RP-HPLC-DAD Analysis of Mitragynine Content in Mitragyna speciosa Korth. (Ketum) Leaf Extracts Prepared Using Ultrasound Assisted Extraction Technique and Their Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Analytical Instruments
2.3. Plant Material
2.3.1. Preparation of Plant Extracts
2.3.2. Isolation and Quantification of the Mitragynine
2.4. Total Phenolic Flavonoid Content Analysis
2.4.1. Total Phenolic Contents (TPC)
2.4.2. Total Flavonoid Contents (TFC)
2.5. Cell Cultures and Conditions
2.5.1. Preparation of Extracts and Cell Treatment
2.5.2. MTT Cell Viability Assay
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.; Chear, N.J.Y.; Narayanan, S.; Leon, F.; Sharma, A.; McCurdy, C.R.; Avery, B.A.; Balasingam, V. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly-drug users. J. Ethnopharmacol. 2020, 249, 112462. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, T.; Ngew, K.Z.; Zailan, A.A.D.; Jong, V.Y.M.; Abu Bakar, M.H. The chemical and pharmacological properties of mitragynine and its diastereomers: An insight review. Front. Pharmacol. 2022, 13, 805986. [Google Scholar] [CrossRef] [PubMed]
- Vicknasingam, B.; Narayanan, S.; Beng, G.T.; Mansor, S.M. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int. J. Drug Policy 2010, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ilmie, M.U.; Mansor, S.M.; Abdullah, J.M. Behavioural and electrophysiological evidence of impaired learning and memory in male sprague dawley rats following subchronic exposure to standardized methanolic extract of Mitragyna speciosa Korth. Malays. J. Med. Sci. 2015, 22, 45–51. [Google Scholar] [PubMed]
- Raffa, R.B. Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Parthasarathy, S.; Ramanathan, S.; Murugaiyah, V.; Hamdan, M.R.; Said, M.I.M.; Lai, C.S.; Mansor, S.M. A simple HPLC–DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for the application in forensic investigation. Forensic Sci. Int. 2013, 226, 183–187. [Google Scholar] [CrossRef]
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.; Suhaimi, F.W.; Vadivelu, R.; Vicknasingam, B.K.; Amato, D.; von Hörsten, S.; Ismail, N.I.; et al. From kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef]
- Kislik, V.S. Solvent Extraction: Classical and Novel Approaches; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Patil, A.S. Plant Secondary Metabolites: Isolation, Characterization & Biological Properties; Studera Press: Delhi, India, 2020. [Google Scholar]
- Kikura-Hanajiri, R.; Kawamura, M.; Maruyama, T.; Kitajima, M.; Takayama, H.; Goda, Y. Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom” (Mitragyna speciosa) by LC-ESI-MS. Forensic Toxicol. 2009, 27, 67–74. [Google Scholar] [CrossRef]
- Chan, K.B.; Pakiam, C.; Rahim, R.A. Psychoactive plant abuse: The identification of mitragynine in ketum and in ketum preparations. Bull. Narc. 2005, 2005, 249–256. [Google Scholar]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and classical methods for the extraction of Mitragyna speciosa leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef]
- Haris, H.M. An optimized recovery of mitragynine from Mitragyna speciosa using freeze drying and ultrasonic-assisted extraction method. Int. J. Sci. Technol. 2013, 15, 1077–1083. [Google Scholar]
- Goh, Y.S.; Karunakaran, T.; Murugaiyah, V.; Santhanam, R.; Abu Bakar, M.H.; Ramanathan, S. Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) leaves: Evaluation of its cytotoxicity and antinociceptive activity. Molecules 2021, 26, 3704. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.H.; Wang, M.; Ali, Z.; Smillie, T.J.; Zweigenbaum, J.; Khan, I.A. Identification and characterization of indole and oxindole alkaloids from leaves of Mitragyna speciosa Korth using liquid chromatography–accurate QToF mass spectrometry. J. AOAC Int. 2015, 98, 13–21. [Google Scholar] [CrossRef]
- Farooq, M.U.; Mumtaz, M.W.; Mukhtar, H.; Rashid, U.; Akhtar, M.T.; Raza, S.A.; Nadeem, M. UHPLC-QTOF-MS/MS based phytochemical characterization and anti-hyperglycemic prospective of hydro-ethanolic leaf extract of Butea monosperma. Sci. Rep. 2020, 10, 3530. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, T.; Firouz, N.S.; Santhanam, R.; Jong, V.Y.M. Phytochemicals from Calophyllum macrocarpum Hook. f. and its cytotoxic activities. Nat. Prod. Res. 2022, 36, 654–659. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Metabolomics: What you see is what you extract. Phytochem. Anal. 2014, 25, 289–290. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, H.; Aslam, M.; Muntaha, S.T. Effect of solvent polarity and extraction method on phytochemical composition and antioxidant potential of corn silk. Free Radic. Antioxid. 2019, 9, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Kothari, V.; Seshadri, S. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr. Food Sci. 2010, 40, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Azieana, J.; Zainon, M.N.; Noriham, A.; Rohana, M.N. Total phenolic and flavonoid content and antioxidant activities of ten Malaysian wild mushrooms. Open Access Libr. 2017, 4, 80503. [Google Scholar] [CrossRef]
- Wiji, P.P.; Bahtiar, A.; Hayun, H. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci. Pharm. 2018, 86, 25. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Williamson, E.M.; Gibbons, S.; Barnes, J.; Prieto-Garcia, J. Fundamentals of Pharmacognosy and Phytotherapy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
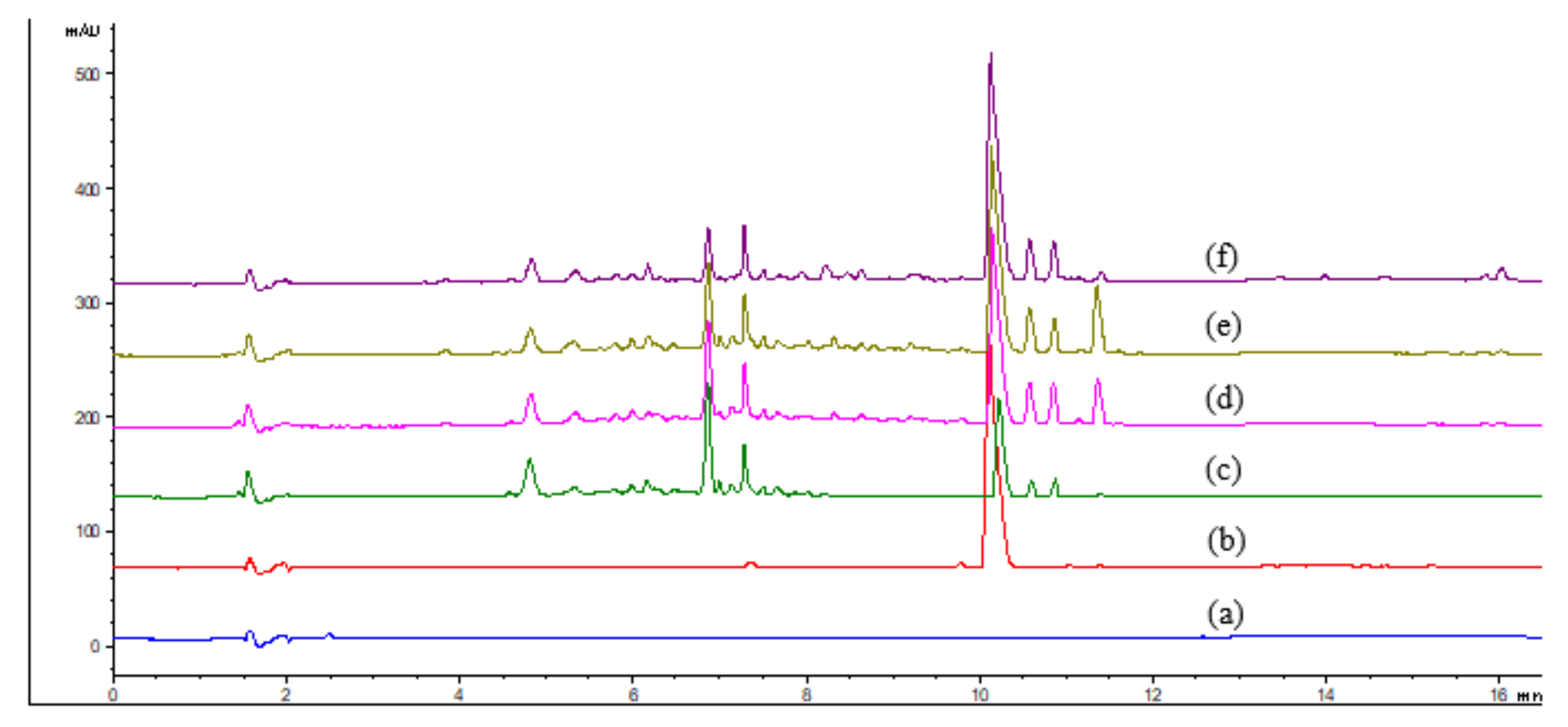
| Sample | Time (min) | Mean of Dry Yield (g) | Mean Percentage of Mitragynine (%) Mean ± SEM (n) |
|---|---|---|---|
| UAE aqueous | 20 | 1.88 ± 0.13 | 2.27 ± 0.13 |
| 40 | 1.80 ± 0.26 | 2.39 ± 0.21 | |
| 60 | 2.24 ± 0.11 | 2.41 ± 0.17 |
| Sample | Time (min) | Mean of Dry Yield (g) | Mean Percentage of Mitragynine (%) Mean ± SEM |
|---|---|---|---|
| UAE aqueous | 20 | 1.88 ± 0.13 c | 2.27 ± 0.13 a |
| UAE MeOH | 20 | 1.92 ± 0.22 c | 7.22 ± 0.48 b |
| UAE EtOH | 20 | 0.87 ± 0.04 b | 9.40 ± 0.91 b |
| UAE EtOAc | 20 | 0.22 ± 0.03 a | 8.10 ± 1.12 b |
| Extract | Mean of Absorbance | Total Phenolic Content in Dry Extract (GAE mg/g), Mean ± SEM |
|---|---|---|
| UAE aqueous | 0.220 | 192.83 ± 4.17 a |
| UAE MeOH | 0.492 | 419.50 ± 2.50 d |
| UAE EtOH | 0.344 | 295.75 ± 1.25 c |
| UAE EtOAc | 0.258 | 224.08 ± 0.59 b |
| Extract | Mean of Absorbance | Total Flavonoids Content in Dry Extract (QE mg/g), Mean ± SEM |
|---|---|---|
| UAE aqueous | 0.036 | 69.00 ± 2.89 a |
| UAE MeOH | 0.079 | 177.33 ± 3.00 c |
| UAE EtOH | 0.068 | 148.17 ± 2.20 b |
| UAE EtOAc | 0.081 | 181.50 ± 2.89 c |
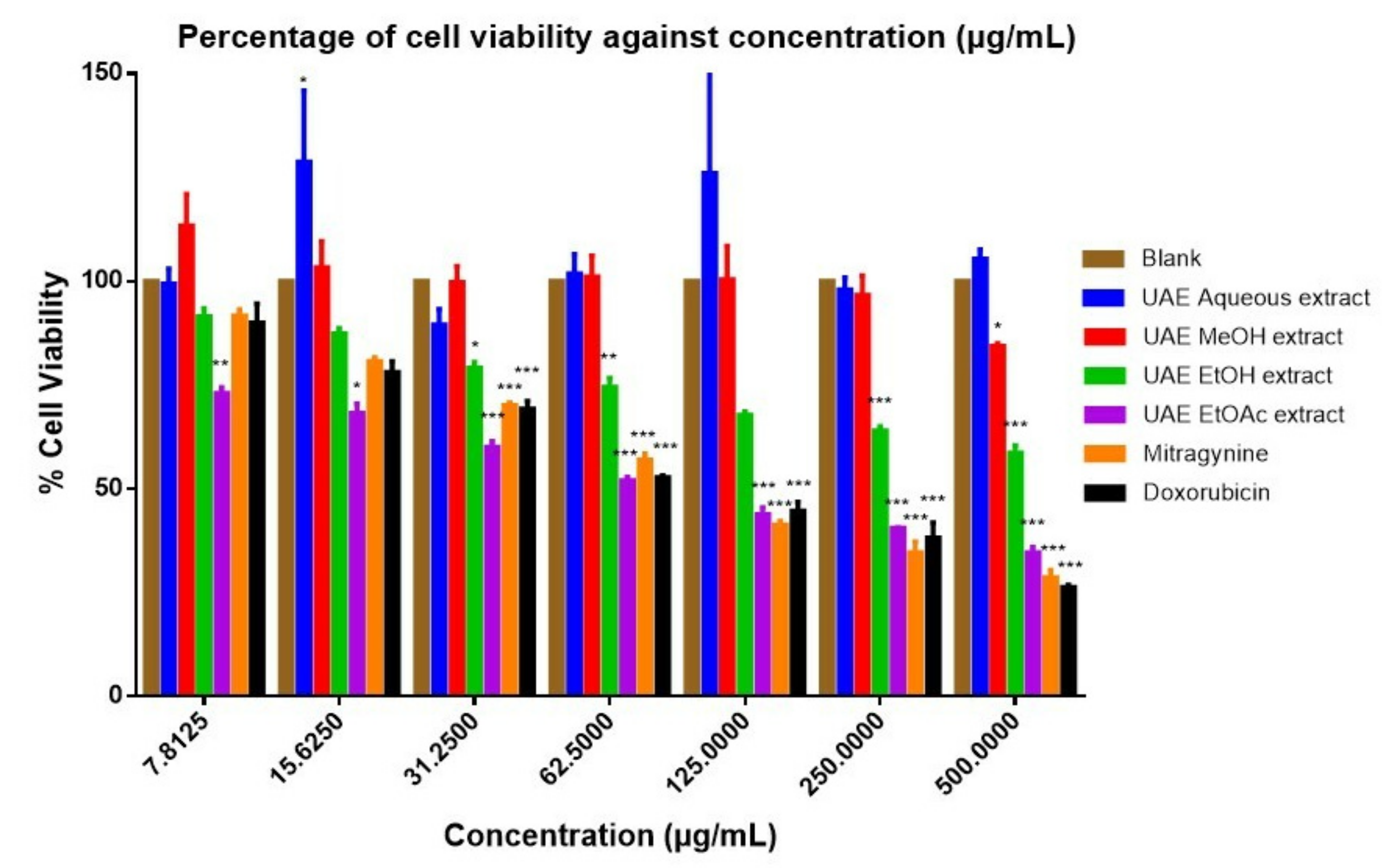
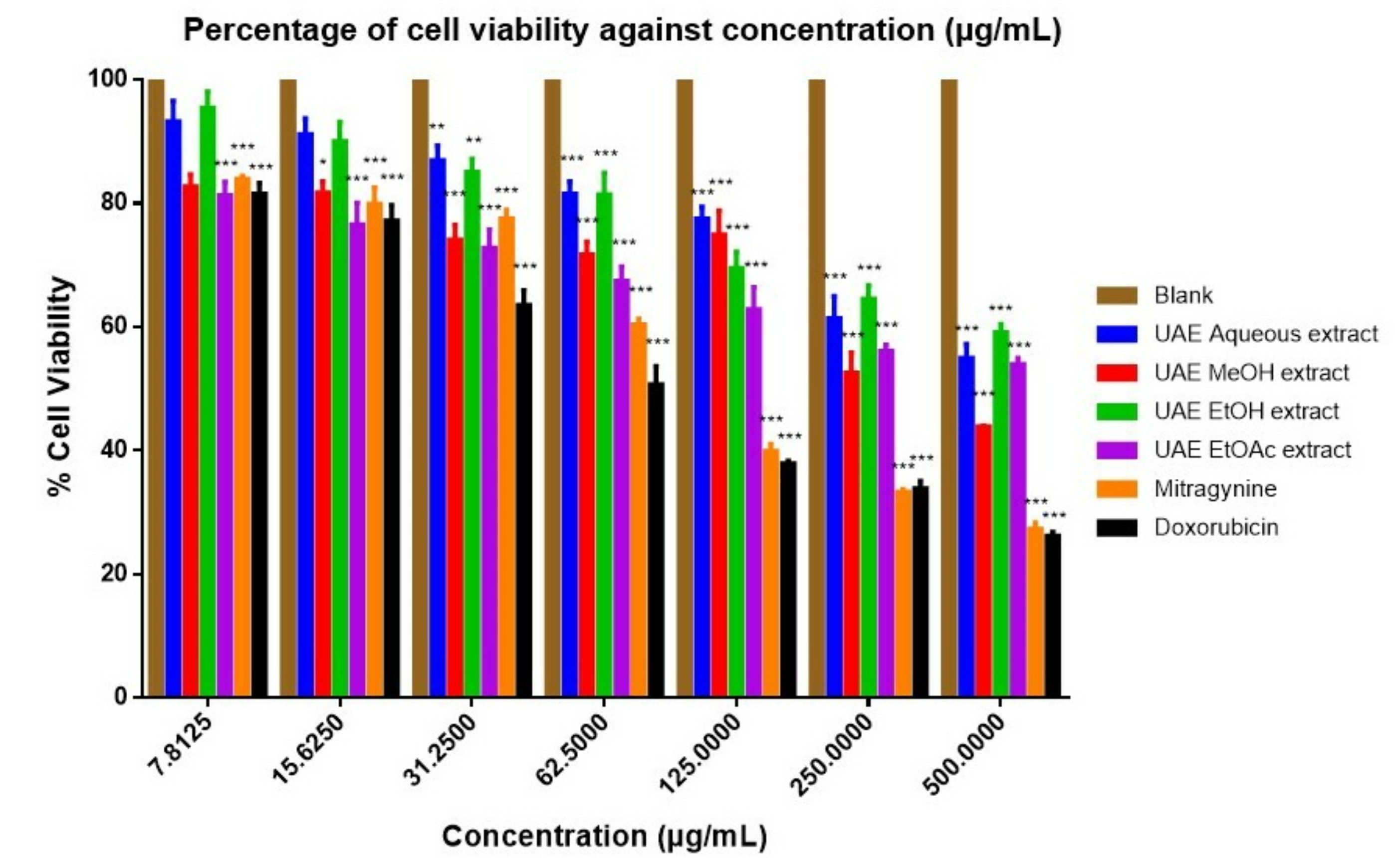
| Sample | IC50 Value | |
|---|---|---|
| HEK-293 Kidney Cells | HeLa Chang Liver Cells | |
| UAE aqueous extract | >100 µg/mL | >100 µg/mL |
| UAE MeOH extract | >100 µg/mL | >100 µg/mL |
| UAE EtOH extract | 48.46 ± 11.93 µg/mL | 86.61 ± 14.95 µg/mL |
| UAE EtOAc extract | 46.16 ± 9.37 µg/mL | 70.58 ± 8.10 µg/mL |
| Mitragynine | 112.30 ± 17.59 µM | 210.04 ± 0.80 µM |
| Doxorubicin a | 80.82 ± 12.05 µM | 86.23 ± 27.49 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunakaran, T.; Goh, Y.S.; Santhanam, R.; Murugaiyah, V.; Abu Bakar, M.H.; Ramanathan, S. RP-HPLC-DAD Analysis of Mitragynine Content in Mitragyna speciosa Korth. (Ketum) Leaf Extracts Prepared Using Ultrasound Assisted Extraction Technique and Their Cytotoxicity. Separations 2022, 9, 345. https://doi.org/10.3390/separations9110345
Karunakaran T, Goh YS, Santhanam R, Murugaiyah V, Abu Bakar MH, Ramanathan S. RP-HPLC-DAD Analysis of Mitragynine Content in Mitragyna speciosa Korth. (Ketum) Leaf Extracts Prepared Using Ultrasound Assisted Extraction Technique and Their Cytotoxicity. Separations. 2022; 9(11):345. https://doi.org/10.3390/separations9110345
Chicago/Turabian StyleKarunakaran, Thiruventhan, Yong Sean Goh, Rameshkumar Santhanam, Vikneswaran Murugaiyah, Mohamad Hafizi Abu Bakar, and Surash Ramanathan. 2022. "RP-HPLC-DAD Analysis of Mitragynine Content in Mitragyna speciosa Korth. (Ketum) Leaf Extracts Prepared Using Ultrasound Assisted Extraction Technique and Their Cytotoxicity" Separations 9, no. 11: 345. https://doi.org/10.3390/separations9110345