Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer
Abstract
1. Introduction
2. Materials and Methods
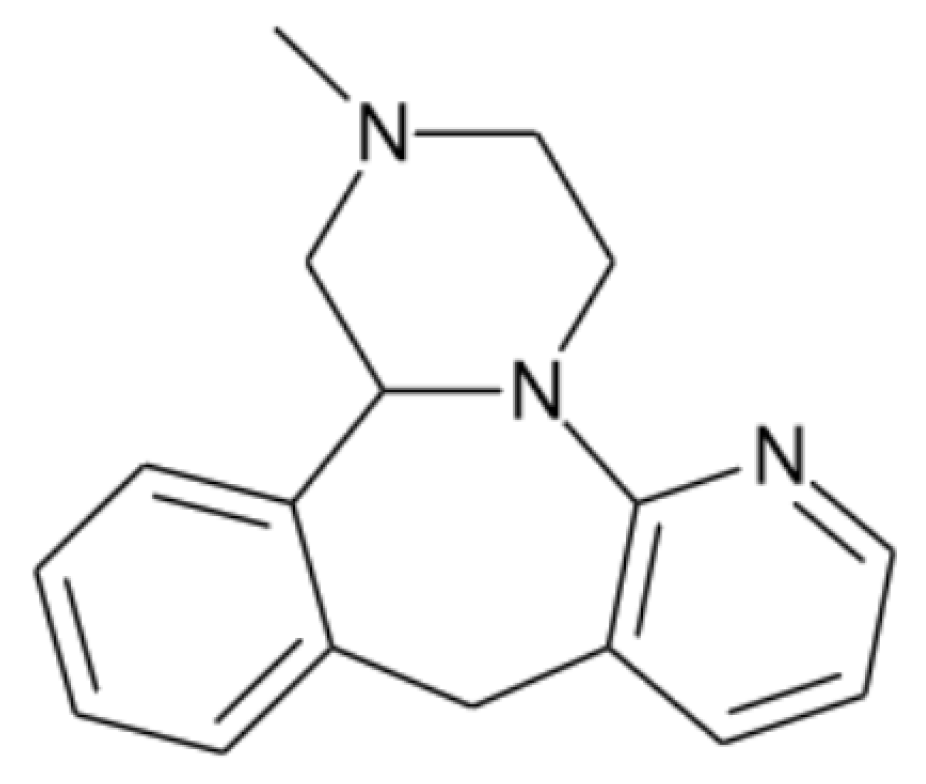
2.1. Materials
2.2. Rheological Characterization
2.3. Oral Fast-Dissolving Film Loading Mirtazapine Preparation
2.3.1. Casting Method
2.3.2. 3D-Printing Method
2.4. Printability Study
2.5. Thickness and Weight Validation
2.6. Morphological Characterization
2.7. X-Ray Powder Diffractometry (XRD)
2.8. In Vitro Disintegration Time
2.9. Mechanical Properties Test
2.10. MTZ Content
2.11. In Vitro MTZ Release Profile
2.12. Statistical Analysis
3. Results and Discussion
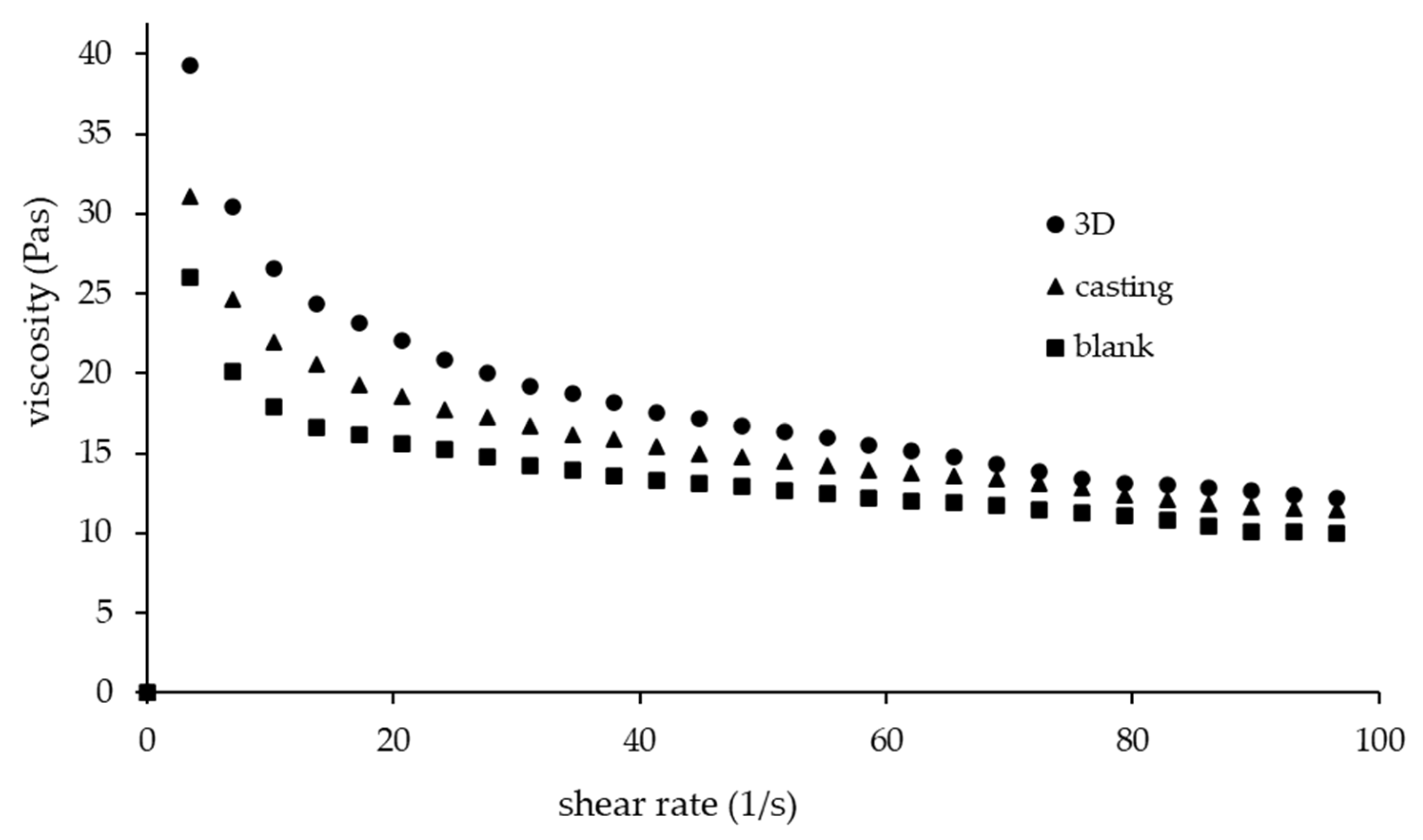
3.1. Rheological Characteristic
3.2. Printability of the Formulations
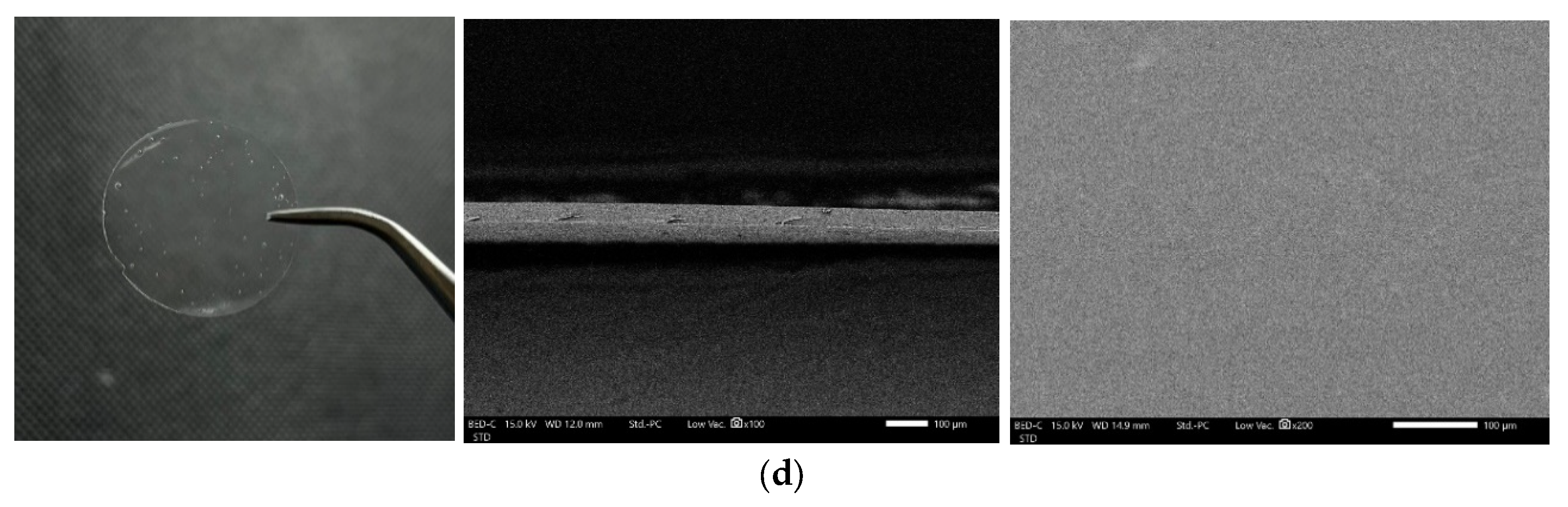
3.3. Morphology of ODFs
3.4. Thickness and Weight Variation of ODFs
3.5. Mechanical Properties of ODFs
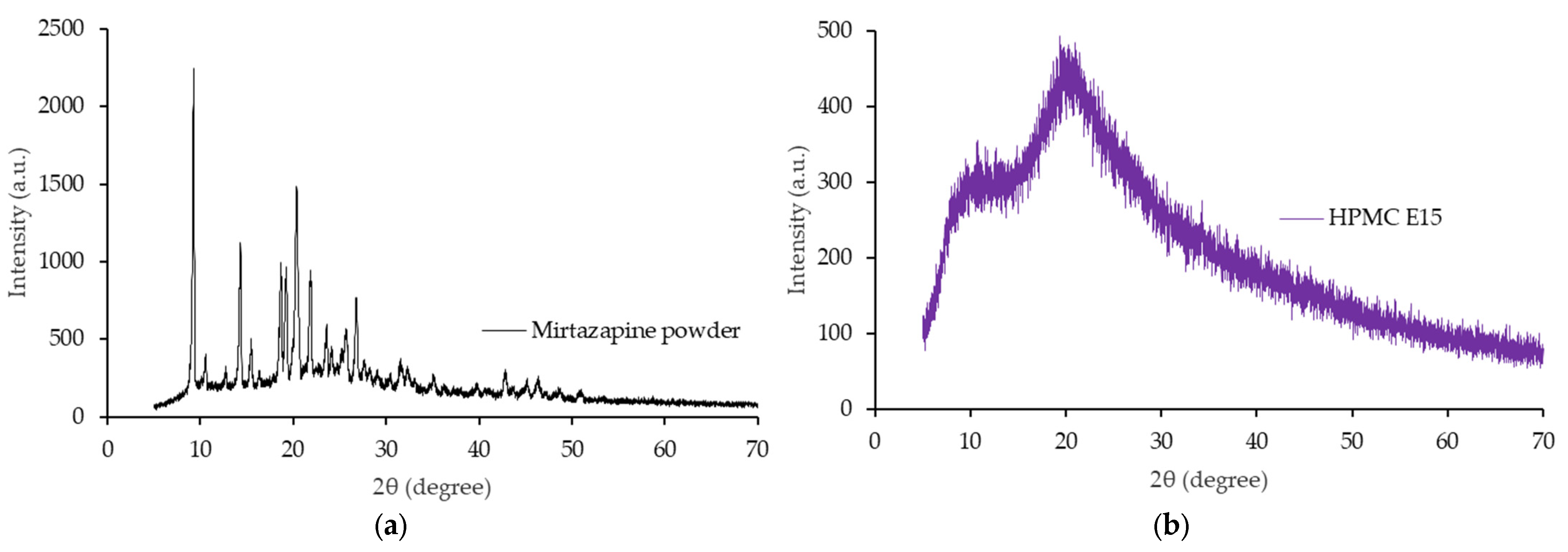
3.6. X-Rays Powder Diffractometry
3.7. Disintegration Time of ODFs
3.8. MTZ Content
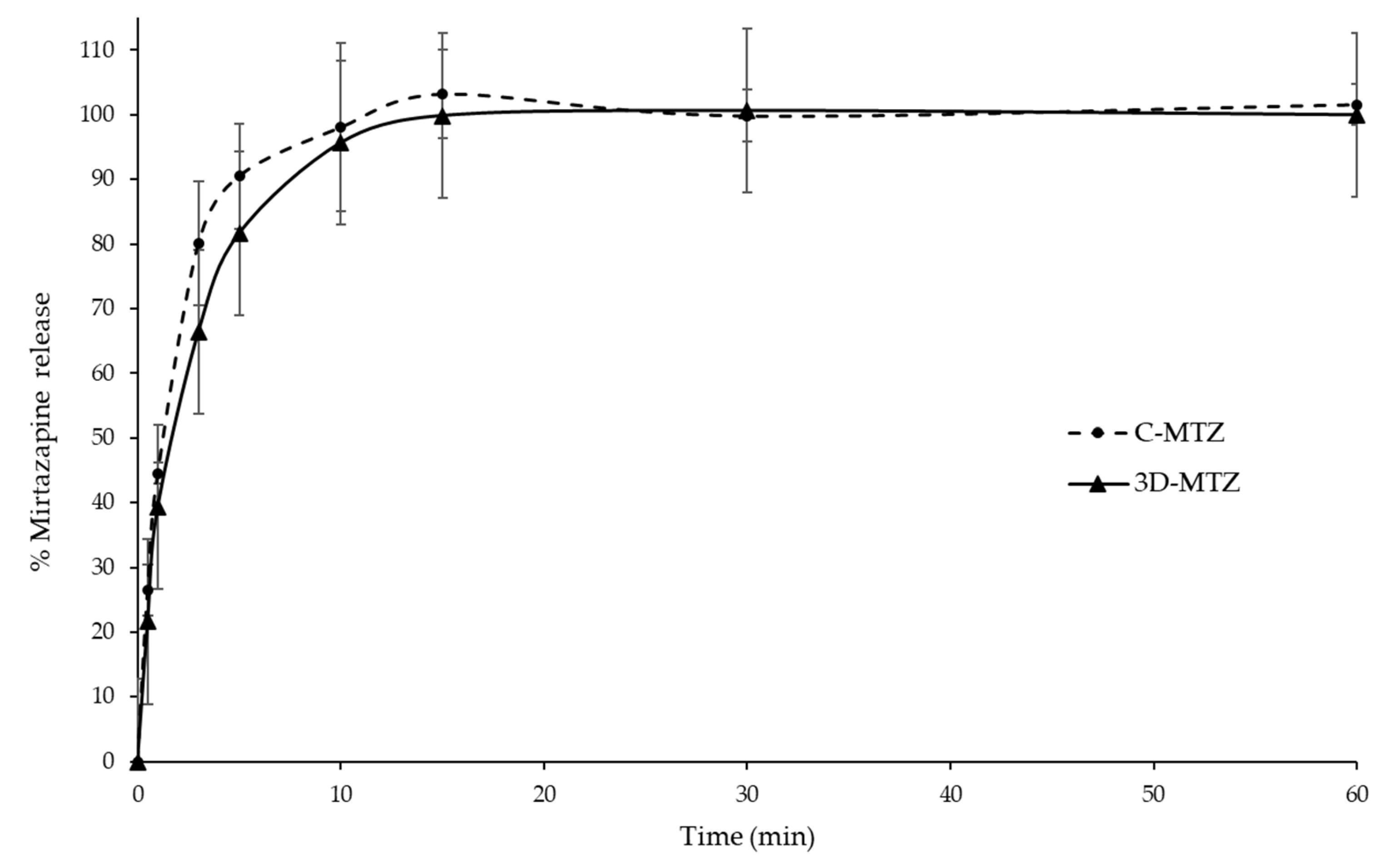
3.9. MTZ Release Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World health organization (WHO). Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 3 October 2022).
- Matreja, P.S.; Badyal, D.K.; Deswal, R.S.; Sharma, A. Efficacy and safety of add on low-dose mirtazapine in depression. Indian J. Pharmacol. 2012, 44, 173–177. [Google Scholar] [PubMed]
- Papakostas, G.I.; Homberger, C.H.; Fava, M. A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. J. Psychopharmacol. 2008, 22, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Ezealisiji, K.E.; Mbah, C.J.; Osadebe, P.O. Aqueous Solubility Enhancement of Mirtazapine: Effect of Cosolvent and Surfactant. Pharmacol. Pharm. 2015, 6, 471–476. [Google Scholar] [CrossRef]
- Meineke, I.; Steinmetz, H.; Kirchheiner, J.; Brockmöller, J. Therapeutic drug monitoring of mirtazapine, desmethylmirtazapine, 8-hydroxymirtazapine, and mirtazapine-N-oxide by enantioselective HPLC with fluorescence detection. Ther. Drug Monit. 2006, 28, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef]
- Krause, J.; Bogdahn, M.; Schneider, F.; Koziolek, M.; Weitschies, W. Design and characterization of a novel 3D printed pressure-controlled drug delivery system. Eur. J. Pharm. Sci. 2019, 140, 105060. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B. Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Redekop, W.K.; Mladsi, D. The Faces of Personalized Medicine: A Framework for Understanding Its Meaning and Scope. Value Health 2013, 16, S4–S9. [Google Scholar] [CrossRef]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, M.; Saha, S.; Shahiwala, F.A. A Review on Mouth Dissolving Films. Curr. Drug Deliv. 2009, 6, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef]
- Yan, T.T.; Lv, Z.F.; Tian, P.; Lin, M.M.; Lin, W.; Huang, S.Y.; Chen, Y.Z. Semi-solid extrusion 3D printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538. [Google Scholar] [CrossRef]
- Naureen, F.; Shah, Y.; Shah, S.I.; Abbas, M.; Rehman, I.U.; Muhammad, S.; Hamdullah, H.; Goh, K.W.; Khuda, F.; Khan, A.; et al. Formulation Development of Mirtazapine Liquisolid Compacts: Optimization Using Central Composite Design. Molecules 2022, 27, 4005. [Google Scholar] [CrossRef]
- Junmahasathien, T.; Panraksa, P.; Protiarn, P.; Hormdee, D.; Noisombut, R.; Kantrong, N.; Jantrawut, P. Preparation and Evaluation of Metronidazole-Loaded Pectin Films for Potentially Targeting a Microbial Infection Associated with Periodontal Disease. Polymers 2018, 10, 1021. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef]
- Cooke, M.; Rosenzweig, D. The rheology of direct and suspended extrusion bioprinting. APL Bioeng. 2021, 5, 011502. [Google Scholar] [CrossRef] [PubMed]
- Polamaplly, P.; Cheng, Y.; Shi, X.; Manikandan, K.; Zhang, X.; Kremer, G.E.; Qin, H. 3D printing and characterization of hydroxypropyl methylcellulose and methylcellulose for biodegradable support structures. Polymer 2019, 173, 119–126. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of Orodispersible Polymer Films with Micronized Loratadine-Influence of Different Drug Loadings on Film Properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Rohani, S. Molecular salts and co-crystals of mirtazapine with promising physicochemical properties. J. Pharm. Biomed. Anal. 2015, 110, 93–99. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Yamashita, K.; Nakate, T.; Okimoto, K.; Ohike, A.; Tokunaga, Y.; Ibuki, R.; Higaki, K.; Kimura, T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm. 2003, 267, 79–91. [Google Scholar] [CrossRef]
- Oh, D.H.; Park, Y.-J.; Kang, J.H.; Yong, C.S.; Choi, H.-G. Physicochemical characterization and in vivo evaluation of flurbiprofen-loaded solid dispersion without crystalline change. Drug Deliv. 2011, 18, 46–53. [Google Scholar] [CrossRef]
- Janßen, E.M.; Schliephacke, R.; Breitenbach, A.; Breitkreutz, J. Drug-printing by flexographic printing technology—A new manufacturing process for orodispersible films. Int. J. Pharm. 2013, 441, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Banarjee, T.; Ansari, V.A.; Singh, S.; Mahmood, T.; Akhtar, J. A review on fast dissolving films for buccal delivery of low dose drugs. Int. J. Life Sci. Rev. 2015, 1, 117–123. [Google Scholar]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- USP. The United States Pharmacopeia, 43rd ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2020; p. 2980. [Google Scholar]
- Yıldız, S.; Aytekin, E.; Yavuz, B.; Bozdağ Pehlivan, S.; Ünlü, N. Formulation studies for mirtazapine orally disintegrating tablets. Drug Dev. Ind. Pharm. 2016, 42, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L. Driving forces for drug loading in drug carriers. J. Microencapsul. 2015, 32, 255–272. [Google Scholar] [CrossRef] [PubMed]



| Formulations | Flow Behaviour Index (n) | Consistency Coefficient (Pa.s) | Shape Fidelity Factor | Diameter of Printed Filament (mm) |
|---|---|---|---|---|
| 3D-MTZ | 0.67 | 59.38 | 1.05 ± 0.04 a | 0.56 ± 0.01 a |
| C-MTZ | 0.72 | 43.69 | NA | NA |
| Blank | 0.74 | 34.74 | 1.01 ± 0.08 a | 0.90 ± 0.02 b |
| Formulations | Thickness (mm) | Weight (g) | Puncture Strength (N/mm2) | Elongation at Break (%) | Young’s Modulus (N/mm2) |
|---|---|---|---|---|---|
| 3D-MTZ | 0.128 ± 0.008 a | 0.0344 ± 0.0006 a | 1.11 ± 0.05 a | 5.39 ± 0.67 a | 22.06 ± 0.95 a |
| C-MTZ | 0.145 ± 0.004 b | 0.0515 ± 0.0045 b | 1.53 ± 0.13 b | 1.55 ± 0.36 b | 27.89 ± 3.66 b |
| 3D-blank | 0.104 ± 0.012 c | 0.0308 ± 0.0012 c | 3.30 ± 0.49 c | 12.61 ± 1.55 c | 62.03 ± 2.84 c |
| C-blank | 0.074 ± 0.019 c | 0.0252 ± 0.0034 d | 4.92 ± 0.51 d | 12.79 ± 0.40 c | 98.13 ± 2.74 d |
| Formulations | Disintegration Time (s) | MTZ Content (%) |
|---|---|---|
| 3D-MTZ | 24.38 ± 1.53 a | 106.25 ± 3.85 a |
| C-MTZ | 46.75 ± 2.52 b | 100.74 ± 1.80 a |
| 3D-blank | 17.85 ± 1.87 c | NA |
| C-blank | 15.26 ± 1.17 c | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwarit, T.; Aodsab, N.; Promyos, P.; Panraksa, P.; Udomsom, S.; Jantrawut, P. Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer. Sci. Pharm. 2022, 90, 68. https://doi.org/10.3390/scipharm90040068
Chaiwarit T, Aodsab N, Promyos P, Panraksa P, Udomsom S, Jantrawut P. Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer. Scientia Pharmaceutica. 2022; 90(4):68. https://doi.org/10.3390/scipharm90040068
Chicago/Turabian StyleChaiwarit, Tanpong, Niphattha Aodsab, Pimonnart Promyos, Pattaraporn Panraksa, Suruk Udomsom, and Pensak Jantrawut. 2022. "Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer" Scientia Pharmaceutica 90, no. 4: 68. https://doi.org/10.3390/scipharm90040068
APA StyleChaiwarit, T., Aodsab, N., Promyos, P., Panraksa, P., Udomsom, S., & Jantrawut, P. (2022). Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer. Scientia Pharmaceutica, 90(4), 68. https://doi.org/10.3390/scipharm90040068







