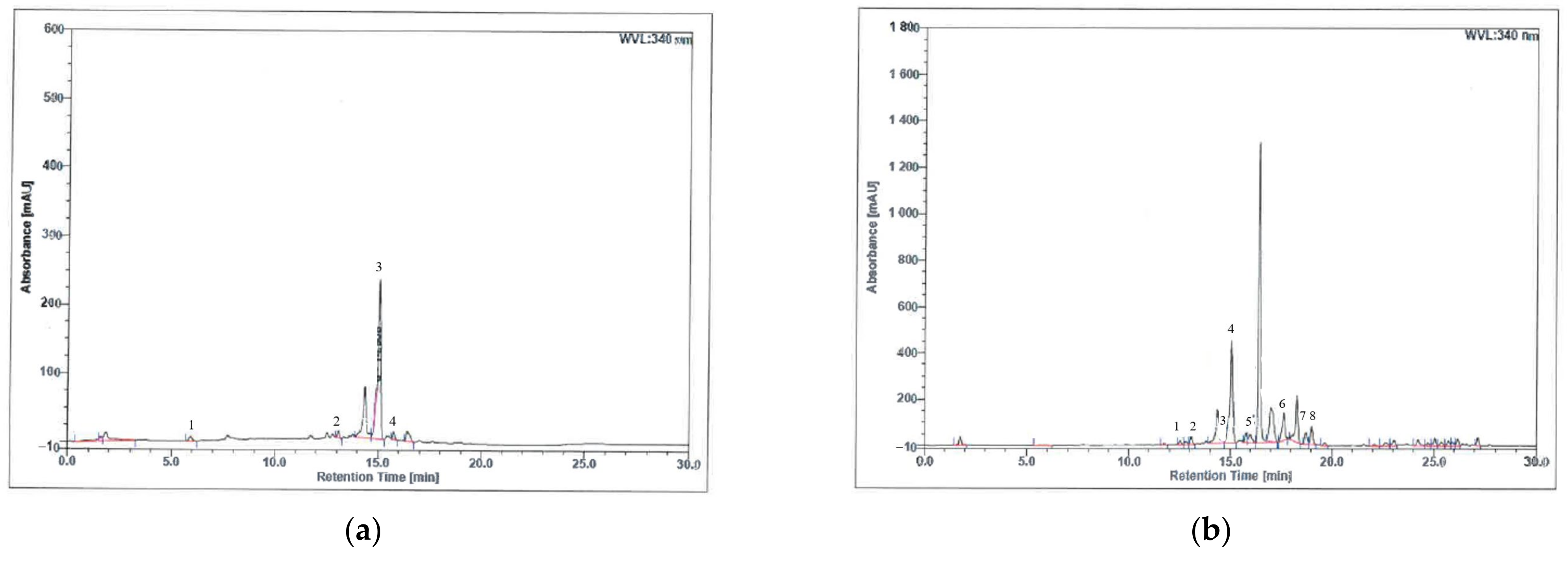
3.1. Phenolic Compound Analysis of H. itama Bee Bread Using High-Performance Liquid Chromatography (HPLC)
3.2. Effects of H. itama Bee Bread on Obesity Parameters and Nutritional Composition
3.3. Effects of H. itama Bee Bread on Serum Glucose, Insulin Resistance and Lipid Profile
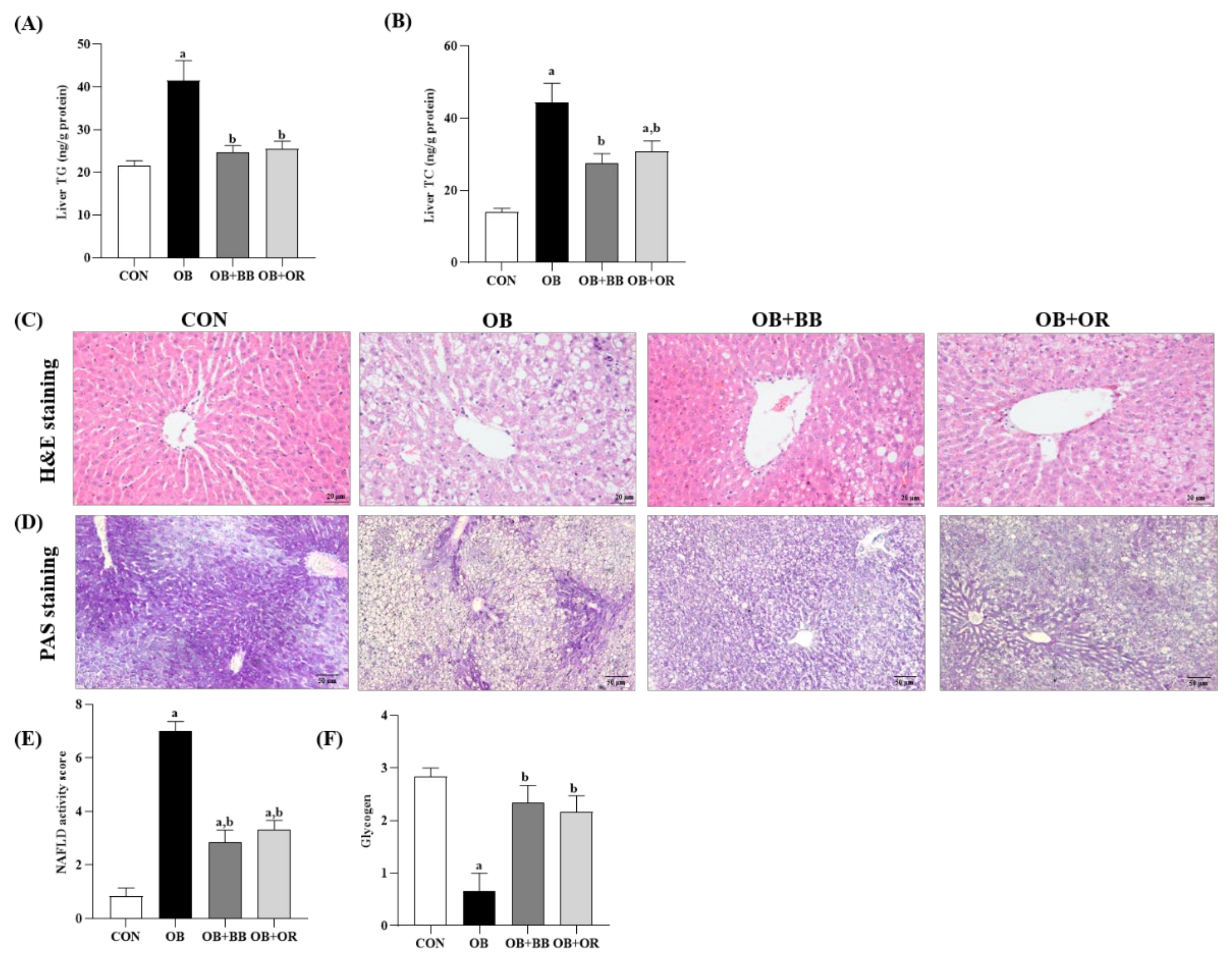
3.4. Effects of H. itama Bee Bread on Accumulations of Hepatic Lipid, NASH Activity and Glycogen
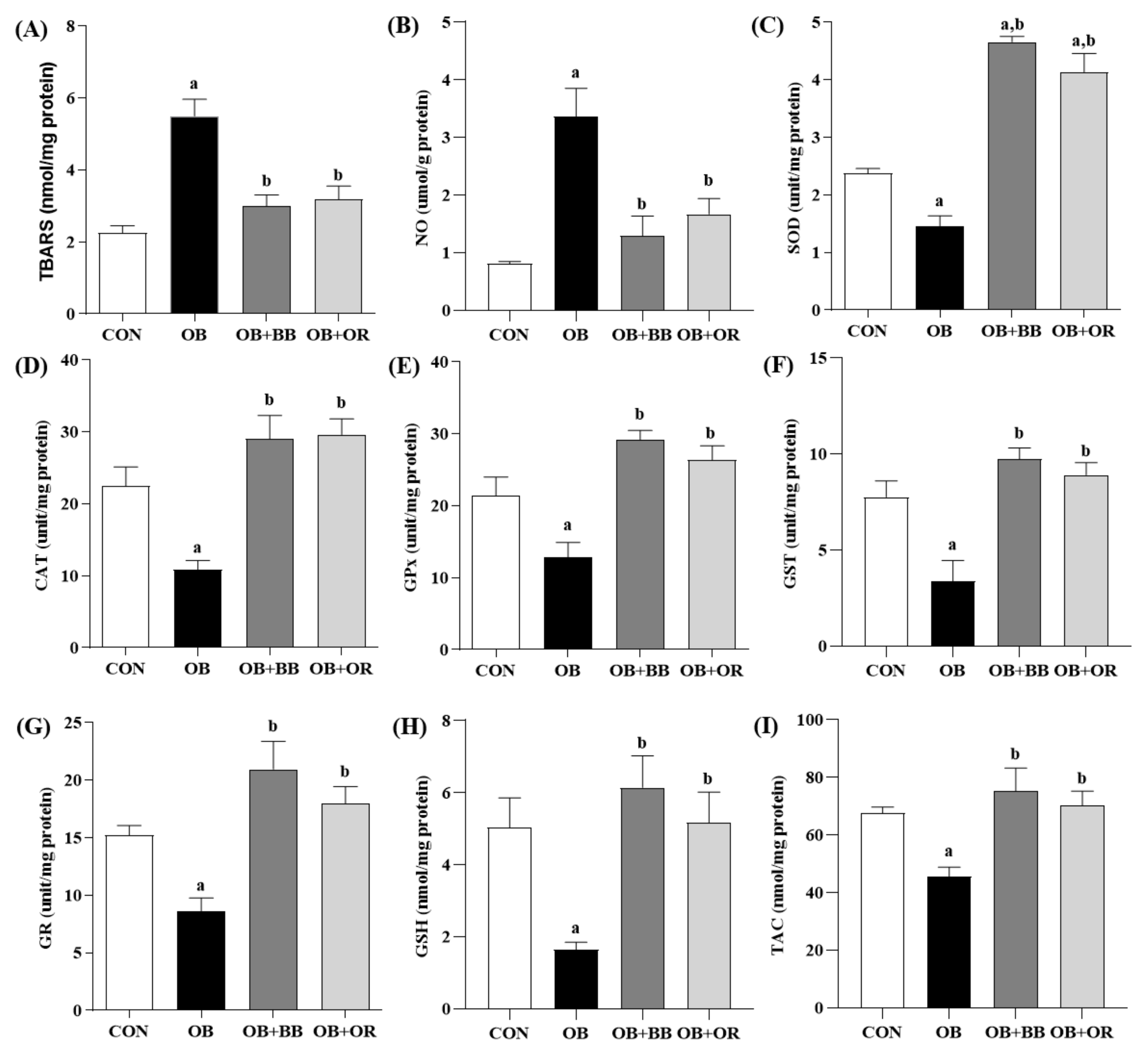
3.5. Effect of H. itama Bee Bread on Liver Oxidant–Antioxidant Parameters
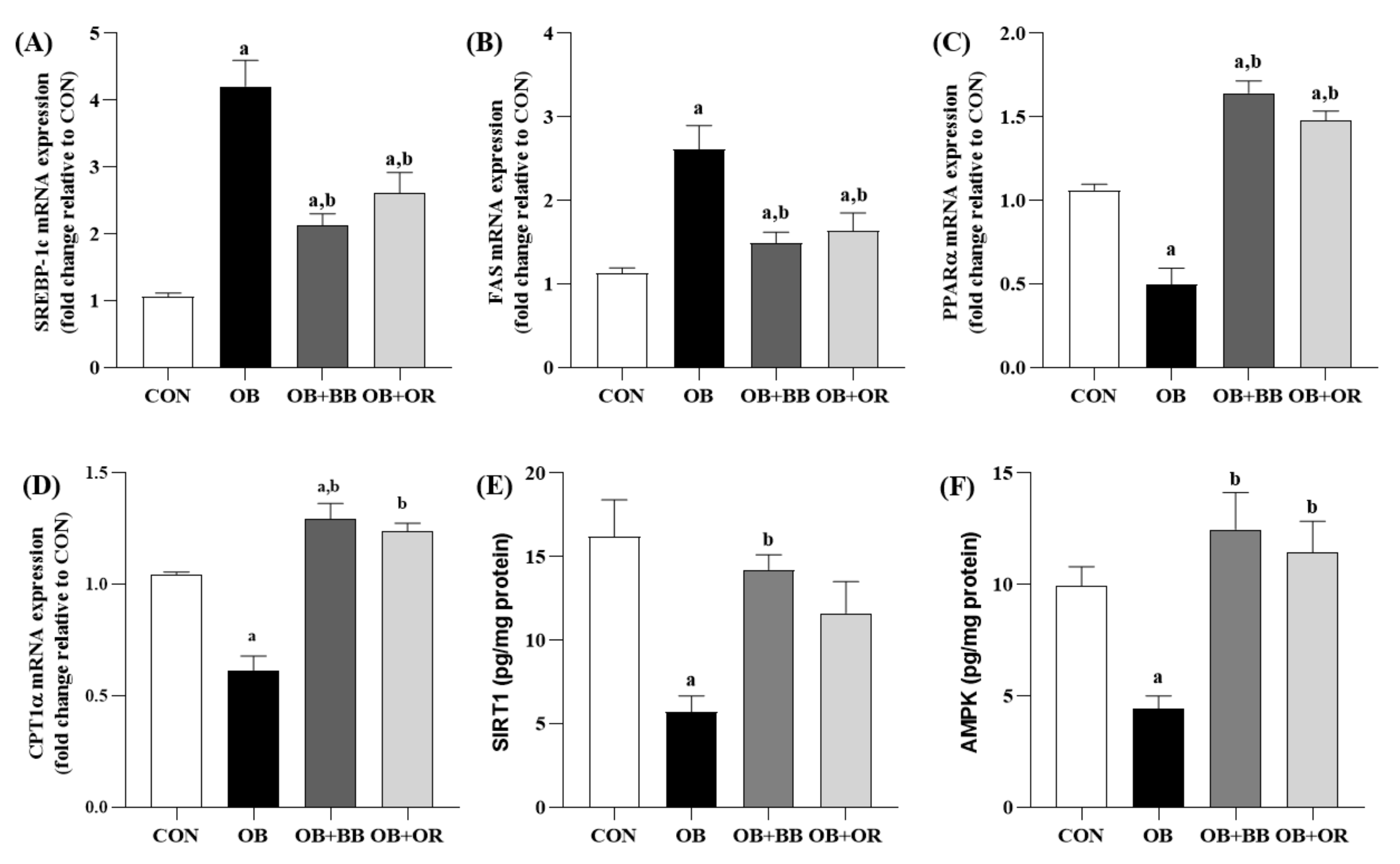
3.6. Effects of H. itama Bee Bread on the Expression of Hepatic Lipid Metabolism-Related Genes, and SIRT1 and AMPK Protein Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, T.K.; Dhurandhar, E.J.; Allison, D.B. Regarding obesity as a disease: Evolving policies and their implications. Endocrinol. Metab. Clin. N. Am. 2016, 45, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romer-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufor, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial oxidative stress and antioxidants balance in fatty liver disease. Hepatol. Commun. 2018, 2, 1425–1439. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Bae, J.H.; Im, S.S.; Hwang, I.; Ha, H.; Song, D.K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch.-Eur. J. Physiol. 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Donizzetti, I.; Gifuni, G.; Sica, R.; Pignalosa, A.; Cavaliere, G.; Gaita, M.; De Filippo, C.; Zorzano, A.; et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS ONE 2014, 9, e92753. [Google Scholar] [CrossRef] [Green Version]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxidat. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Lotfi, A.; Saneei, P.; Hekmatdost, A.; Salehisahlabadi, A.; Shiranian, A.; Ghiasvand, R. The relationship between dietary antioxidant intake and physical activity rate with nonalcoholic fatty liver disease (NAFLD): A case-control study. Clin. Nutr. ESPEN 2019, 34, 45–49. [Google Scholar] [CrossRef]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Fatahi, S.; Sayyari, A.; Olang, B.; Shidfar, F. Associations between dietary total antioxidant capacity and odds of non-alcoholic fatty liver disease (NAFLD) in adults: A case–control study. J. Nutr. Sci. 2020, 9, e48. [Google Scholar] [CrossRef] [PubMed]
- Braud, L.; Battault, S.; Meyer, G.; Nascimento, A.; Gaillard, S.; de Sousa, G.; Rahmani, R.; Riva, C.; Armand, M.; Maixent, J.M.; et al. Antioxidant properties of tea blunt ROS-dependent lipogenesis: Beneficial effect on hepatic steatosis in a high fat-high sucrose diet NAFLD obese rat model. J. Nutr. Biochem. 2017, 40, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental nonalcoholic steatohepatitis and liver fibrosis are ameliorated by pharmacologic activation of Nrf2 (NF-E2 p45-related factor 2). Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 367–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Xie, Z.; Li, E.; Yuan, Y.; Fu, Y.; Wang, P.; Zhang, X.; Qiao, Y.; Xu, J.; Hölscher, C.; et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J. Nat. Med. 2021, 75, 540–552. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.S.; Mestry, S.N.; Gursahani, M.S.; Juvekar, A.R. Plumbagin reduces obesity and nonalcoholic fatty liver disease induced by fructose in rats through regulation of lipid metabolism, inflammation and oxidative stress. Biomed. Pharmacother. 2019, 111, 686–694. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef]
- Silva, A.K.S.; Peixoto, C.A. Role of peroxisome proliferator-activated receptors in non-alcoholic fatty liver disease inflammation. Cell. Mol. Life Sci. 2018, 75, 2951–2961. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Karbasforooshan, H.; Karimi, G. The role of SIRT1 in diabetic retinopathy. Biomed. Pharmacother. 2018, 97, 190–194. [Google Scholar] [CrossRef]
- Lopez, M. Hypothalamic AMPK and energy balance. Eur. J. Clin. Investig. 2018, 48, e12996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, C.-J.; Wu, S.-J.; Shen, S.-C.; Chen, L.-C.; Chen, Y.-L.; Huang, W.-C. Phloretin ameliorates hepatic steatosis through regulation of lipogenesis and Sirt1/AMPK signaling in obese mice. Cell Biosci. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.F.; Shao, J.; Zhao, S.Y.; Qiu, Y.Y.; Teng, L.P.; Huang, W.; Wang, S.S.; Cheng, X.R.; Jiang, Y.Y. Niga-ichigoside F1 ameliorates high-fat diet-induced hepatic steatosis in male mice by Nrf2 activation. Food Funct. 2018, 9, 906–916. [Google Scholar] [CrossRef]
- Mohd, N.; Mohd, F.; Sajap, A.S.; Rosliza, J.; Suri, R. Conservation and sustainable utilization of stingless bees for pollination services in agricultural ecosystems in Malaysia. In Proceedings of the International Seminar on Enhancement of Functional Biodiversity Relevant to Sustainable Food Production in ASPAC, Tsukuba, Japan, 9–11 November 2010; pp. 1–11. Available online: http://www.niaes.affrc.go.jp/sinfo/sympo/h22/1109/paper_04.pdf (accessed on 16 June 2022).
- Mustafa, M.Z.; Yaacob, N.S.; Sulaiman, S.A. Reinventing the Honey Industry: Opportunities of the Stingless Bee. Malays. J. Med. Sci. 2018, 25, 1–5. [Google Scholar] [CrossRef]
- Barajas, J.; Cortes-Rodriguez, M.; Rodríguez-Sandoval, E. Effect of temperature on the drying process of bee pollen from two zones of Colombia. J. Food Process Eng. 2012, 35, 134–148. [Google Scholar] [CrossRef]
- Vásquez, A.; Olofsson, T.C. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 2009, 48, 189–195. [Google Scholar] [CrossRef]
- Andjelkovic, B.; Jevtic, G.; Markovic, J.; Mladenovic, M.; Pseva, V. Quality of honey bee bread collected in spring. In Proceedings of the International Symposium on Animal Science 2014, Belgrade, Serbia, 23–25 September 2014; pp. 450–454. Available online: http://arhiva.nara.ac.rs/handle/123456789/699 (accessed on 19 April 2021).
- Khalifa, S.A.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Xiao, J.; Zou, X.; Khatib, A.; Göransson, U.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Urcan, A.; Al-Marghitas, L.; Dezmirean, D.S.; Bobis, O.; Bonta v Murusen, C.L.; Margaoan, R. Chemical composition and biological activities of beebread—Review. Bull. UASVM Anim. Sci. Biotechnol. 2017, 74, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Antibacterial activity of honey and beebread of different origin against S. aureus and S. epidermidis. Food Technol. Biotechnol. 2007, 45, 201–208. Available online: https://web.p.ebscohost.com/ehost/detail/detail?vid=0&sid=55dd179b-73d0-46bd-83dd-2a2087e2c989%40redis&bdata=#AN=25978276&db=fsr (accessed on 26 February 2018).
- Othman, Z.A.; Ghazali, W.S.W.; Noordin, L.; Yusof, N.A.M.; Mohamed, M. Phenolic compounds and the anti-atherogenic effect of bee bread in high-fat diet-induced obese rats. Antioxidants 2020, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Nna, V.U.; Romli, A.C.; Ghazali, W.S.W.; Mohamed, M. Bee bread ameliorates vascular inflammation and impaired vasorelaxation in obesity-induced vascular damage rat model: The role of eNOS/NO/cGMP-signaling pathway. Int. J. Mol. Sci. 2021, 22, 4225. [Google Scholar] [CrossRef]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Jalil, N.A.C.; Ghazali, W.S.W.; Mohamed, M. Bee bread attenuates the progression of atherosclerosis by activating Nrf2/Keap1 and modulating TNF-α/NF-κβ-associated mast cell migration and a mitochondrial-dependent apoptotic pathway in the obese rat model. Food Funct. 2022, 13, 8119–8130. [Google Scholar] [CrossRef]
- Eleazu, C.; Suleiman, J.B.; Othman, Z.A.; Zakaria, Z.; Nna, V.U.; Hussain, N.H.N.; Mohamed, M. Bee bread attenuates high fat diet induced renal pathology in obese rats via modulation of oxidative stress, downregulation of NF-kB mediated inflammation and Bax signalling. Arch. Physiol. Biochem. 2020, 128, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Abu Bakar, A.B.; Noor, M.M.; Nna, V.U.; Othman, Z.A.; Zakaria, Z.; Eleazu, C.O.; Mohamed, M. Bee bread mitigates downregulation of steroidogenic genes, decreased spermatogenesis, and epididymal oxidative stress in male rats fed with high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2021, 321, e351–e366. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Nna, V.U.; Zakaria, Z.; Othman, Z.A.; Eleazu, C.O.; Bakar, A.B.A.; Ahmad, A.; Usman, U.Z.; Rahman, W.F.W.A.; Mohamed, M. Protective effects of bee bread on testicular oxidative stress, NF-κB-mediated inflammation, apoptosis and lactate transport decline in obese male rats. Biomed. Pharmacother. 2020, 131, 110781. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Mohamed, M.; Bakar, A.B.A.; Zakaria, Z.; Othman, Z.A.; NNa, V.U. Therapeutic effects of bee bread on obesity-induced testicular-derived oxidative stress, inflammation, and apoptosis in high-fat diet obese rat model. Antioxidants 2022, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Othman, Z.A.; Suleiman, J.B.; Jalil, N.A.C.; Ghazali, W.S.W.; Nna, V.U.; Mohamed, M. Hepatoprotective effect of bee bread in metabolic dysfunction-associated fatty liver disease (MAFLD) rats: Impact on oxidative stress and inflammation. Antioxidants 2021, 10, 2031. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Q.; Liu, Y.; Peng, C.; Zeng, Z. Natural bee bread positively regulates lipid metabolism in rats. Int. J. Agric. Sci. Food Technol. 2021, 7, 266–271. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic acid inhibits lipid accumulation via AMPK pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Z.; Cheng, B.; Liu, M.; Tao, S.; Guan, S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp. Ther. Med. 2019, 18, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2021, 99, 108840. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Mohamed, M.; Bakar, A.B.A.; Nna, V.U.; Zakaria, Z.; Othman, Z.A.; Aroyehun, A.B. Chemical Profile, Antioxidant Properties and Antimicrobial Activities of Malaysian Heterotrigona itama Bee Bread. Molecules 2021, 26, 4943. [Google Scholar] [CrossRef]
- Othman, Z.A.; Noordin, L.; Omar, N.; Mohd Yusof, N.A.; Mohamaed, M. Protective Effects of orlistat on lipid profile, cardiac oxidative stress biomarkers and histology in high-fat diet-induced obese rats. IIUM Med. J. Malays. 2019, 18, 1–6. [Google Scholar] [CrossRef]
- Bellinger, L.L.; Bernardis, L.L. Effect of dorsomedial hypothalamic nuclei knife cuts on ingestive behavior. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R1772–R1779. [Google Scholar] [CrossRef] [Green Version]
- Malafaia, A.B.; Nassif, P.A.N.; Ribas, C.A.P.M.; Ariede, B.L.; Sue, K.N.; Cruz, M.A. Obesity induction with high fat sucrose in rats. ABCD Arquiros Bras. Cir. Dig. 2013, 26, 17–21. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Essawy, S. Addition of a low dose of rimonabant to orlistat therapy decreases weight gain and reduces adiposity in dietary obese rats. Clin. Exp. Pharmacol. Physiol. 2012, 39, 551–559. [Google Scholar] [CrossRef]
- Roza, N.A.; Possignolo, L.F.; Palanch, A.C.; Gontijo, J.A. Effect of long-term high-fat diet intake on peripheral insulin sensibility, blood pressure, and renal function in female rats. Food Nutr. Res. 2016, 60, 28536. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Cuzzocrea, S.; Brown, P.A.; Zacharowski, K.; Stewart, K.N.; Mota-Filipe, H.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000, 58, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Al Batran, R.; Al-Bayaty, F.; Jamil Al-Obaidi, M.M.; Abdualkader, A.M.; Hadi, H.A.; Ali, H.M.; Abdulla, M.A. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS ONE 2013, 8, e64751. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Doğan, P.; Tanrikulu, G.; Soyuer, Ü.; Köse, K. Oxidative enzymes of polymorphonuclear leucocytes and plasma fibrinogen, ceruloplasmin, and copper levels in Behcet’s disease. Clin. Biochem. 1994, 27, 413–418. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Luchese, C.; Pinton, S.; Nogueira, C.W. Brain and lungs of rats are differently affected by cigarette smoke exposure: Antioxidant effect of an organoselenium compound. Pharmacol. Res. 2009, 59, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Annuk, M.; Zilmer, M.; Lind, L.; Linde, T.; Fellström, B. Oxidative stress and endothelial function in chronic renal failure. J. Am. Soc. Nephrol. 2001, 12, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Skinner, R.C.; Warren, D.C.; Lateef, S.N.; Benedito, V.A.; Tou, J.C. Apple pomace consumption favorably alters hepatic lipid metabolism in young female Sprague-Dawley rats fed a western diet. Nutrients 2018, 10, 1882. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Deng, J.; Zhang, Y.; Yang, J.; He, Y.; Fu, W.; Xing, P.; Wan, H.T. Lipid-lowering effects of Danhong injection on hyperlipidemia rats. J. Ethnopharmacol. 2014, 154, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.F.; Li, J.; Zhang, Y.M.; Zhu, L.; Chen, H.; Yuan, L.; Hu, J.; Yi, X.L.; Wu, Q.T.; Wan, M.H.; et al. Sheng-jiang powder ameliorates obesity-induced pancreatic inflammatory injury via stimulating activation of the AMPK signalling pathway in rats: Basic Study. World J. Gastroenterol. 2018, 24, 4448–4461. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Hu, X.F.; Yao, J.; Gao, S.G.; Wang, X.S.; Peng, X.Q.; Yang, Y.T.; Feng, X.S. Nrf2 overexpression predicts prognosis and 5-FU resistance in gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5231–5235. [Google Scholar] [CrossRef] [Green Version]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2020, 15, 790–800. [Google Scholar] [CrossRef]
- Liou, C.-J.; Wei, C.-H.; Chen, Y.-L.; Cheng, C.-Y.; Wang, C.-L.; Huang, W.-C. Fisetin protects against hepatic steatosis through regulation of the Sirt1/AMPK and fatty acid β-oxidation signaling pathway in high-fat diet-induced obese mice. Cell. Physiol. Biochem. 2018, 49, 1870–1884. [Google Scholar] [CrossRef]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Mărgăoan, R.; Caeiro, A.; Campos, M.G. Similarity of data from bee bread with the same taxa collected in India and Romania. Molecules 2018, 23, 2491. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-J.; Lee, Y.-J.; Hwang, J.-H.; Kim, K.-J.; Lee, B.-Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, R.; Chotimarkorn, C.; Shafiqul, I.M.; Hori, M.; Ozaki, H.; Ushio, H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007, 358, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Zakaria, Z.; Othman, Z.A.; Bagi Suleiman, J.; Che Jalil, N.A.; Wan Ghazali, W.S.; Mohamed, M. Protective and therapeutic effects of orlistat on metabolic syndrome and oxidative stress in high-fat diet-induced metabolic dysfunction-associated fatty liver disease (MAFLD) in rats: Role on Nrf2 activation. Vet. Sci. 2021, 8, 274. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, Y.; Liu, Y.; Ping, J.; Shou, Q.; Chen, F.; Ruo, R. Metformin improves hepatic IRS2/PI3K/Akt signaling in insulin-resistant rats of NASH and cirrhosis. J. Endocrinol. 2016, 229, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, W.; Liu, Y.; Li, Y.; Gao, L.; Zhao, J.J. Alpha-lipoic acid attenuates insulin resistance and improves glucose metabolism in high fat diet-fed mice. Acta. Pharmacol. Sin. 2014, 35, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Liu, Q.; Zhang, Q.; Liu, D.; Zhang, C.; Fan, D.; Deng, J.; Yang, H. Kiwifruit seed oil ameliorates inflammation and hepatic fat metabolism in high-fat diet-induced obese mice. J. Funct. Foods 2019, 52, 715–723. [Google Scholar] [CrossRef]
- Nah, E.-H.; Cho, S.; Park, H.; Kim, S.; Cho, H.-I. Associations of complete blood count parameters with pancreatic beta-cell function and insulin resistance in prediabetes and type 2 diabetes mellitus. J. Clin. Lab. Anal. 2022, 36, e24454. [Google Scholar] [CrossRef]
- Guo, H.; Ma, C.; Wu, X.; Pan, C. Functional status of pancreatic α and β cells in type 2 diabetes mellitus patients with different plasma triglyceride levels: A retrospective analysis. Int. J. Endocrinol. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Joukar, F.; Mobaraki, S.N.; Mavaddati, S.; Hassanipourd, S.; Sepehrimaneshad, M. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: A cross-sectional study in north of Iran. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shao, S.; Cai, H.; Han, J.; Guo, T.; Fu, Y.; Yu, C.; Zhao, M.; Bo, T.; Yao, Z.; et al. Comparison of erythrocyte membrane lipid profiles between NAFLD patients with or without hyperlipidemia. Int. J. Endocrinol. 2020, 2020, 9501826. [Google Scholar] [CrossRef] [PubMed]
- Begum, T.A.K.M.; Ramamurthy, V. The modulating effect of ferulic acid on high fat diet induced hyperlipidemia and obesity: A dose response study in male Sprague Dawleyrats. Biomedicine 2021, 41, 413–420. [Google Scholar] [CrossRef]
- Feng, K.; Zhu, X.; Chen, T.; Peng, B.; Lu, M.; Zheng, H.; Huang, Q.; Ho, C.T.; Chen, Y.; Cao, Y. Prevention of obesity and hyperlipidemia by heptamethoxyflavone in high-fat diet-induced rats. J. Agric. Food Chem. 2019, 67, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Jeepipallia, S.P.K.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Lai, Y.S.; Chen, W.C.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Tseng, H.C.; Lin, S.Y.; Sheen, L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef]
- Cai, S.M.; Yang, R.Q.; Li, Y.; Ning, Z.W.; Zhang, L.L.; Zhou, G.S.; Luo, W.; Li, D.H.; Chen, Y.; Pan, M.X.; et al. Angiotensin-(1-7) improves liver fibrosis by regulating the NLRP3 inflammasome via redox balance modulation. Antioxid. Redox Signal. 2016, 24, 795–812. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, L.; Yuan, X.; Chen, H.; Xiong, R.; Zhang, S.; Cheng, H.; Shen, Y.; An, H.; Li, T.; et al. Britanin ameliorates cerebral ischemia–reperfusion injury by inducing the Nrf2 protective pathway. Antioxid. Redox Signal. 2017, 27, 754–768. [Google Scholar] [CrossRef]
- Wu, K.C.; Liu, J.; Klaassen, C.D. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol. Appl. Pharmacol. 2012, 262, 321–329. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Z.H.; Li, C.G.; Zhu, Y.J.; Li, Z.; Tang, Y.Z.; Ni, C.L. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed. Pharmacother. 2018, 105, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Chen, L.; Zhao, Y.; Pan, Y.; Yang, Y.Z.; Sun, Y.; Jiao, R.Q.; Kong, L.D. Polygonum cuspidatum extract attenuates fructose-induced liver lipid accumulation through inhibiting Keap1 and activating Nrf2 antioxidant pathway. Phytomedicine 2019, 63, 152986. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, Z.; Othman, Z.A.; Nna, V.U.; Mohamed, M. The promising roles of medicinal plants and bioactive compounds on hepatic lipid metabolism in the treatment of non-alcoholic fatty liver disease in animal models: Molecular targets. Arch. Physiol. Biochem. 2021, 1–17. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, F.; Xu, W.; Wu, X.; Lian, N.; Jin, H.; Chen, Q.; Chen, L.; Shao, J.; Wu, L.; et al. Curcumin attenuates ethanol-induced hepatic steatosis through modulating Nrf2/FXR signaling in hepatocytes. IUBMB Life 2015, 67, 645–658. [Google Scholar] [CrossRef]
- Li, J.; Liu, M.; Yu, H.; Wang, W.; Han, L.; Chen, Q.; Ruan, J.; Wen, S.; Zhang, Y.; Wang, T. Mangiferin improves hepatic lipid metabolism mainly through its metabolite-norathyriol by modulating SIRT-1/AMPK/SREBP-1c signaling. Front. Pharmacol. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Bonfleur, M.L.; Borck, P.C.; Ribeiro, R.A.; Caetano, L.C.; Soares, G.M.; Carneiro, E.M.; Balbo, S.L. Improvement in the expression of hepatic genes involved in fatty acid metabolism in obese rats supplemented with taurine. Life Sci. 2015, 135, 15–21. [Google Scholar] [CrossRef]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, H.; Lee, H.; Kim, E.; Mu, L.; Rhee, Y.K.; Cho, C.W.; Chung, J. Inhibitory effect of a Cirsium setidens extract on hepatic fat accumulation in mice fed a high-fat diet via the induction of fatty acid β-oxidation. Biosci. Biotech. Biochem. 2013, 77, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Li, S.; Ma, L.; Cheng, L.; Deng, C.; Chen, Z.; Xie, C.; Xiang, M.; Jiang, W.; Chen, L. A novel agonist of PPAR-γ based on barbituric acid alleviates the development of non-alcoholic fatty liver disease by regulating adipocytokine expression and preventing insulin resistance. Eur. J. Pharmacol. 2011, 659, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Lee, M.S.; Chang, E.; Kim, Y. Chrysanthemum morifolium flower extract ameliorates obesity-induced inflammation and increases the muscle mitochondria content and AMPK/SIRT1 activities in obese rats. Nutrients 2021, 13, 3660. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwarkanath, A.; Dwivedi, U.N.; Kakkar, P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. 2021, 12, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Tao, X.; Xu, L.; Qi, Y.; Yin, L.; Han, X.; Xu, Y.; Zheng, L.; Peng, J. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacol. Res. 2018, 131, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary polyphenols protect against oleic acid-induced steatosis in an in vitro model of NAFLD by modulating lipid metabolism and improving mitochondrial function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84. [Google Scholar] [CrossRef]
- Li, X.; Gong, H.; Yang, S.; Yang, L.; Fan, Y.; Zhou, Y. Pectic bee pollen polysaccharide from Rosa rugosa alleviates diet-induced hepatic steatosis and insulin resistance via induction of AMPK/mTOR-mediated autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.M.; Peyton, K.J.; Shebib, A.R.; Wang, H.; Korthuis, R.J.; Durante, W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H84–H93. [Google Scholar] [CrossRef] [Green Version]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [Green Version]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Accession Number | Sequence | Reference |
|---|---|---|---|
| SREBP-1c | NM_001276707.1 | Forward: 5′-GCCTGCTTGGCTCTTCTCT-3′ Reverse: 5′-GCTTGTTTGCGATGTCTCC-3′ | [62] |
| FAS | NM_017332.1 | Forward: 5′-TCGACTTCAAAGGACCCAGC-3′ Reverse: 5′-ACTGCACAGAGGTGTTAGGC-3′ | [63] |
| PPARα | NM_013196.1 | Forward: 5′-ATTCGGCTAAAGCTGGCGTA-3′ Reverse: 5′-TGCATTGTGTGACATCCCGA-3′ | [63] |
| CPT1α | BC072522.1 | Forward: 5′-GGACATTCCTCTCTCAGGTTTC-3′ Reverse: 5′-ACCTCCTCCTTTGAACACATAC-3′ | [63] |
| GAPDH | NM_017008 | Forward: 5′-TCACCACCATGGAGAAGGC-3′ Reverse: 5′-GCTAAGCAGTTGGTGGTGCA-3′ | [39] |
| Phenolic Compound | Molecular Formula | Retention Time (min) | Area (mAU × min) | Relative Area (%) |
|---|---|---|---|---|
| Aqueous extract: | ||||
| Gallic acid | C7H6O5 | 5.93 | 1.106 | 1.59 |
| Mangiferin | C19H18O11 | 13.09 | 1.093 | 1.57 |
| Trans 3-hydroxycinnamic acid | C9H8O3 | 15.05 | 40.235 | 57.77 |
| 2-hydroxycinnamic acid | C9H8O3 | 15.77 | 1.379 | 1.98 |
| Methanol extract: | ||||
| Caffeic acid | C9H8O4 | 12.53 | 2.153 | 0.50 |
| Mangiferin | C19H18O11 | 13.09 | 4.170 | 0.97 |
| Trans ferulic acid | C10H10O4 | 14.62 | 1.035 | 0.24 |
| Trans 3-hydroxycinnamic acid | C9H8O3 | 15.05 | 74.221 | 17.31 |
| 2-hydroxycinnamic acid | C9H8O3 | 15.77 | 4.909 | 1.15 |
| Quercetin | C15H10O7 | 17.64 | 18.878 | 4.40 |
| Kaempferol | C15H10O6 | 18.73 | 7.999 | 1.87 |
| Apigenin | C15H10O5 | 19.01 | 9.499 | 2.22 |
| CON | OB | OB + BB | OB + OR | |
|---|---|---|---|---|
| Lee obesity index | 306.4 ± 1.60 | 331.5 ± 2.69 a | 311.4 ± 2.45 b | 316.9 ± 2.64 a,b |
| Body weight gain (g) | 103.0 ± 9.26 | 204.7 ± 10.98 a | 154.9 ± 14.47 a,b | 157.0 ± 6.98 a,b |
| Average daily food intake (g/day) | 20.97 ± 0.50 | 20.65 ± 0.71 | 18.58 ± 0.70 | 19.08 ± 0.72 |
| Average daily calorie intake (kJ/day) | 282.7 ± 6.47 | 446.2 ± 15.35 a | 401.5 ± 15.17 a | 412.3 ± 15.46 a |
| CON | OB | OB + BB | OB + OR | |
| Fasting glucose (mg/dL) | 70.50 ± 1.45 | 83.00 ± 3.54 a | 73.22 ± 1.36 b | 74.50 ± 1.93 b |
| Fasting insulin (ng/mL) | 0.64 ± 0.09 | 3.71 ± 1.33 a | 1.22 ± 0.22 b | 1.80 ±0.09 b |
| HOMA-IR | 0.12 ± 0.01 | 0.38 ± 0.07 a | 0.22 ± 0.03 b | 0.26 ± 0.05 a |
| TG (mmol/L) | 0.49 ± 0.03 | 0.92 ± 0.03 a | 0.60 ± 0.06 b | 0.77 ± 0.08 a |
| TC (mmol/L) | 1.66 ± 0.10 | 2.79 ± 0.40 a | 1.97 ± 0.06 b | 2.01 ± 0.09 b |
| LDL-C (mmol/L) | 0.80 ± 0.04 | 1.80 ± 0.24 a | 1.12 ± 0.10 b | 1.03 ± 0.08 b |
| HDL-C (mmol/L) | 0.53 ± 0.02 | 0.40 ± 0.02 a | 0.49 ± 0.04 | 0.66 ± 0.03 a,b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, Z.; Othman, Z.A.; Suleiman, J.B.; Mustaffa, K.M.F.; Jalil, N.A.C.; Ghazali, W.S.W.; Zulkipli, N.N.; Mohamed, M.; Kamaruzaman, K.A. Therapeutic Effects of Heterotrigona itama (Stingless Bee) Bee Bread in Improving Hepatic Lipid Metabolism through the Activation of the Keap1/Nrf2 Signaling Pathway in an Obese Rat Model. Antioxidants 2022, 11, 2190. https://doi.org/10.3390/antiox11112190
Zakaria Z, Othman ZA, Suleiman JB, Mustaffa KMF, Jalil NAC, Ghazali WSW, Zulkipli NN, Mohamed M, Kamaruzaman KA. Therapeutic Effects of Heterotrigona itama (Stingless Bee) Bee Bread in Improving Hepatic Lipid Metabolism through the Activation of the Keap1/Nrf2 Signaling Pathway in an Obese Rat Model. Antioxidants. 2022; 11(11):2190. https://doi.org/10.3390/antiox11112190
Chicago/Turabian StyleZakaria, Zaida, Zaidatul Akmal Othman, Joseph Bagi Suleiman, Khairul Mohd Fadzli Mustaffa, Nur Asyilla Che Jalil, Wan Syaheedah Wan Ghazali, Ninie Nadia Zulkipli, Mahaneem Mohamed, and Khaidatul Akmar Kamaruzaman. 2022. "Therapeutic Effects of Heterotrigona itama (Stingless Bee) Bee Bread in Improving Hepatic Lipid Metabolism through the Activation of the Keap1/Nrf2 Signaling Pathway in an Obese Rat Model" Antioxidants 11, no. 11: 2190. https://doi.org/10.3390/antiox11112190
APA StyleZakaria, Z., Othman, Z. A., Suleiman, J. B., Mustaffa, K. M. F., Jalil, N. A. C., Ghazali, W. S. W., Zulkipli, N. N., Mohamed, M., & Kamaruzaman, K. A. (2022). Therapeutic Effects of Heterotrigona itama (Stingless Bee) Bee Bread in Improving Hepatic Lipid Metabolism through the Activation of the Keap1/Nrf2 Signaling Pathway in an Obese Rat Model. Antioxidants, 11(11), 2190. https://doi.org/10.3390/antiox11112190











