Chemerin-Induced Down-Regulation of Placenta-Derived Exosomal miR-140-3p and miR-574-3p Promotes Umbilical Vein Endothelial Cells Proliferation, Migration, and Tube Formation in Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Mouse Model Establishment
2.3. Preparation of Placenta-Derived Exosomes from Placenta Tissue and Exosomes from Trophoblast Cells
2.4. Cell Culture and Treatment
2.5. Small RNA Sequencing
2.6. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Immunofluorescence Staining
2.8. Western Blotting
2.9. Enzyme-Linked Immunosorbent Assay
2.10. Cell Counting Kit-8 Assay
2.11. Transwell Assay
2.12. Tube Formation Assay
2.13. Statistical Analysis
3. Results
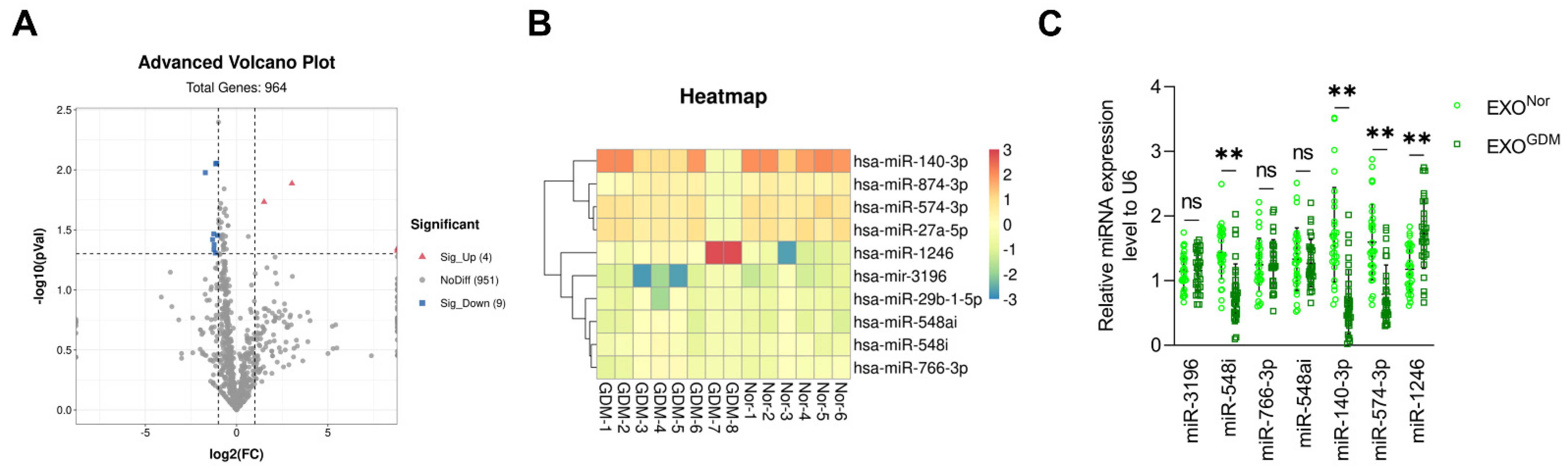
3.1. Expression Levels of miR-140-3p and miR-574-3p Are Significantly Reduced in Placenta-Derived Exosomes from GDM
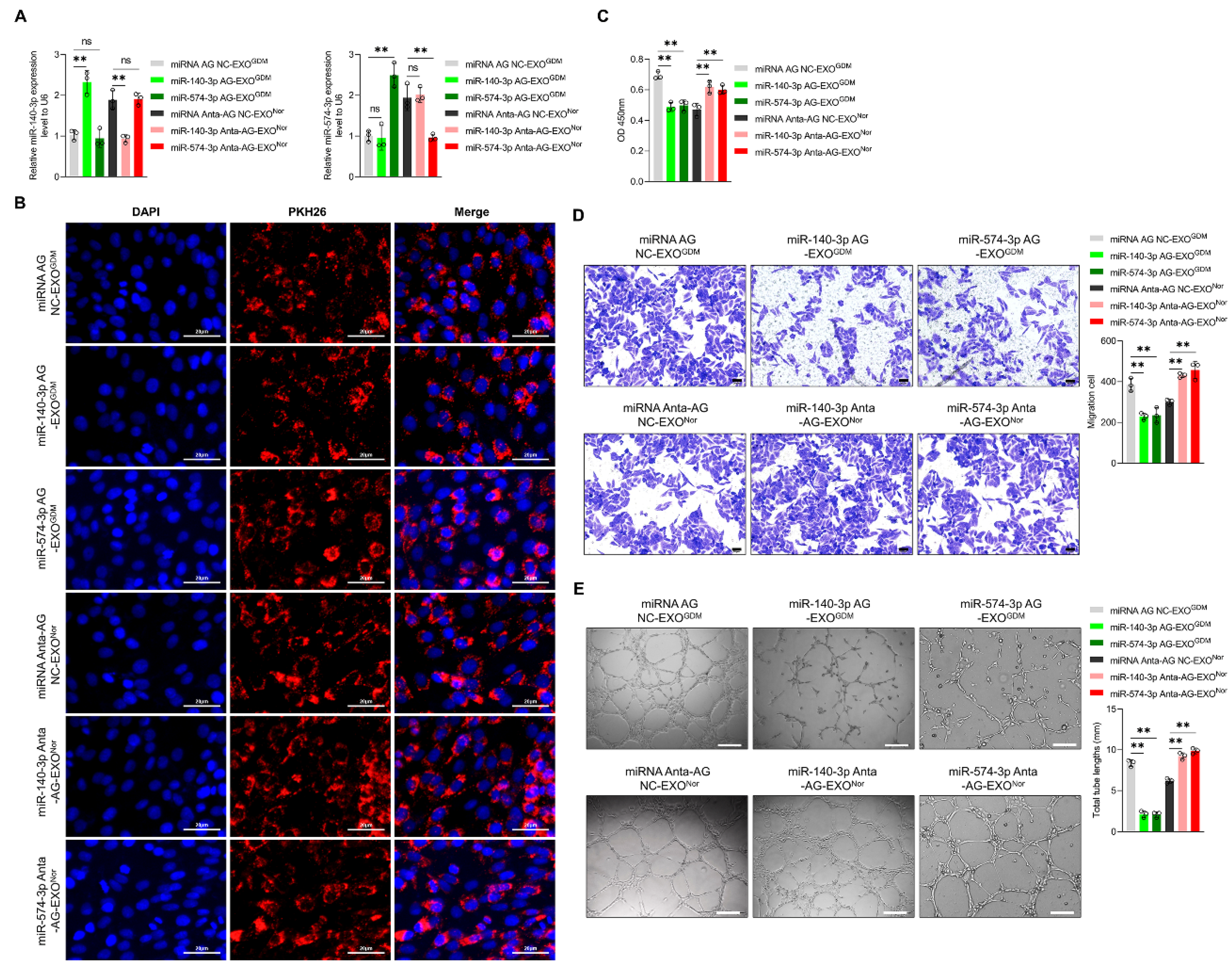
3.2. Placenta-Derived Exosomes from GDM Altered Endothelial Cell Function Depending on miR-140-3p and miR-574-3p In Vitro
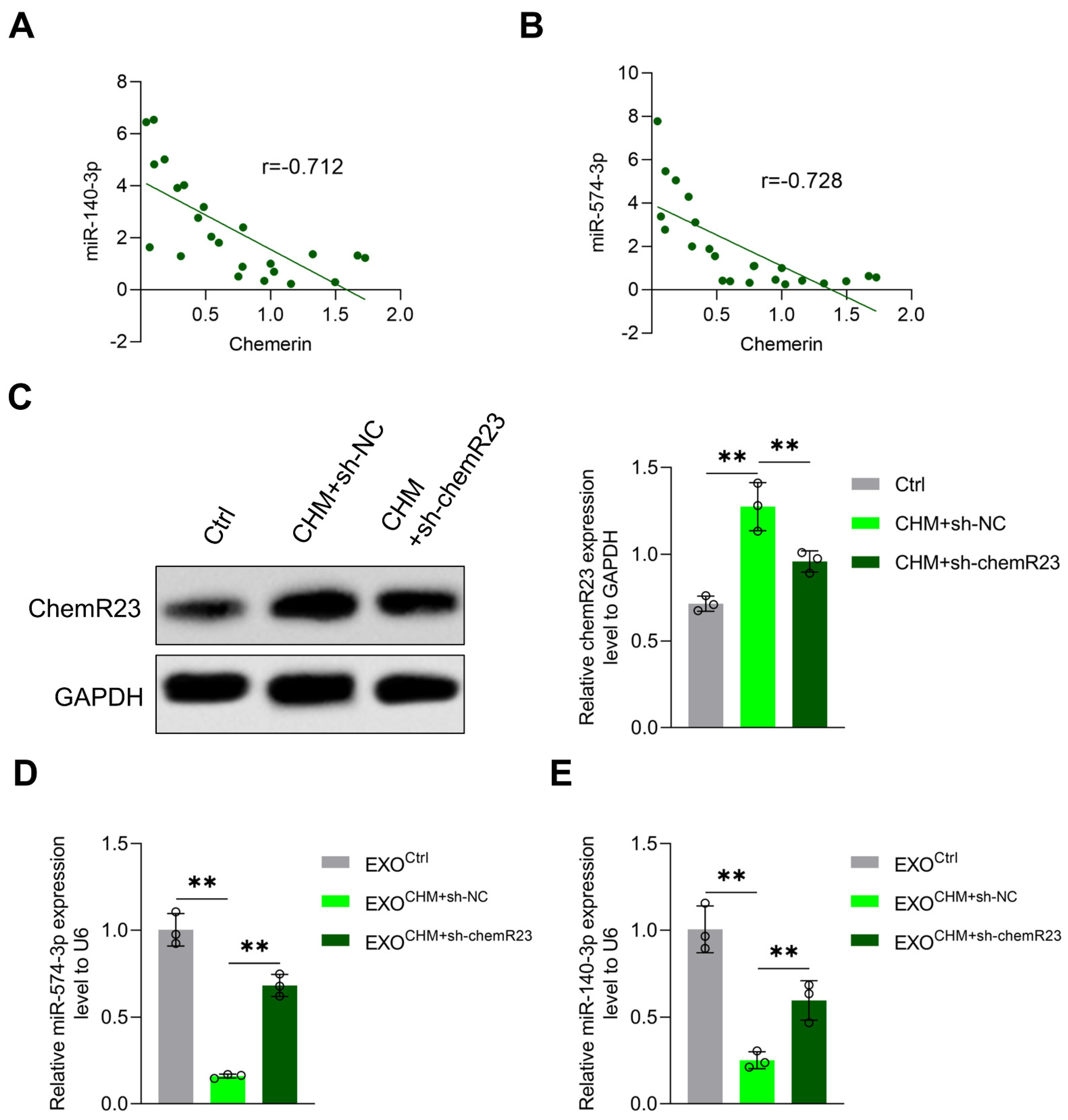
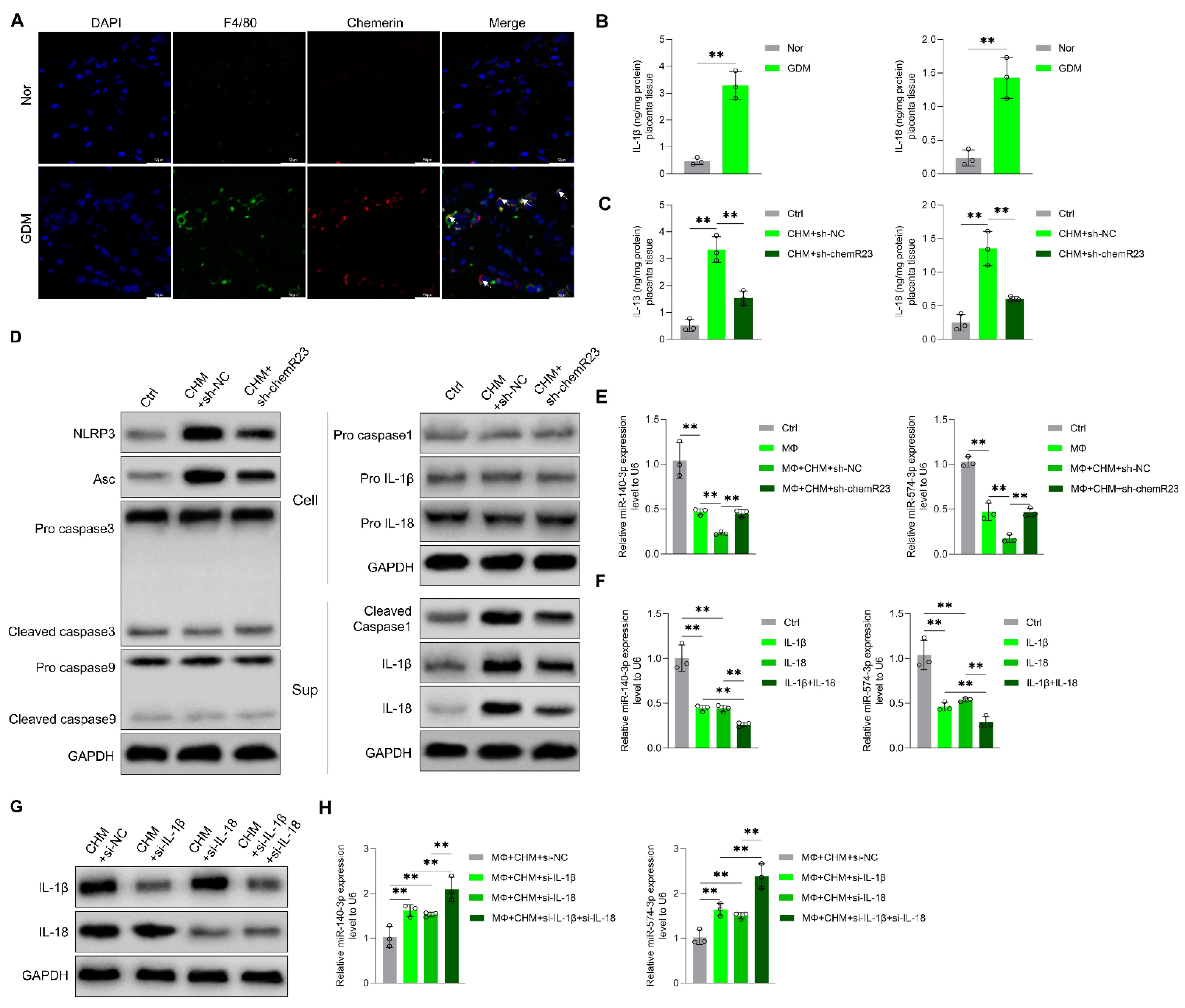
3.3. Chemerin Reduces Placenta-Derived Exosomal miR-140-3p and miR-574-3p Expression by Inducing Placental Inflammation
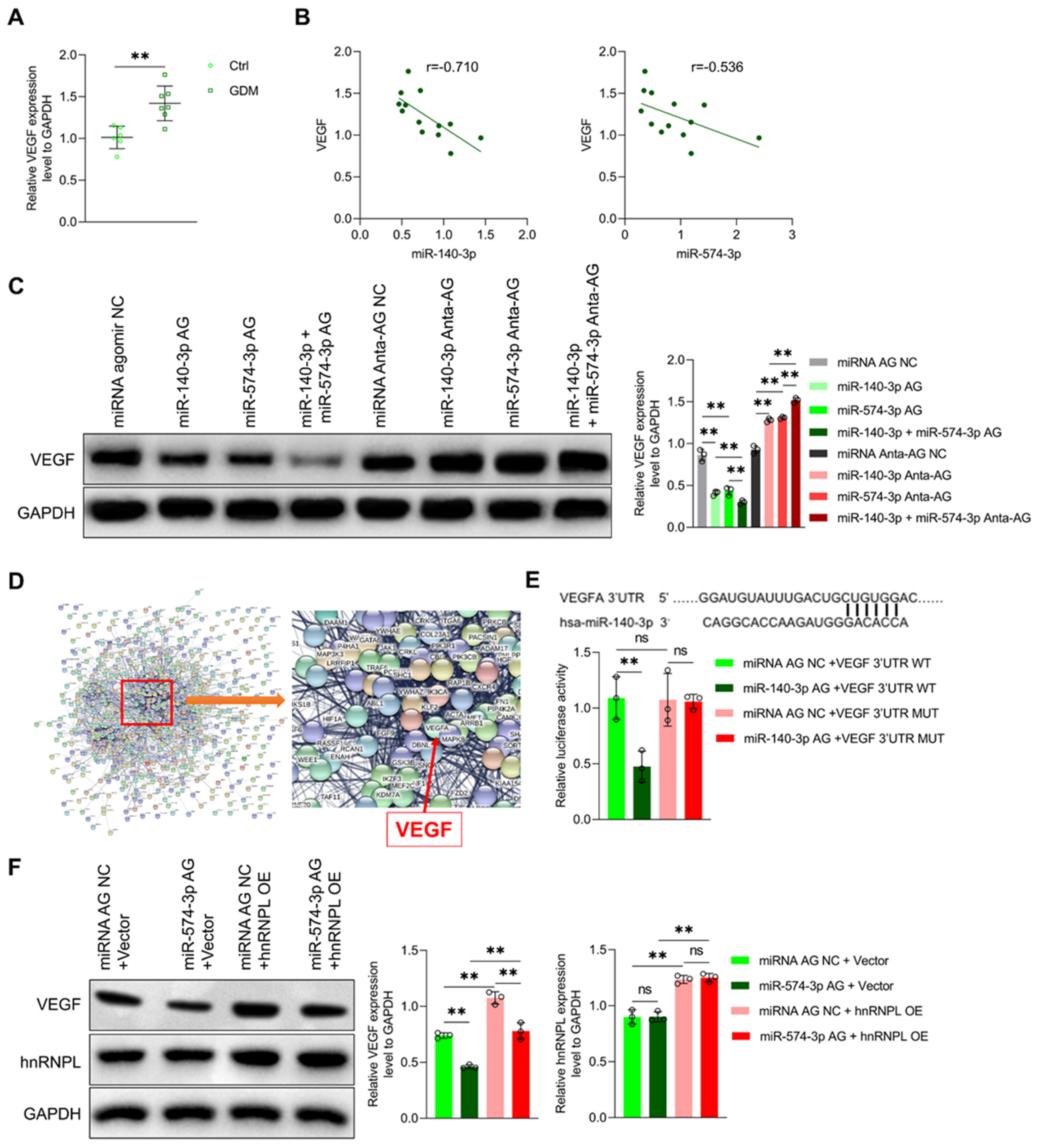
3.4. MiR-140-3p and miR-574-3p Down-Regulate VEGF Expression in Endothelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L., Jr.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.E.; Josefson, J.L. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes. Curr. Diabetes Rep. 2019, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.A.; Liu, W.; Peng, Y.; Roberts, W.; Whitelaw, D.; Graham, A.M. The Role of Maternal Gestational Diabetes in Inducing Fetal Endothelial Dysfunction. J. Cell. Physiol. 2015, 230, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Cvitic, S.; Novakovic, B.; Gordon, L.; Ulz, C.M.; Mühlberger, M.; Diaz-Perez, F.I.; Joo, J.E.; Svendova, V.; Schimek, M.G.; Trajanoski, S.; et al. Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes. Diabetologia 2018, 61, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Knapp, M.; Tu, X.; Wu, R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol. Sin. 2019, 40, 1–8. [Google Scholar] [CrossRef]
- Mrizak, I.; Grissa, O.; Henault, B.; Fekih, M.; Bouslema, A.; Boumaiza, I.; Zaouali, M.; Tabka, Z.; Khan, N.A. Placental infiltration of inflammatory markers in gestational diabetic women. Gen. Physiol. Biophys. 2014, 33, 169–176. [Google Scholar] [CrossRef]
- Pan, X.; Jin, X.; Wang, J.; Hu, Q.; Dai, B. Placenta inflammation is closely associated with gestational diabetes mellitus. Am. J. Transl. Res. 2021, 13, 4068–4079. [Google Scholar]
- Kuzmicki, M.; Telejko, B.; Wawrusiewicz-Kurylonek, N.; Citko, A.; Lipinska, D.; Pliszka, J.; Wilk, J.; Kalejta, K.; Lemancewicz, A.; Grabiec, M.; et al. The expression of suppressor of cytokine signaling 1 and 3 in fat and placental tissue from women with gestational diabetes. Gynecol. Endocrinol. 2012, 28, 841–844. [Google Scholar] [CrossRef]
- Keckstein, S.; Pritz, S.; Amann, N.; Meister, S.; Beyer, S.; Jegen, M.; Kuhn, C.; Hutter, S.; Knabl, J.; Mahner, S.; et al. Sex Specific Expression of Interleukin 7, 8 and 15 in Placentas of Women with Gestational Diabetes. Int. J. Mol. Sci. 2020, 21, 8026. [Google Scholar] [CrossRef] [PubMed]
- Kleiblova, P.; Dostalova, I.; Bartlova, M.; Lacinova, Z.; Ticha, I.; Krejci, V.; Springer, D.; Kleibl, Z.; Haluzik, M. Expression of adipokines and estrogen receptors in adipose tissue and placenta of patients with gestational diabetes mellitus. Mol. Cell. Endocrinol. 2010, 314, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Subiabre, M.; Villalobos-Labra, R.; Silva, L.; Fuentes, G.; Toledo, F.; Sobrevia, L. Role of insulin, adenosine, and adipokine receptors in the foetoplacental vascular dysfunction in gestational diabetes mellitus. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165370. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Rehman, R.; Baig, M.; Khan, T.A. New roles of the multidimensional adipokine: Chemerin. Peptides 2014, 62, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rytelewska, E.; Kiezun, M.; Zaobidna, E.; Gudelska, M.; Kisielewska, K.; Dobrzyn, K.; Kaminski, T.; Smolinska, N. Chemerin as a modulator of angiogenesis and apoptosis processes in the corpus luteum of pigs: An in vitro study†. Biol. Reprod. 2021, 105, 1002–1015. [Google Scholar] [CrossRef]
- Tan, L.; Chen, Z.; Sun, F.; Zhou, Z.; Zhang, B.; Wang, B.; Chen, J.; Li, M.; Xiao, T.; Neuman, R.I.; et al. Placental trophoblast-specific overexpression of chemerin induces preeclampsia-like symptoms. Clin. Sci. 2022, 136, 257–272. [Google Scholar] [CrossRef]
- Ji, Z.S.; Jiang, H.; Xie, Y.; Wei, Q.P.; Yin, X.F.; Ye, J.H.; Quan, X.Z.; Lan, Y.L.; Zhao, M.; Tian, X.L.; et al. Chemerin promotes the pathogenesis of preeclampsia by activating CMKLR1/p-Akt/CEBPɑ axis and inducing M1 macrophage polarization. Cell Biol. Toxicol. 2022, 38, 611–628. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, M.; Xu, X.K.; Qu, F.; Chen, D. Is Chemerin associated with gestational diabetes mellitus? An evidence-based clinical research from Chinese women. J. Obstet. Gynaecol. 2018, 38, 482–487. [Google Scholar] [CrossRef]
- Tsiotra, P.C.; Halvatsiotis, P.; Patsouras, K.; Maratou, E.; Salamalekis, G.; Raptis, S.A.; Dimitriadis, G.; Boutati, E. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides 2018, 101, 157–166. [Google Scholar] [CrossRef]
- Liang, Z.; Han, L.; Sun, D.; Chen, Y.; Wu, Q.; Zhang, L.; Zhou, M.; Chen, D. Chemerin-induced macrophages pyroptosis in fetal brain tissue leads to cognitive disorder in offspring of diabetic dams. J. Neuroinflammation 2019, 16, 226. [Google Scholar] [CrossRef]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Sáez, T.; Salsoso, R.; Leiva, A.; Toledo, F.; de Vos, P.; Faas, M.; Sobrevia, L. Human umbilical vein endothelium-derived exosomes play a role in foetoplacental endothelial dysfunction in gestational diabetes mellitus. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2018, 1864, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sáez, T.; de Vos, P.; Kuipers, J.; Sobrevia, L.; Faas, M.M. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta 2018, 66, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Guanzon, D.; Jayabalan, N.; Lai, A.; Scholz-Romero, K.; Kalita de Croft, P.; Ormazabal, V.; Palma, C.; Diaz, E.; McCarthy, E.A.; et al. Extracellular vesicle-associated miRNAs are an adaptive response to gestational diabetes mellitus. J. Transl. Med. 2021, 19, 360. [Google Scholar] [CrossRef]
- Zhao, X.; Su, L.; He, X.; Zhao, B.; Miao, J. Long noncoding RNA CA7-4 promotes autophagy and apoptosis via sponging MIR877-3P and MIR5680 in high glucose-induced vascular endothelial cells. Autophagy 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Shang, F.; Wang, S.C.; Hsu, C.Y.; Miao, Y.; Martin, M.; Yin, Y.; Wu, C.C.; Wang, Y.T.; Wu, G.; Chien, S.; et al. MicroRNA-92a Mediates Endothelial Dysfunction in CKD. J. Am. Soc. Nephrol. 2017, 28, 3251–3261. [Google Scholar] [CrossRef]
- Lip, S.V.; Boekschoten, M.V.; Hooiveld, G.J.; van Pampus, M.G.; Scherjon, S.A.; Plösch, T.; Faas, M.M. Early-onset preeclampsia, plasma microRNAs, and endothelial cell function. Am. J. Obstet. Gynecol. 2020, 222, e491–e497. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bhattarai, J.P.; Park, S.J.; Lee, J.C.; Cho, D.H.; Han, S.K. Enhanced GABA action on the substantia gelatinosa neurons of the medullary dorsal horn in the offspring of streptozotocin-injected mice. J. Diabetes Its Complicat. 2015, 29, 629–636. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Ju, H.; Sun, M. Circulating chemerin levels and gestational diabetes mellitus: A systematic review and meta-analysis. Lipids Health Dis. 2018, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Chen, J.K.; Zhang, Z.T.; Ma, X.J.; Chen, Y.C.; Du., X.M. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrow-derived macrophages. Cell Death Dis. 2017, 8, e2579. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Wu, J.; Lindner, D.; Fox, P.L. Interplay between miR-574-3p and hnRNP L regulates VEGFA mRNA translation and tumorigenesis. Nucleic Acids Res. 2017, 45, 7950–7964. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Dawson, D.; Roberts, D.; Bentley-Lewis, R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta 2015, 36, 101–114. [Google Scholar] [CrossRef]
- Sáez, T.; de Vos, P.; Sobrevia, L.; Faas, M.M. Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus? Placenta 2018, 61, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.; Donald, A.; Ferri, C.; Giannattasio, C.; Halcox, J.; Halligan, S.; Lerman, A.; Mancia, G.; Oliver, J.J.; Pessina, A.C.; et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sáez, T.; Toledo, F.; Sobrevia, L. Impaired signalling pathways mediated by extracellular vesicles in diabesity. Mol. Asp. Med. 2019, 66, 13–20. [Google Scholar] [CrossRef]
- Silva, L.; Subiabre, M.; Araos, J.; Sáez, T.; Salsoso, R.; Pardo, F.; Leiva, A.; San Martín, R.; Toledo, F.; Sobrevia, L. Insulin/adenosine axis linked signalling. Mol. Asp. Med. 2017, 55, 45–61. [Google Scholar] [CrossRef]
- Guarino, E.; Delli Poggi, C.; Grieco, G.E.; Cenci, V.; Ceccarelli, E.; Crisci, I.; Sebastiani, G.; Dotta, F. Circulating MicroRNAs as Biomarkers of Gestational Diabetes Mellitus: Updates and Perspectives. Int. J. Endocrinol. 2018, 2018, 6380463. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Li, Q.; Fei, X.; Ma, H.; Hu, R. miR-140-3p functions as a tumor suppressor in squamous cell lung cancer by regulating BRD9. Cancer Lett. 2019, 446, 81–89. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, X.; Wang, K. miR-140-3p inhibits bladder cancer cell proliferation and invasion by targeting FOXQ1. Aging 2020, 12, 20366–20379. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Ishikawa, A.; Shoji, Y.; Imai, T. A genotoxic stress-responsive miRNA, miR-574-3p, delays cell growth by suppressing the enhancer of rudimentary homolog gene in vitro. Int. J. Mol. Sci. 2014, 15, 2971–2990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, J.; Zheng, Y.; Zhang, H.; Sun, H.; Gao, S. miRNA-574-3p inhibits metastasis and chemoresistance of epithelial ovarian cancer (EOC) by negatively regulating epidermal growth factor receptor (EGFR). Am. J. Transl. Res. 2019, 11, 4151–4165. [Google Scholar] [PubMed]
- Liang, S.; Ren, K.; Li, B.; Li, F.; Liang, Z.; Hu, J.; Xu, B.; Zhang, A. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1α/VEGF signal pathway. Mol. Cell. Biochem. 2020, 465, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qin, T.; Li, J.; Wang, L.; Zhang, Q.; Jiang, Z.; Mao, J. MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2017, 24, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, Z.; Chen, Y.; Mai, Z.; Jiang, M.; Di, Q.; Sun, B. MicroRNA-140-3p represses the proliferation, migration, invasion and angiogenesis of lung adenocarcinoma cells via targeting TYMS (thymidylate synthetase). Bioengineered 2021, 12, 11959–11977. [Google Scholar] [CrossRef]
- He, J.; Xue, Y.; Wang, Q.; Zhou, X.; Liu, L.; Zhang, T.; Shang, C.; Ma, J.; Ma, T. Long non-coding RNA MIAT regulates blood tumor barrier permeability by functioning as a competing endogenous RNA. Cell Death Dis. 2020, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Li, M.; Zhao, R. Chemerin levels and its genetic variants are associated with Gestational diabetes mellitus: A hospital-based study in a Chinese cohort. Gene 2022, 807, 145888. [Google Scholar] [CrossRef]
- Saavedra, P.H.; Demon, D.; Van Gorp, H.; Lamkanfi, M. Protective and detrimental roles of inflammasomes in disease. Semin. Immunopathol. 2015, 37, 313–322. [Google Scholar] [CrossRef]
- Huang, X.; Fu, C.; Liu, W.; Liang, Y.; Li, P.; Liu, Z.; Sheng, Q.; Liu, P. Chemerin-induced angiogenesis and adipogenesis in 3 T3-L1 preadipocytes is mediated by lncRNA Meg3 through regulating Dickkopf-3 by sponging miR-217. Toxicol. Appl. Pharmacol. 2019, 385, 114815. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Miyaki, S.; Ishitobi, H.; Nakamura, Y.; Nakasa, T.; Lotz, M.K.; Ochi, M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014, 16, R163. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wu, Q.; Zhu, S.; Tang, Y.; Chen, Y.; Chen, D.; Liang, Z. Chemerin-Induced Down-Regulation of Placenta-Derived Exosomal miR-140-3p and miR-574-3p Promotes Umbilical Vein Endothelial Cells Proliferation, Migration, and Tube Formation in Gestational Diabetes Mellitus. Cells 2022, 11, 3457. https://doi.org/10.3390/cells11213457
Zhang L, Wu Q, Zhu S, Tang Y, Chen Y, Chen D, Liang Z. Chemerin-Induced Down-Regulation of Placenta-Derived Exosomal miR-140-3p and miR-574-3p Promotes Umbilical Vein Endothelial Cells Proliferation, Migration, and Tube Formation in Gestational Diabetes Mellitus. Cells. 2022; 11(21):3457. https://doi.org/10.3390/cells11213457
Chicago/Turabian StyleZhang, Lixia, Qi Wu, Shuqi Zhu, Yibo Tang, Yanmin Chen, Danqing Chen, and Zhaoxia Liang. 2022. "Chemerin-Induced Down-Regulation of Placenta-Derived Exosomal miR-140-3p and miR-574-3p Promotes Umbilical Vein Endothelial Cells Proliferation, Migration, and Tube Formation in Gestational Diabetes Mellitus" Cells 11, no. 21: 3457. https://doi.org/10.3390/cells11213457
APA StyleZhang, L., Wu, Q., Zhu, S., Tang, Y., Chen, Y., Chen, D., & Liang, Z. (2022). Chemerin-Induced Down-Regulation of Placenta-Derived Exosomal miR-140-3p and miR-574-3p Promotes Umbilical Vein Endothelial Cells Proliferation, Migration, and Tube Formation in Gestational Diabetes Mellitus. Cells, 11(21), 3457. https://doi.org/10.3390/cells11213457






