Sustainable Agricultural Systems for Fruit Orchards: The Influence of Plant Growth Promoting Bacteria on the Soil Biodiversity and Nutrient Management
Abstract
:1. Introduction
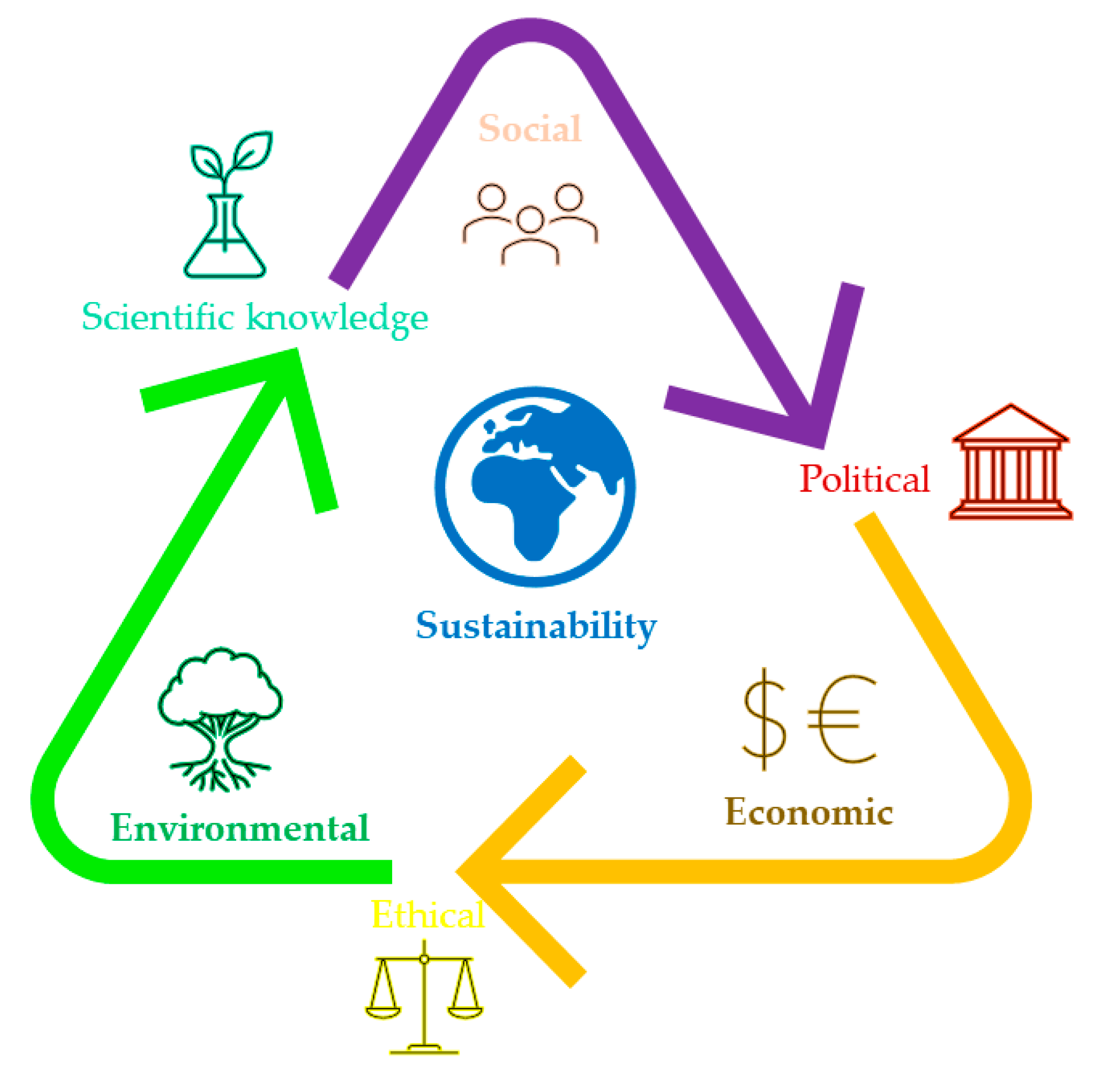
2. Sustainability Concept
3. Sustainable Agriculture Systems
3.1. Sustainable Practices
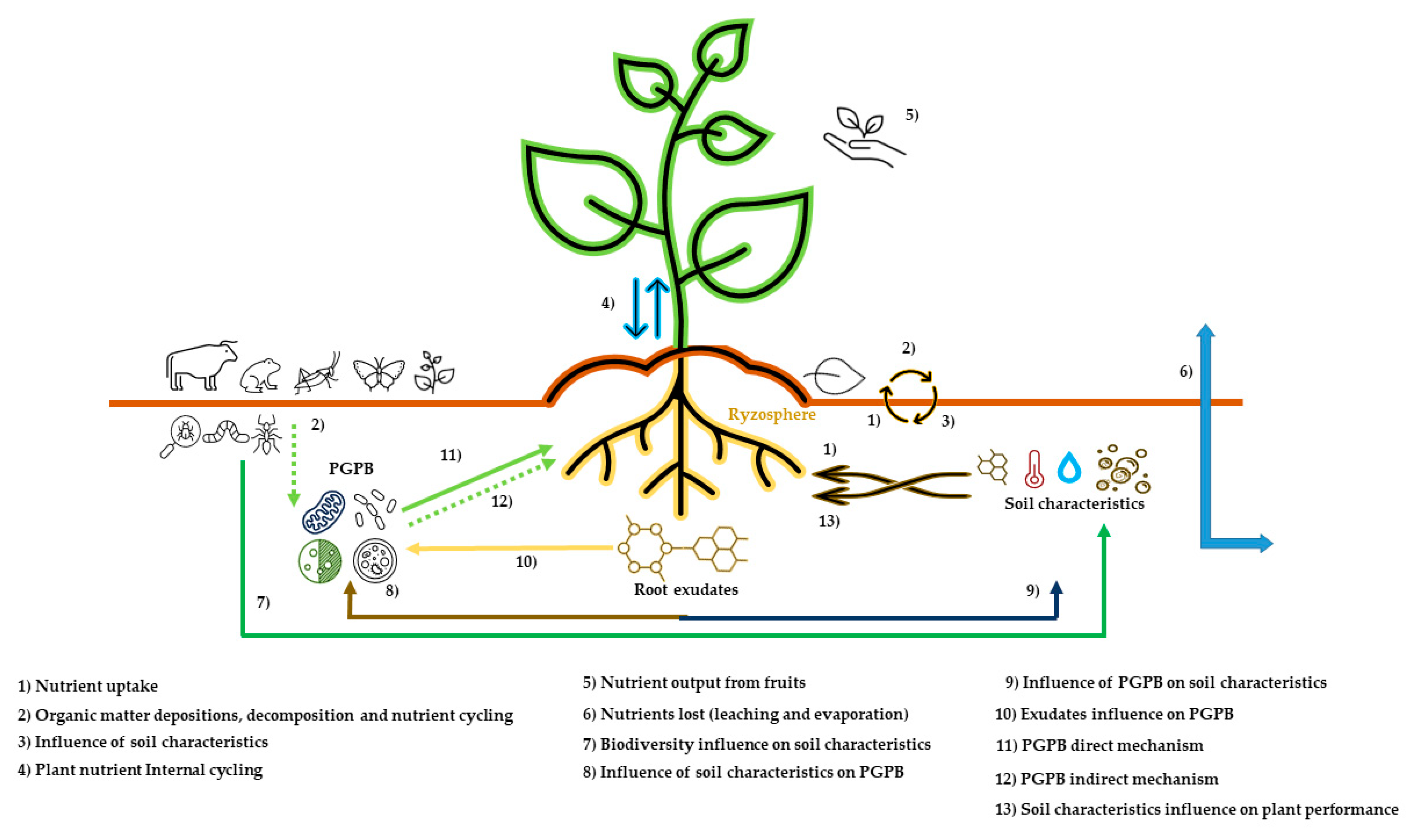
3.2. Nutritional Management
3.3. Plant Growth Promoting Bacteria (PGPB)
4. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aeron, A.; Khare, E.; Jha, C.K.; Meena, V.S.; Aziz, S.M.A.; Islam, M.T.; Kim, K.; Meena, S.K.; Pattanayak, A.; Rajashekara, H.; et al. Revisiting the plant growth-promoting rhizobacteria: Lessons from the past and objectives for the future. Arch. Microbiol. 2020, 202, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; Enshasy, H. El Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Geisen, S.; Hartmann, M.; Tebbe, C.C. The European Journal of Soil Biology: A catalyst for soil biodiversity research. Eur. J. Soil Biol. 2021, 102, 103262. [Google Scholar] [CrossRef]
- FAO; ITPS; GSBI; CBD; EC. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities; FAO: Rome, Italy, 2020. [Google Scholar]
- Herencia, J.F.; Pérez-Romero, L.F.; Daza, A.; Arroyo, F.T. Chemical and biological indicators of soil quality in organic and conventional Japanese plum orchards. Biol. Agric. Hortic. 2021, 37, 71–90. [Google Scholar] [CrossRef]
- Dobbs, M.; Gravey, V.; Petetin, L. Driving the european green deal in turbulent times. Polit. Gov. 2021, 9, 316–326. [Google Scholar] [CrossRef]
- Prandecki, K.; Wrzaszcz, W.; Zieliński, M. Environmental and climate challenges to agriculture in poland in the context of objectives adopted in the european green deal strategy. Sustainability 2021, 13, 10318. [Google Scholar] [CrossRef]
- McNeill, D. The Contested Discourse of Sustainable Agriculture. Glob. Policy 2019, 10, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Trigo, A.; Marta-Costa, A.; Fragoso, R. Principles of sustainable agriculture: Defining standardized reference points. Sustainability 2021, 13, 4086. [Google Scholar] [CrossRef]
- Dilnashin, H.; Birla, H.; Hoat, T.X.; Singh, H.B.; Singh, S.P.; Keswani, C. Applications of agriculturally important microorganisms for sustainable crop production. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; INC: New York, NY, USA, 2020; pp. 403–415. ISBN 9780128184691. [Google Scholar]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.K.; Kumar, A. Plant Growth Promoting Rhizobacteria; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128158791. [Google Scholar]
- Demestihas, C.; Plénet, D.; Génard, M.; Raynal, C.; Lescourret, F. Ecosystem services in orchards. A review. Agron. Sustain. Dev. 2017, 37, 12. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental Impact of Different Agricultural Management Practices: Conventional vs. Organic Agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Norris, C.; Congreves, K.A. Alternative Management Practices Improve Soil Health Indices in Intensive Vegetable Cropping Systems: A Review. Front. Environ. Sci. 2018, 6, 50. [Google Scholar] [CrossRef]
- Rosa-Schleich, J.; Loos, J.; Mußhoff, O.; Tscharntke, T. Ecological-economic trade-offs of Diversified Farming Systems—A review. Ecol. Econ. 2019, 160, 251–263. [Google Scholar] [CrossRef]
- Fess, T.L.; Benedito, V.A. Organic versus conventional cropping sustainability: A comparative system analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Yadav, S.K.; Babu, S.; Yadav, M.K.; Singh, K.; Yadav, G.S.; Pal, S. A Review of Organic Farming for Sustainable Agriculture in Northern India. Int. J. Agron. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Seufert, V.; Ramankutty, N. Many shades of gray—The context-dependent performance of organic agriculture. Sci. Adv. 2017, 3, e1602638. [Google Scholar] [CrossRef] [Green Version]
- Röös, E.; Mie, A.; Wivstad, M.; Salomon, E.; Johansson, B.; Gunnarsson, S.; Wallenbeck, A.; Hoffmann, R.; Nilsson, U.; Sundberg, C.; et al. Risks and opportunities of increasing yields in organic farming. A review. Agron. Sustain. Dev. 2018, 38, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Bruins, R.J.F.; Heberling, M.T. Factors influencing farmers’ adoption of best management practices: A review and synthesis. Sustainability 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.M.; Iqbal, U.; Mir, S.A. Proudction Technology of Stone Fruits; Springer Nature Singapore Pte Ltd.: Singapore, 2021; ISBN 9789811589195. [Google Scholar]
- Bustamante, M.; Muñoz, A.; Romero, I.; Osorio, P.; Mánquez, S.; Arriola, R.; Reyes-Díaz, M.; Ribera-Fonseca, A. Impact of potassium pre-harvest applications on fruit quality and condition of sweet cherry (Prunus avium L.) cultivated under plastic covers in southern chile orchards. Plants 2021, 10, 2778. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Celador-Lera, L.; Fradejas-Bayón, M.; Rivas, R. Plant probiotic bacteria enhance the quality of fruit and horticultural crops. AIMS Microbiol. 2017, 3, 483–501. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.K.; Malhotra, S.K. Nutrient use efficiency in perennial fruit crops—A review. J. Plant Nutr. 2017, 40, 1928–1953. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef] [PubMed]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Andrews, E.; Kassama, S.; Smith, E.; Brown, P.; Khalsa, S. A Review of Potassium-Rich Crop Residues Used as Organic Matter Amendments in Tree Crop Agroecosystems. Agriculture 2021, 11, 580. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A. Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization. Plants 2021, 10, 2624. [Google Scholar] [CrossRef]
- Lima, A.P.; Lourenzi, C.R.; Comin, J.J.; Loss, A.; Brunetto, G.; Souza, M.; Ventura, B.S.; Trapp, T.; Ferreira, G.W. Soil phosphorus fractions in an apple orchard with different weed managements. Res. Soc. Dev. 2020, 9, e3449108767. [Google Scholar] [CrossRef]
- Gulbagca, F.; Burhan, H.; Elmusa, F.; Sen, F. Calcium nutrition in fruit crops: Agronomic and physiological implications. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 173–190. ISBN 9780128187326. [Google Scholar]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Hu, C.; Dong, Z.; Zhao, Y.; Jia, W.; Cai, M.; Zhan, T.; Tan, Q.; Li, J. Floral analysis in fruit crops: A potential tool for nutrient constraints diagnosis. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 157–172. ISBN 9780128187326. [Google Scholar]
- Kalcsits, L.; Lotze, E.; Tagliavini, M.; Hannam, K.D.; Mimmo, T.; Neilsen, D.; Neilsen, G.; Atkinson, D.; Biasuz, E.C.; Borruso, L.; et al. Recent achievements and new research opportunities for optimizing macronutrient availability, acquisition, and distribution for perennial fruit crops. Agronomy 2020, 10, 1738. [Google Scholar] [CrossRef]
- Mosa, W.F.A.E.-G.; Paszt, L.S.; Frąc, M.; Trzciński, P. The Role of Biofertilization in Improving Apple Productivity―A Review. Adv. Microbiol. 2015, 05, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Tipathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M.; Dubey, N.K. Role of Macronutrients in Plant Growth and Acclimation: Recent Advances and Future Prospective. In Improvement of Crops in the Era of Climatic Changes; Springer: New York, NY, USA, 2014; Volume 2, pp. 1–368. ISBN 9781461488248. [Google Scholar]
- Shahrokh, V.; Khademi, H.; Faz Cano, A.; Acosta, J.A. Different forms of soil potassium and clay mineralogy as influenced by the lemon tree rhizospheric environment. Int. J. Environ. Sci. Technol. 2019, 16, 3979–3988. [Google Scholar] [CrossRef]
- Jat, R.K.; Kumar, M.; Jat, M.L.; Shivran, J.S. A Review on Use of Micronutrients in Tropical and Subtropical Fruit Crops. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2744–2753. [Google Scholar] [CrossRef]
- Shahane, A.A.; Shivay, Y.S. Agronomic Biofortification of Crops: Current Research Status and Future Needs. Indian J. Fertil. 2022, 18, 164–179. [Google Scholar]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V. Physiological disorders in perennial woody tropical and subtropical fruit crops: A review. Indian J. Agric. Sci. 2016, 86, 703–717. [Google Scholar]
- Zia, M.H.; Ahmad, R.; Khaliq, I.; Ahmad, A.; Irshad, M. Micronutrients status and management in orchards soils: Applied aspects. Soil Environ. 2006, 25, 6–16. [Google Scholar]
- Wang, N.; He, H.; Lacroix, C.; Morris, C.; Liu, Z.; Ma, F. Soil fertility, leaf nutrients and their relationship in kiwifruit orchards of China’s central Shaanxi province. Soil Sci. Plant Nutr. 2019, 65, 369–376. [Google Scholar] [CrossRef]
- Bright, J. Apple and pear nutrition. NSW Dep. Prim. Ind. Primefact 2005, 85, 1–12. [Google Scholar]
- Stiles, W.C.; Hoying, S.; Fargione, M.; Stiles, W.C. Soil Analysis and Interpretation Interpretation. N. Y. Fruit Q. 2004, 12, 1. [Google Scholar]
- Milošević, T.; Milošević, N. Soil fertility: Plant nutrition vis-à-vis fruit yield and quality of stone fruits. In Fruit Crops: Diagnosis and Management of Nutrient Constraints, 1st ed.; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 583–606. [Google Scholar] [CrossRef]
- Chater, J.M.; Merhaut, D.J.; Preece, J.E. Diagnosis and management of nutrient constraints in pomegranate. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 681–691. ISBN 9780128187326. [Google Scholar]
- Lal, R. Soil conservation and ecosystem services. Int. Soil Water Conserv. Res. 2014, 2, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil health and sustainable agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Soil macrofauna: A key factor for increasing soil fertility and promoting sustainable soil use in fruit orchard agrosystems. Agronomy 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Mosqueda, M.; del, C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Singh, I. Plant Growth Promoting Rhizobacteria (PGPR) and their various mechanisms for plant growth enhancement in stressful conditions: A review. Eur. J. Biol. Res. 2018, 8, 191–213. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A.; Stepantsova, L.; Pugachev, G. Soil fertility management in apple orchard with microbial biofertilizers. In Proceedings of the E3S Web of Conferences, Constanta, Romania, 26–27 June 2020; Volume 222. [Google Scholar]
- Aslantaş, R.; Çakmakçi, R.; Şahin, F. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci. Hortic. 2007, 111, 371–377. [Google Scholar] [CrossRef]
- Pirlak, L.; Turan, M.; Sahin, F.; Esitken, A. Floral and foliar application of Plant Growth Promoting Rhizobacteria (PGPR) to apples increases yield, growth, and nutrient element contents of leaves. J. Sustain. Agric. 2007, 30, 145–155. [Google Scholar] [CrossRef]
- Aras, S.; Arıkan, Ş.; Ipek, M.; Eşitken, A.; Pırlak, L.; Dönmez, M.F.; Turan, M. Plant growth promoting rhizobacteria enhanced leaf organic acids, FC-R activity and Fe nutrition of apple under lime soil conditions. Acta Physiol. Plant. 2018, 40, 120. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Qi, M.; Mustafad, N.S.; Ahmed, N.; Anees, M.; Ahanger, M.A.; Zhang, L. Effects of plant growth-promoting rhizobacteria on uptake and utilization of phosphorus and root architecture in apple seedlings under water limited regimes Plant material. Int. J. Appl. Exp. Biol. 2022, 1, 1–8. [Google Scholar] [CrossRef]
- Treder, W.; Klamkowski, K.; Wójcik, K.; Tryngiel-Gać, A.; Sas-Paszt, L.; Mika, A.; Kowalczyk, W. Apple leaf macro- and micronutrient content as affected by soil treatments with fertilizers and microorganisms. Sci. Hortic. 2022, 297, 110975. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, Y.; Li, Z.; Chen, X.; Yin, C.; Mao, Z. Effects of Bacillus amyloliquefaciens QSB-6 on the Growth of Replanted Apple Trees and the Soil Microbial Environment. Horticulturae 2022, 8, 83. [Google Scholar] [CrossRef]
- Vahedi, R.; Rasouli-Sadaghiani, M.H.; Barin, M.; Vetukuri, R.R. Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil. Processes 2022, 10, 343. [Google Scholar] [CrossRef]
- Przybyłko, S.; Kowalczyk, W.; Wrona, D. The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production. Agronomy 2021, 11, 1402. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Wu, Q.S.; Mousavi, S.M.; Hota, D. Integrated Soil Fertility Management in Fruit Crops: An Overview. Int. J. Fruit Sci. 2021, 21, 413–439. [Google Scholar] [CrossRef]
- Essalimi, B.; Esserti, S.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Rifai, S.; Venisse, J.S.; Faize, L.; Alburquerque, N.; et al. Enhancement of plant growth, acclimatization, salt stress tolerance and verticillium wilt disease resistance using plant growth-promoting rhizobacteria (PGPR) associated with plum trees (Prunus domestica). Sci. Hortic. 2022, 291, 110621. [Google Scholar] [CrossRef]
- Karakurt, H.; Kotan, R.; Aslantas, R.; Dadasoglu, F.; Karagöz, K. Inoculation effects of pantoea agglomerans strains on growth and chemical composition of plum. J. Plant Nutr. 2010, 33, 1998–2009. [Google Scholar] [CrossRef]
- Bonaterra, A.; Ruz, L.; Badosa, E.; Pinochet, J.; Montesinos, E. Growth promotion of Prunus rootstocks by root treatment with specific bacterial strains. Plant Soil 2003, 255, 555–569. [Google Scholar] [CrossRef]
- Gani, G.; Asif, M.; Wani, P.A.; Malik, M.A.; Dar, Z.M.; Masood, A.; Shafi, S. Chlorpyrifos degradation, biocontrol potential and antioxidant defence activation under pesticide stress by rhizosphere bacteria isolated from rhizosphere of peach (Prunus persica) plants. Chem. Ecol. 2021, 37, 866–881. [Google Scholar] [CrossRef]
- Arıkan, Ş.; Eşitken, A.; İpek, M.; Aras, S.; Şahin, M.; Pırlak, L.; Dönmez, M.F.; Turan, M. Effect of Plant Growth Promoting Rhizobacteria on Fe Acquisition in Peach (Prunus persica L.) Under Calcareous Soil Conditions. J. Plant Nutr. 2018, 41, 2141–2150. [Google Scholar] [CrossRef]
- Gharbi-Hajji, H.; Sanaa, M. Enhancement of Nutrient Uptake in Peach Rootstock with Arbuscular Mycorrhizal Fungi and Plant-Growth Promoting Rhizo-Bacteria Inoculation in Nursery. In Proceedings of the Fifth International Scientific Agricultural Symposium “Agrosym 2014”, Jahorina, Bosnia and Herzegovina, 23–26October 2014; University of East Sarajevo, Faculty of Agriculture: Lukavica, Bosnia and Herzegovina, 2014. [Google Scholar]
- Chang, H.; Yang, H.; Han, T.; Wang, F.; Liu, Y. Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach. Open Life Sci. 2020, 15, 890–901. [Google Scholar] [CrossRef]
- Ipek, M.; Arıkan, Ş.; Eşitken, A.; Pırlak, L.; Dönmez, M.F.; Turan, M. Influence of Bacterial Inoculation on Growth and Plant Nutrition of Peach Grafted in Different Rootstocks in Calcareous Soil. Sains Malays. 2021, 50, 2615–2624. [Google Scholar] [CrossRef]
- Gallart, M.; Paungfoo-Lonhienne, C.; Trueman, S.J. Effects of a growth-promoting Paraburkholderia species on nitrogen acquisition by avocado seedlings. Sci. Hortic. 2022, 295, 110767. [Google Scholar] [CrossRef]
- Tzec-Interián, J.A.; Desgarennes, D.; Carrión, G.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Ferrera-Rodríguez, O.; Santos-Rodríguez, D.L.; Liahut-Guin, N.; Caballero-Reyes, G.E.; Ortiz-Castro, R. Characterization of plant growth-promoting bacteria associated with avocado trees (Persea americana Miller) and their potential use in the biocontrol of Scirtothrips perseae (avocado thrips). PLoS ONE 2020, 15, e0231215. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Luo, J.; Ahmed, T.; Zhang, J.; Xie, T.; Dai, D.; Jiang, J.; Zhu, J.; Hassan, S.; Alorabi, J.A.; et al. Pseudomonas bijieensis Strain XL17 within the P. corrugata Subgroup Producing 2,4-Diacetylphloroglucinol and Lipopeptides Controls Bacterial Canker and Gray Mold Pathogens of Kiwifruit. Microorganisms 2022, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; He, X.; Liu, Y.; Chen, Y.; Tang, J.; Guo, T. A complex inoculant of N2-fixing, P- and K-solubilizing bacteria from a purple soil improves the growth of kiwifruit (Actinidia chinensis) plantlets. Front. Microbiol. 2016, 7, 841. [Google Scholar] [CrossRef] [PubMed]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercisli, S.; Esitken, A.; Cangi, R.; Şahin, F. Adventitious root formation of kiwifruit in relation to sampling date, IBA and Agrobacterium rubi inoculation. Plant Growth Regul. 2003, 41, 133–137. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, X.; Li, Y.; Ji, L.; Wu, G.; Li, B.; Cheng, L.; Long, M.; Deng, W.; Zou, L. The influence of effective microorganisms on microbes and nutrients in kiwifruit planting soil. Appl. Sci. 2016, 6, 168. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, W.; Ren, Y.; Huang, S.; Liu, Y. Azospirillum Actinidiae sp. nov., a Nitrogen-Fixing Bacterium Isolated from The Roots of Kiwifruit Plants. Res. Sq. 2021, 1–18. [Google Scholar] [CrossRef]
- Giassi, V.; Kiritani, C.; Kupper, K.C. Bacteria as growth-promoting agents for citrus rootstocks. Microbiol. Res. 2016, 190, 46–54. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Thokchom, E.; Kalita, M.C.; Talukdar, N.C. Isolation, screening, characterization, and selection of superior rhizobacterial strains as bioinoculants for seedling emergence and growth promotion of Mandarin orange (Citrus reticulata Blanco). Can. J. Microbiol. 2014, 60, 85–92. [Google Scholar] [CrossRef]
- Andra, C.B.; Carlos, I.A.V.; Aline, A.C.N.; Welington, L.A. Effects of growth-promoting endophytic Methylobacterium on development of Citrus rootstocks. Afr. J. Microbiol. Res. 2016, 10, 646–653. [Google Scholar] [CrossRef] [Green Version]
- De Queiroz, B.P.V.; De Melo, I.S. Antagonism of Serratia marcescens towards Phytophthora parasitica and its effects in promoting the growth of citrus. Braz. J. Microbiol. 2006, 37, 448–450. [Google Scholar] [CrossRef] [Green Version]
- Sudyoung, N.; Tokuyama, S.; Krajangsang, S.; Pringsulaka, O.; Sarawaneeyaruk, S. Bacterial antagonists and their cell-free cultures efficiently suppress canker disease in citrus lime. J. Plant Dis. Prot. 2020, 127, 173–181. [Google Scholar] [CrossRef]
- Riera, N.; Handique, U.; Zhang, Y.; Dewdney, M.M.; Wang, N. Characterization of antimicrobial-producing beneficial bacteria isolated from Huanglongbing escape citrus trees. Front. Microbiol. 2017, 8, 2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginnan, N.A.; Dang, T.; Bodaghi, S.; Ruegger, P.M.; McCollum, G.; England, G.; Vidalakis, G.; Borneman, J.; Rolshausen, P.E.; Caroline Roper, M. Disease-induced microbial shifts in citrus indicate microbiome-derived responses to huanglongbing across the disease severity spectrum. Phytobiomes J. 2020, 4, 375–387. [Google Scholar] [CrossRef]
- Keswani, C.; Prakash, O.; Bharti, N.; Vílchez, J.I.; Sansinenea, E.; Lally, R.D.; Borriss, R.; Singh, S.P.; Gupta, V.K.; Fraceto, L.F.; et al. Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total Environ. 2019, 690, 841–852. [Google Scholar] [CrossRef]

| Description | Examples of Expected Impact | Reference | |
|---|---|---|---|
| Management Practices | [15,16,17] | ||
| Cover crops and green manure | Provide constant soil cover. | Increase SOM; nutrient mobilization; decrease emergence of pests and pathogens. | |
| Crop rotation | Sequence of different crops cultivated on the same land, with different temporal frames. | Use of different soil nutrient combinations to avoid over-exploiting the soil ecosystem. | |
| Reduced tillage | Reduce mechanical disturbance of the soil. | Avoid soil parameters degradation (e.g., carbon sequestration, soil density, water holding capacity). | |
| Intercropping | Cultivating different crops species on the same field simultaneously. | Improve nitrogen fixation and other nutrient cycles; obtain soil cover; decrease emergence of pests and pathogens. | |
| Structural elements | Features in the landscape and techniques implemented or managed by the farmer. | Decrease impact of weather; improve light accessibility. | |
| Irrigation management | Control the amount of water supplied. | Maintain optimal humidity levels; avoid nutrients leaching; decrease water loss. | |
| Allowed External Inputs | |||
| Organic fertilizer | Application of animal manure. | Supplement the Orchard with the required nutrients; improve soil quality. | |
| Compost | Aerobically decomposed organic matter | ||
| Vermicompost | Organic material decomposed by earthworms. | ||
| Biofertilizers | Growth-promoting bacteria or fungus. | ||
| Fertigation | Water fortified with nutrients and controlled administration. | ||
| Sustainable Agriculture Systems | |||
| Conservation Agriculture | Combination of three principals
| ||
| Crop-Livestock systems | Integration of crops with live stocks. | ||
| Organic Agriculture | Absence of agrochemical inputs, relying on ecological processes and biodiversity. | ||
| Agroforestry | Integrations of trees and crops in the same land. Integration of woody and herbaceous layers. | ||
| Nutrients | Uptake Form | Soil Conditions | Biological Functions | Plant Impact/Deficiency | Stratification | References |
|---|---|---|---|---|---|---|
| Nitrogen (N) | NH4+ | Low pH and reducing soil conditions. | Contribute to amino acid formation; energy homeostasis, signaling and protein regulation. Essential for co-enzymes, photosynthetic pigments, secondary metabolites and polyamines. | Stunted growth, small leaves, reduced shoot branching and early flowering. Often anthocyanosis on leaf and stem. | acropetal | [31,33,34,36,37,38,39,40] |
| NO3− | Higher pH and aerobic conditions. | |||||
| Phosphorous (P) | H2PO4− | Available form is pH dependent. Natural availability is very slow. Uptake is improved by the presence of mycorrhizal symbioses. | Cellular energy homoeostasis; component of nucleic acids; structural role in cellular membranes; reversible protein phosphorylation; cellular metabolism. | P deficiency causes a rapid decrease in photosynthetic rates. Anthocyanosis. Dark-green and/or purple leaves. | Acropetal | |
| Potassium (K) | Other forms: solution K, exchangeable K, “fixed” K, structural K in primary minerals | K+ is dehydrated and coordinated with oxygen atoms not available to plants. K solubilization is driven by water. | Metabolic reactions and enzyme activity. Ribosome mediated protein synthesis. Accumulation of reducing sugars and depletion of organic acids; turgor provision and water homeostasis. K demand is strongest during fruit development. | Accelerate premature leaf senescence and reduce numbers of flowers and fruits in subsequent years. Chlorosis on tip of oldest leaves that develop into marginal necrosis. Bronzing. Slack appearance due to poor turgor and stomatal control. | Acropetal | |
| Calcium (Ca) | Ca2+ | Ca2+ adsorbed to colloids can be exchanged with the soil solution where much of the ‘free’ Ca2+ forms nearly insoluble compounds with other elements such as phosphorus, thus making P less available. | Structural and secondary messenger. Rigidity to cell walls and membrane structure. | Ca2+ levels may fall below a critical level in fast-growing tissues causing diseases such as ‘black heart’ in celery, ‘blossom end rot’ in tomatoes or ‘bitter-pit’ in apples. Disintegration of root tissue. Necrotic lesions on leaf edges and tips. Meristem death. Necrotic spots on fruits and vegetables. Leaf deformity. | Basipetal | |
| Magnesium (Mg) | Mg2+ | Adsorption to soil particles is relatively weak which results in high leaching rates and Mg2+ deficiency is therefore common. | Central position in the chlorophyll molecule; signal element in chloroplast development; roles as enzyme cofactors associated with energy transfer. | Intravenous chlorosis on oldest leaves that eventually develop into necrosis. Accumulation of sucrose and starch in chloroplast. | Acropetal | |
| Sulfur (S) | In saline and sodic soils, inorganic salts are predominant. | Amino acids; protein activity reductant in the detoxification of reactive oxygen species.o | Chlorosis of young leaves. Stunted growth. Anthocyanosis; S toxicity is rare but can occur in saline soils with high levels of SO42− salts, and atmospheric pollution. | Basipetal | ||
| SO42− | In aerobic conditions. | |||||
| FeS, FeS2 and H2S | Reducing environment created by flooding. | |||||
| SO42 and H2S. | Extracted from atmosphere. |
| Nutrient | Soil Availability | Biological Function | Deficiency | Stratification | Reference |
|---|---|---|---|---|---|
| Iron (Fe) | Maximum availability in acidic pH range and decreases drastically with increase in pH. Excessive water and poor aeration, organic matter, interaction with other nutrients effect control Fe availability. | Synthesis of chlorophyll | Leaves exhibit pale color and veins remains green or interveinal chlorosis of the whole leaves. Papery white color of the leaves occurs under severe deficiency. | Basipetal | [41,42] |
| Manganese (Mn) | Soluble Mn (Mn2+) is rapidly converted to plant-unavailable Mn oxides, particularly in sandy alkaline soils. Disorder in soils with high pH and high partial pressure of O2. | Enzyme activity. Oxidation-reduction processes. Synthesis of chlorophyll, | Similar to Fe deficiency, with pale leaves and green veins. Sometimes brown, black or grey spots are observed next to leaf veins. Chlorosis up to leaf margins followed by browning and necrosis. | Acropetal | [41,43] |
| Zinc (Zn) | Low content in the rocks/minerals, soil pH, presence of calcium carbonate, soil redox potential, clay content, soil moisture status. Positive interaction with nitrogen (N) and potassium (K). Negative interactions with phosphorus (P), calcium (Ca), iron (Fe), and copper (Cu). | Regulation of plant growth and transformation of carbohydrates. Required for nucleic acid synthesis and enzyme activation | Interveinal chlorosis | Basipetal | [41,42] |
| Copper (Cu) | Availability decreases with high pH, high soil organic carbon and high clay content | Enzyme system that utilizes carbohydrates and proteins and is important for reproductive growth. | Dieback of shoot tips; old leaves develop brown spots. Male flower sterility, delay flowering and senescence. | Acropetal | [41,44] |
| Boron (B) | Increasing soil pH decreases B availability by increasing B adsorption onto clay and Al and Fe hydroxyl surfaces, especially at high soil pH | Required for nucleic acid synthesis, pollen germination and the growth of the pollen tube. Promotes root development, enzyme activity, lignin synthesis, sugar transport, seed and cell wall formation, calcium uptake and water relations. Imparts drought tolerance to the crops | Curled, brittle leaves; discolored or cracked fruits. Leaf symptoms found on leaf tips and terminal buds or the youngest leaves, which become discolored and may die under acute deficient conditions. Development of water-soaked areas on the leaves, development of corky tissues and purpling or yellowing of interveinal portion of young leaves. | Basipetal | [41,45,46] |
| Nutrients | Kiwi | Apple | Peach and Plum | Pomegranate | Citrus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deficit | Normal | Excess | Deficit | Normal | Excess | Deficit | Normal | Excess | Deficit | Normal | Excess | Deficit | Normal | Excess | |
| N | - | 23–28 e | - | <1.6 a | 2.0–2.4 a | >3.0 a | 1.7 | 2.4–3.0 | 4.0 | - | >2.0 | - | <2.2 a | 2.5–2.7 a | >3.0 a |
| P | - | 1.6–2.0 e | - | <0.10 a | 0.15–0.20 a | >0.3 a | 0.09 | 0.14–0.25 | 0.4 | - | 0.13–0.15 | - | <0.09 a | 0.12–0.16 a | >0.30 a |
| K | - | 12–19 e | - | <0.8 a | 1.1–1.5 a | >2.0 a | 1.0 | 1.6–3.0 | 4.0 | - | 1.0–1.2 | - | <0.7 a | 1.2–1.7 a | >2.4 a |
| Ca | - | 33–44 e | - | <0.7 a | 1.1–2.0 a | >2.5 a | 1.0 | 1.5–3.0 | 4.0 | - | 4.5–4.9 | - | <1.5 a | 3.0–4.9 a | >7.0 a |
| Mg | - | 4.0–11 e | - | <0.18 a | 0.25–0.35 a | >0.5 a | 0.2 | 0.3–0.8 | 1.10 | - | 0.38–0.42 | - | <0.2 a | 0.30–0.49 a | >0.7 a |
| Cl | - | 6.0–10 e | - | - | <0.4 a | >1.0 a | - | - | - | - | - | - | - | 0.05–0.10 a | >0.25 a |
| Na | - | <500 b | - | - | <0.02 a | >0.5 a | - | - | - | - | - | - | - | - | >0.25 a |
| Mn | - | 44–173 b | - | <20 c | 25–100 c | >200 c | 20 c | 40–160 c | 400 c | - | 30–45 d | - | <17 b | 25–100 b | >300 b |
| Zn | - | 26–44 b | - | <10 c | 16–50 c | >50 c | 15 c | 20–50 c | 70 c | - | 14–15 d | - | <17 b | 25–100 b | >300 b |
| Cu | - | 7.0–22 b | - | <4 c | 6–20 c | >21 c | 4 c | 4–16 c | 30 c | - | 4.5–7.0 d | - | <3 b | 5–16 b | >20 b |
| Fe | - | 90–268 b | - | - | >50 c | - | 60 c | 100–250 c | 500 c | - | 70–85 d | - | <35 b | 60–120 b | >200 b |
| B | - | 39–80 b | - | <15 c | 20–60 c | >200 c | 20 c | 25–60 c | 80 c | - | 20–22 d | - | <20 b | 36–100 b | >200 b |
| Reference | [47] | [48,49] | [22,50] | [26,51] | [35] | ||||||||||
| Fruit Crop | Microorganisms | Parameters Evaluated | Reference |
|---|---|---|---|
| Apple | Azotobacter chroococcum, Bacillus subtilis, Bacillus megaterium | Fruit yield, Nutrient efficiency | [62] |
| Bacillus spp., Burkholderia spp., Pseudomonas spp. | Growth, Fruit yield | [63] | |
| Pseudomonas putid, Bacillus subtilis | Foliar application | [64] | |
| Alcaligenes spp., Agrobacterium spp., Staphylococcus spp., Bacillus spp., Pantoea sp. | Iron acquisition | [65] | |
| Pseudomonas fluorescens | Drought stress, Nutrient uptake, root grow | [66] | |
| Bacillus sp., Bacillus amyloliquefaciens, Paenibacillus polymyxa | Nutrient composition of apple leaves | [67] | |
| Bacillus amyloliquefaciens | Growth | [68] | |
| Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas putida | Soil properties; Nutrient availability | [69] | |
| Bacillus subtilis; Streptomyces spp. | Nutritional status; Growth | [70] | |
| Pomegranate | Azotobacter chroococcum | Plant canopy, Pruned material, Fruit yield | [71] |
| Plum | Pseudomonas stutzeri; Bacillus toyonensis | Growth; Acclimatization; Disease tolerance | [72] |
| Pantoea agglomerans | Fruit traits; Chemical composition | [73] | |
| Pseudomonas fluorescens; Pantoea agglomerans | Rootstock growth | [74] | |
| Peach | Bacillus flexus | Disease tolerance; Growth | [75] |
| Alcaligenes sp., Agrobacterium sp., Staphylococcus sp., Bacillus sp. and Pantoea sp. | Iron acquisition | [76] | |
| Azospirillum sp.; Frateuria aurantia; Bacillus megaterium | Nutrient uptake; Growth | [77] | |
| Bacillussubtilis; Bacillustequilensis; Bacillusmethylotrophicus | Disease tolerance | [78] | |
| Alcaligenes spp., Agrobacterium spp., Staphylococcus spp., Bacillus spp. and Pantoea spp. | Growth and Nutrient content | [79] | |
| Avocado | Paraburkholderia sp. | Growth; Nitrogen acquisition, | [80] |
| Pseudomonas sp., Serratia sp. and Stenotrophomona sp. | Disease tolerance | [81] | |
| Kiwi | Pseudomonas bijieensis | Disease tolerance | [82] |
| Bacillus amyloliquefaciens, Bacillus pumilus, Bacillus circulans, | Growth promotion; Nutrient uptake | [83] | |
| Paenibacillus polymyxa; Comamonas acidovorans, Bacillus sp. | Root growth | [84] | |
| Agrobacterium rubi | Root growth | [85] | |
| Bacillus subtilis, Bacillus stearothermophilus, Bacillu amyloliquefaciens, Actinobacteria sp. | Impact on soil nutrients | [86] | |
| Azospirillum actinidiae | Nitrogen fixation | [87] | |
| Citrus | Bacillus sp.; Lactic acid bacteria; Actinobacteria sp.; | IAA production; Nutrient availability | [88] |
| Pseudomonas putida; Novosphingobium sp. | Salt stress | [89] | |
| Enterobacter hormaechei; Enterobacter asburiae; Enterobacter ludwigii; Klebsiella pneumoniae | Growth performance | [90] | |
| Methylobacterium sp. | Rootstock development | [91] | |
| Serratia marcescen | Disease tolerance; Growth promotion | [92] | |
| Bacillus velezensis, Pseudomonas aeruginosa | Disease tolerance | [93] | |
| Rhodococcus sp., Burkholderia sp. | Disease tolerance; Growth promotion | [94] | |
| Bacillus sp., Lactobacillus sp., Streptomyces sp., Methylobacterium sp., Hymenobacter sp., Pantoea sp., Curtobacterium sp., Spirosoma sp. | Disease tolerance | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, J.; Silva, P. Sustainable Agricultural Systems for Fruit Orchards: The Influence of Plant Growth Promoting Bacteria on the Soil Biodiversity and Nutrient Management. Sustainability 2022, 14, 13952. https://doi.org/10.3390/su142113952
Freitas J, Silva P. Sustainable Agricultural Systems for Fruit Orchards: The Influence of Plant Growth Promoting Bacteria on the Soil Biodiversity and Nutrient Management. Sustainability. 2022; 14(21):13952. https://doi.org/10.3390/su142113952
Chicago/Turabian StyleFreitas, Jorge, and Pedro Silva. 2022. "Sustainable Agricultural Systems for Fruit Orchards: The Influence of Plant Growth Promoting Bacteria on the Soil Biodiversity and Nutrient Management" Sustainability 14, no. 21: 13952. https://doi.org/10.3390/su142113952
APA StyleFreitas, J., & Silva, P. (2022). Sustainable Agricultural Systems for Fruit Orchards: The Influence of Plant Growth Promoting Bacteria on the Soil Biodiversity and Nutrient Management. Sustainability, 14(21), 13952. https://doi.org/10.3390/su142113952






