Changes in Left Heart Geometry, Function, and Blood Serum Biomarkers in Patients with Obstructive Sleep Apnea after Treatment with Continuous Positive Airway Pressure
Abstract
1. Introduction
1.1. OSA Impact on Cardiovascular System
1.2. OSA and Blood Serum Biomarkers
1.2.1. Galectin-3
1.2.2. sST2
1.2.3. Endothelin-1
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection and Biomarker Testing
2.3. Echocardiography
2.4. Interventions and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Echocardiographic Parameters
3.3. Blood Serum Biomarkers
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jun, J.C.; Chopra, S.; Schwartz, A.R. Sleep apnoea. Eur. Respir. Rev. 2016, 139, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, A.C.; Mentz, R.J. Sleep Breathing Disorders in Heart Failure. Heart Fail Clin. 2020, 1, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 24, 884–894. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, H.; Liu, X.; Fan, J.; Zhu, Q.; Li, J.; Wang, J.A. Left ventricular remodeling and dysfunction in obstructive sleep apnea: Systematic review and meta-analysis. Herz 2020, 45, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Sleep apnea and heart failure: Part I: Obstructive sleep apnea. Circulation 2003, 107, 1671–1678. [Google Scholar] [CrossRef]
- Papanikolaou, J.; Ntalapascha, M.; Makris, D.; Koukoubani, T.; Tsolaki, V.; Zakynthinos, G.; Zakynthinos, E. Diastolic dysfunction in men with severe obstructive sleep apnea syndrome but without cardiovascular or oxidative stress-related comorbidities. Ther. Adv. Respir. Dis. 2019, 13, 1753466619880076. [Google Scholar] [CrossRef]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Mediano, O.; Martinez, I.; Villamor, J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: Effects of nasal continuous positive airway pressure in men. Circulation 2005, 112, 375–383. [Google Scholar] [CrossRef]
- Maripov, A.; Mamazhakypov, A.; Sartmyrzaeva, M.; Akunov, A.; Duishobaev, M.; Cholponbaeva, M.; Sarybaev, A. Right ventricular remodeling and dysfunction in obstructive sleep apnea: A systematic review of the literature and meta-analysis. Can. Respir. J. 2017, 2017, 1587865. [Google Scholar] [CrossRef]
- Hansdottir, S.; Groskreutz, D.J.; Gehlbach, B.K. WHO’s in second?: A practical review of World Health Organization group 2 pulmonary hypertension. Chest 2013, 144, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J. Pulmonary hypertension associated with left-sided heart disease. Clin. Chest Med. 2007, 28, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Montesi, S.B.; Bajwa, E.K.; Malhotra, A. Biomarkers of sleep apnea. Chest 2012, 142, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Steiropoulos, P. Biomarkers to Improve Diagnosis and Monitoring of Obstructive Sleep Apnea Syndrome: Current Status and Future Perspectives. Pulm. Med. 2014, 2014, 930535. [Google Scholar] [CrossRef]
- Maeder, M.; Mueller, C.; Schoch, O.D.; Ammann, P.; Rickli, H. Biomarkers of cardiovascular stress in obstructive sleep apnea. Clin. Chim. Acta. 2016, 460, 152–163. [Google Scholar] [CrossRef]
- Argüeso, P.; Panjwani, N. Focus on molecules: Galectin-3. Exp. Eye Res. 2011, 92, 2–3. [Google Scholar] [CrossRef]
- Merino-Merino, A.; Gonzalez-Bernal, J.; Fernandez-Zoppino, D.; Saez-Maleta, R.; Perez-Rivera, J.A. The Role of Galectin-3 and ST2 in Cardiology: A Short Review. Biomolecules 2021, 11, 1167. [Google Scholar] [CrossRef]
- Singh, M.; Hanis, C.L.; Redline, S.; Ballantyne, C.M.; Hamzeh, I.; Aguilar, D. Sleep apnea and galectin-3: Possible sex-specific relationship. Sleep Breath. 2019, 23, 1107–1114. [Google Scholar] [CrossRef]
- Pusuroglu, H.; Somuncu, U.; Bolat, I.; Akgul, O.; Ornek, V.; Yıldırım, H.A.; Akkaya, E.; Karakurt, H.; Yıldırım, A.; Savaş, A.U. Galectin-3 is associated with coronary plaque burden and obstructive sleep apnoea syndrome severity. Kardiol. Pol. 2017, 75, 351–359. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Kopeva, E.V.; Grakova, A.V.; Yakovlev, S.N.; Shilov, N.F.; Yakovleva, E.N.; Berezikova, A.; Teplyakov, T. Prognostic value of soluble ST2 biomarker in heart failure patients with obstructive sleep apnea syndrome. Eur. J. Cardiovasc. Nurs. 2018, 20, zvab060-126. [Google Scholar] [CrossRef]
- Andreieva, I. Effects of CPAP and mandibular advancement devices on cardiac biomarkers in patients with obstructive sleep apnea. Eur. Respir. J. 2020, 56, 4738. [Google Scholar] [CrossRef]
- Harańczyk, M.; Konieczyńska, M.; Płazak, W. Endothelial dysfunction in obstructive sleep apnea patients. Sleep Breath. 2022, 26, 231–242. [Google Scholar] [CrossRef]
- Lin, G.; Chen, Q.; Huang, J.; Chen, L.; Lin, T.; Lin, Q. Effect of continuous positive airway pressure on endothelin-1 in patients with obstructive sleep apnea: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2019, 276, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kondratavičienė, L.; Malakauskas, K.; Vaitukaitienė, G.; Balsevičius, T.; Žemaitis, M.; Miliauskas, S. Short-Term Continuous Positive Air Pressure Treatment: Effects on Quality of Life and Sleep in Patients with Obstructive Sleep Apnea. Medicina 2022, 58, 350. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Voigt, J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Singh, M.; Sethi, A.; Mishra, A.K.; Subrayappa, N.K.; Stapleton, D.D.; Pellikka, P.A. Echocardiographic Imaging Challenges in Obesity: Guideline Recommendations and Limitations of Adjusting to Body Size. J. Am. Heart Assoc. 2020, 9, e014609. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Evangelista, A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abaraham, T.P.; Aurigemma, G.; Edvardsen, T. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of pulmonary artery pressure by echocardiography—A comprehensive review. IJC Heart Vasc. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Andreieva, I. The effect of CPAP treatment on Galectin-3 level in patients with severe obstructive sleep apnea. Eur. Respir. J. 2018, 52, PA4332. [Google Scholar] [CrossRef]
- Ansari, U.; Behnes, M.; Hoffmann, J.; Natale, M.; Fastner, C.; El-Battrawy, I.; Rusnak, J.; Kim, S.H.; Lang, S.; Hoffmann, U.; et al. Galectin-3 Reflects the Echocardiographic Grades of Left Ventricular Diastolic Dysfunction. Ann. Lab. Med. 2018, 38, 306–315. [Google Scholar] [CrossRef]
- Andrejic, O.M.; Vucic, R.M.; Pavlovic, M.; McClements, L.; Stokanovic, D.; Jevtovic-Stoimenov, T.; Nikolic, V.N. Association between Galectin-3 levels within central and peripheral venous blood, and adverse left ventricular remodelling after first acute myocardial infarction. Sci. Rep. 2019, 9, 13145. [Google Scholar] [CrossRef] [PubMed]
- Ionin, V.; Filatova, A.; Skuridin, D.; Petrischeva, E.; Zaslavskaya, E.; Baranova, E. Obstructive sleep apnea, galectin-3 and atrial fibrillation in patients with obesity and hypertension. J. Hypertens. 2019, 37, e41. [Google Scholar] [CrossRef]
- Cicco, S.; Castellana, G.; Marra, L.; Di Lecce, V.; Carratù, P.; Prete, M.; Ranieri, G.; Resta, O.; Carpagnano, G.E.; Racanelli, V.; et al. Galectin-3 and neutrophil-to-lymphocyte ratio are indicative of heart remodelling and disease severity in patients with obstructive sleep apnoea. Sleep Med. 2021, 82, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Clopton, P.; Iqbal, N.; Tran, K.; Maisel, A.S. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am. Heart J. 2010, 160, 721–728. [Google Scholar] [CrossRef]
- Farcaş, A.D.; Anton, F.P.; Goidescu, C.M.; Gavrilă, I.L.; Vida-Simiti, L.A.; Stoia, M.A. Serum Soluble ST2 and Diastolic Dysfunction in Hypertensive Patients. Dis. Markers 2017, 2017, 2714095. [Google Scholar] [CrossRef]
- Ferri, C.; Bellini, C.; De Angelis, C.; De Siati, L.; Perrone, A.; Properzi, G.; Santucci, A. Circulating endothelin-1 concentrations in patients with chronic hypoxia. J. Clin. Pathol. 1995, 48, 519–524. [Google Scholar] [CrossRef]
- Carratù, P.; Ventura, V.A.; Maniscalco, M.; Dragonieri, S.; Berardi, S.; Ria, R.; Quaranta, V.N.; Vacca, A.; Devito, F.; Ciccone, M.M.; et al. Echocardiographic findings and plasma endothelin-1 levels in obese patients with and without obstructive sleep apnea. Sleep Breath. 2016, 20, 613–619. [Google Scholar] [CrossRef]
- Jankowich, M.D.; Wu, W.C.; Choudhary, G. Association of Elevated Plasma Endothelin-1 Levels With Pulmonary Hypertension, Mortality, and Heart Failure in African American Individuals: The Jackson Heart Study. JAMA Cardiol. 2016, 1, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Egea, C.J.; Aizpuru, F.; Pinto, J.A.; Ayuela, J.M.; Ballester, E.; Zamarrón, C.; Sojo, A.; Montserrat, J.M.; Barbe, F.; Alonso-Gomez, A.M.; et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: A multicenter study. Sleep Med. 2008, 9, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Ikejder, Y.; Sebbani, M.; Hendy, I.; Khramz, M.; Khatouri, A.; Bendriss, L. Impact of Arterial Hypertension on Left Atrial Size and Function. Biomed Res. Int. 2020, 2020, 2587530. [Google Scholar] [CrossRef] [PubMed]
- Bodez, D.; Damy, T.; Soulat-Dufour, L.; Meuleman, C.; Cohen, A. Consequences of obstructive sleep apnoea syndrome on left ventricular geometry and diastolic function. Arch. Cardiovasc. Dis. 2016, 109, 494–503. [Google Scholar] [CrossRef]
- Sascău, R.; Zota, I.M.; Stătescu, C.; Boișteanu, D.; Roca, M.; Maștaleru, A.; Leon Constantin, M.M.; Vasilcu, T.F.; Gavril, R.S.; Mitu, F. Review of Echocardiographic Findings in Patients with Obstructive Sleep Apnea. Can. Respir. J. 2018, 2018, 1206217. [Google Scholar] [CrossRef]
- Giampá, S.Q.C.; Furlan, S.F.; Freitas, L.S.; Macedo, T.A.; Lebkuchen, A.; Cardozo, K.H.M.; Carvalho, V.M.; Martins, F.C.; Azam, I.F.B.; Costa-Hong, V.; et al. Effects of CPAP on Metabolic Syndrome in Patients With OSA: A Randomized Trial. Chest 2022, 161, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Colish, J.; Walker, J.R.; Elmayergi, N.; Almutairi, S.; Alharbi, F.; Lytwyn, M.; Francis, A.; Bohonis, S.; Zeglinski, M.; Kirkpatrick, I.D.C.; et al. Obstructive sleep apnea: Effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest 2012, 141, 674–681. [Google Scholar] [CrossRef]
- Sun, H.; Shi, J.; Li, M.; Chen, X. Impact of continuous positive airway pressure treatment on left ventricular ejection fraction in patients with obstructive sleep apnea: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e62298. [Google Scholar] [CrossRef]
- Javaheri, S.; Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Muriel, A.; Peker, Y. CPAP Adherence for prevention of major adverse cerebrovascular and cardiovascular events in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2019, 201, 607–610. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Anderson, C.S. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Tadic, M.; Gherbesi, E.; Faggiano, A.; Sala, C.; Carugo, S.; Cuspidi, C. The impact of continuous positive airway pressure on cardiac mechanics: Findings from a meta-analysis of echocardiographic studies. J. Clin. Hypertens. 2022, 24, 795–803. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Martone, F.; Liccardo, B.; Mazza, M.; Annunziata, A.; Di Palma, E.; Conte, M.; Sirignano, C.; D’Alto, M.; Esposito, N.; et al. Acute and Chronic Effects of Noninvasive Ventilation on Left and Right Myocardial Function in Patients with Obstructive Sleep Apnea Syndrome: A Speckle Tracking Echocardiographic Study. Echocardiography 2016, 33, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Haruki, N.; Takeuchi, M.; Kanazawa, Y.; Tsubota, N.; Shintome, R.; Nakai, H.; Lang, R.M.; Otsuji, Y. Continuous positive airway pressure ameliorates sleep-induced subclinical left ventricular systolic dysfunction: Demonstration by two-dimensional speckle-tracking echocardiography. Eur. J. Echocardiogr. 2010, 11, 352–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.; Shim, C.Y.; Cho, Y.J.; Park, S.; Lee, C.J.; Park, J.H.; Cho, H.J.; Ha, J.W.; Hong, G.R. Continuous Positive Airway Pressure Therapy Restores Cardiac Mechanical Function in Patients with Severe Obstructive Sleep Apnea: A Randomized, Sham-Controlled Study. J. Am. Soc. Echocardiogr. 2019, 32, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Xiang, G.; Xing, Y.; Hao, S.; Shu, X.; Pan, C.; Li, S. Left atrial dysfunction in patients with obstructive sleep apnea: A combined assessment by speckle tracking and real-time three-dimensional echocardiography. Ann. Palliat. Med. 2021, 10, 2668–2678. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Vural, M.G.; Cetin, S.; Firat, H.; Akdemir, R.; Yeter, E. Impact of continuous positive airway pressure therapy on left atrial function in patients with obstructive sleep apnoea: Assessment by conventional and two-dimensional speckle-tracking echocardiography. Acta Cardiol. 2014, 69, 175–184. [Google Scholar] [CrossRef]
- Chetan, I.M.; Domokos Gergely, B.; Albu, A.; Tomoaia, R.; Todea, D.A. Understanding the role of echocardiography in patients with obstructive sleep apnea and right ventricular subclinical myocardial dysfunction Comparison with other conditions affecting RV deformation. Med. Ultrason. 2021, 23, 213–219. [Google Scholar] [CrossRef]
| OSA Group (n = 34) | Control Group (n = 13) | p-Value * | ||
|---|---|---|---|---|
| Mean (Standard Deviation) | ||||
| Sex | Men (%) | 29 (85.3) | 7 (53.8) | 0.023 * |
| Women (%) | 5 (14.7) | 6 (46.2) | 0.76 | |
| Age, years | 52.15 (8.89) | 45.46 (9.47) | 0.028 * | |
| BMI, kg/m2 | 37.94 (5.31) | 35.33 (5.32) | 0.108 | |
| Chronic diseases | Arterial hypertension, n (%) | 26 (76.5) | 5 (38.5) | 0.014 * |
| Diabetes mellitus, n (%) | 1 (8.3) | 8 (23.5) | 0.254 | |
| Asthma, n (%) | 1 (7.7) | 1 (2.9) | 0.481 | |
| Smoking status, n (%) | 21 (61.8) | 6 (46.2) | 0.33 | |
| AHI, per h | 66.94 (25.33) | 7.02 (4.45) | 0.00 * | |
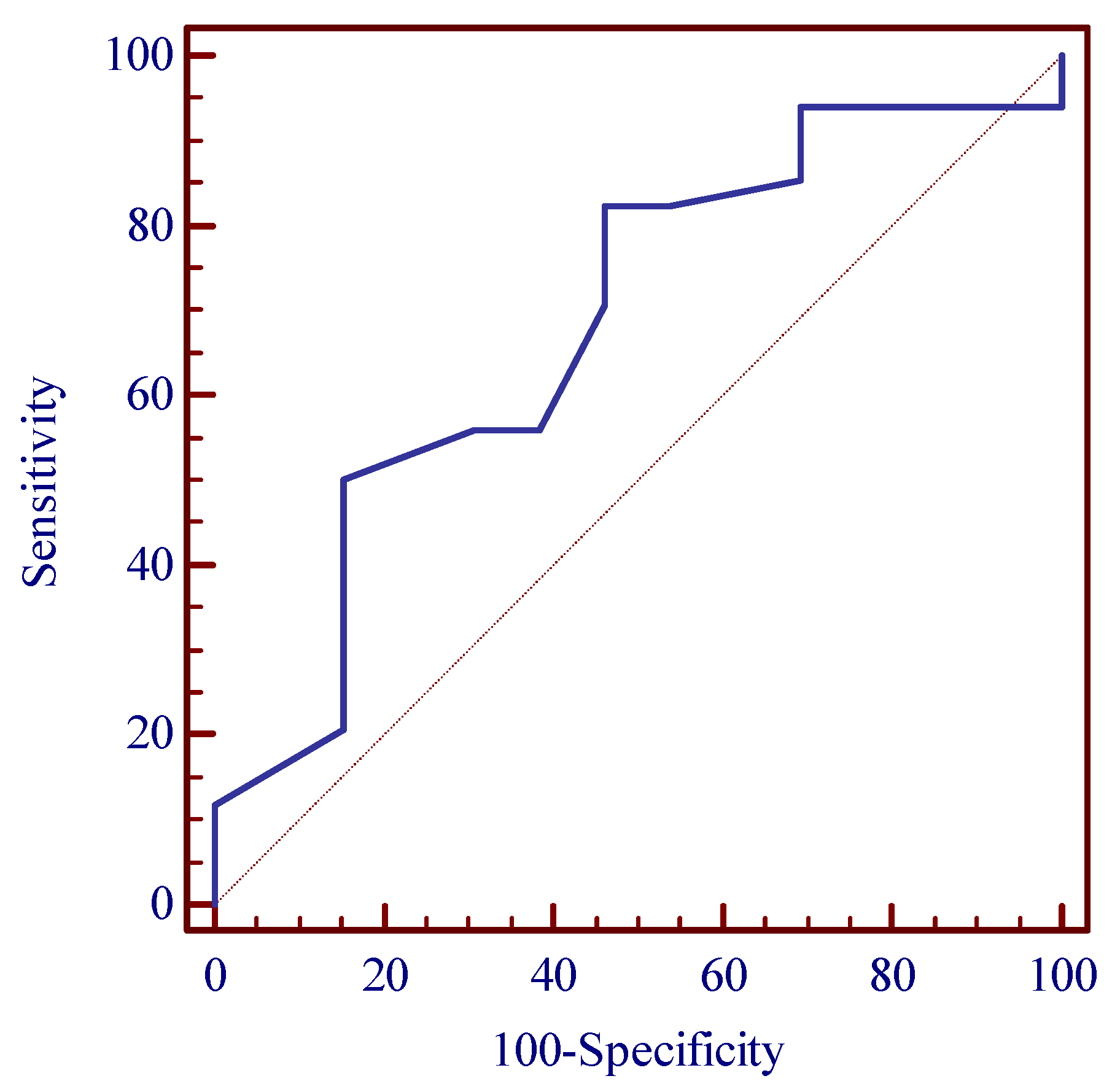
| Parameter/Threshold Value | Area under the ROC Curve (AUC) (%) | Sensitivity/Specificity (%) | Control Group/OSA Group N (%) | p-Value * | OR for OSA Incidence [95% CI] |
|---|---|---|---|---|---|
| Age >44 years | 68.0 | 82.4 53.8 | 6 (46.2) 28 (82.4) | 0.013 * | 5.44 [1.34–22.13] |
| Control Group (n = 13) | OSA Group (n = 34) | p-Value * | ||
|---|---|---|---|---|
| LV EDD (mm) | 47.62 ± 3.97 | 50.08 ± 4.69 | 0.165 | |
| LV EDV (mL) | 102.55 ± 26.05 | 142.25 ± 26.84 | 0.026 * | |
| LV ESV (mL) | 44.08 ± 12.72 | 67.06 (14.77) | 0.015 * | |
| LV Mmi (g/m2) | 79.56 ± 2.92 | 88.98 ± 13.51 | 0.084 | |
| LV Mmi by height (g/m2.7) | 38.96 ± 5.90 | 46.11 ± 8.23 | 0.007 * | |
| IVS (mm) | 10.6 ± 1.09 | 11.42 ± 1.32 | 0.006 * | |
| LV EF (%) | 56.45 ± 2.35 | 54.15 ± 3.59 | 0.055 | |
| LV GLS (%) | −23.20 ± 2.44 | −16.56 ± 3.31 | 0.035 * | |
| LA diameter (mm) | 36.23 ± 3.08 | 43.58 ± 8.19 | <0.001 * | |
| LA volume (mL) | 64.35 ± 3.09 | 70.97 ± 13.80 | 0.191 | |
| LA dilatation (volume index by BSA, >34 mL/m2) | 1 (7.7) | 5 (14.70) | 0.324 | |
| LA dilatation (volume index by height >18.5 mL/m2 men, >16.5 mL/m2 women) | 9 (69.2) | 19 (55.88) | 0.289 | |
| LV diastolic dysfunction | Normal | 7 (53.8) | 4 (15.4) | 0.781 |
| Grade 1 | 6 (46.2) | 22 (80.8) | 0.023 * | |
| Grade 2 | 0 (0) | 1 (3.8) | 0.923 | |
| Grade 3 | 0 (0) | 0 (0) | 0 | |
| LA reservoir strain (%) | 31.01 ± 1.56 | 26.56 ± 1.38 | <0.001 * | |
| RV diameter (mm) | 35.46 ± 4.23 | 36.55 ± 4.57 | 0.419 | |
| RA diameter (mm) | 37.62 ± 4.48 | 40.58 ± 3.4 | 0.043 * | |
| RV S′ (cm/s) | 13.18 ± 2.27 | 14.06 ± 6.16 | 0.941 | |
| RV FAC (%) | 43.72 ± 5.92 | 41.51 ± 4.66 | 0.415 | |
| RV GLS (%) | −25.03 ± 3.21 | −19.65 ± 3.75 | 0.026 * | |
| Mean PAP (mmHg) | 22.19 ± 7.60 | 28.59 ± 7.08 | 0.037 * | |
| OSA Group (n = 13) | ||||
|---|---|---|---|---|
| Before Treatment | 3 Months after CPAP Treatment | p-Value * | ||
| Mean (Standard Deviation) | ||||
| LV EDD (mm) | 50.08 (4.69) | 49.75 (3.57) | 0.504 | |
| LV EDV | 142.25 (26.84) | 127.08 (33.8) | 0.022 * | |
| LV ESV (mL) | 67.06 (14.77) | 53.74 (17.8) | 0.037 * | |
| LV EF (%) | 54.15 (3.59) | 54.83 (3.27) | 0.473 | |
| LV Mmi (g/m2) | 88.98 (13.51) | 80.06 (20.79) | 0.963 | |
| LV Mmi by height (g/m2.7) | 46.11 (8.23) | 43.63 (6.29) | 0.480 | |
| LV GLS (%) | −16.28 (3.82) | −18.82 (3.04) | 0.005 * | |
| LA reservoir strain (%) | 25.82 (7.6) | 32.45 (5.64) | 0.008 * | |
| LA volume (mL) | 64.35 (3.09) | 67.56 (11.24) | 0.328 | |
| LA diameter (mm) | 43.58 ± 8.19 | 43.79 ± 2.27 | 0.874 | |
| LV diastolic dysfunction | Normal | 4 (15.4) | 6 (46.15) | 0.657 |
| Grade 1 | 22 (80.8) | 6 (46.15) | 0.024 * | |
| Grade 2 | 1 (3.8) | 1 (7.7) | 0.973 | |
| Grade 3 | 0 (0) | 0 (0) | 0 | |
| RV diameter (mm) | 36.55 (4.57) | 39.17 (3.83) | 0.411 | |
| RA diameter (mm) | 40.58 (3.4) | 41.92 (3.75) | 0.623 | |
| RV S′ (cm/s) | 14.06 (6.16) | 13.62 (3.72) | 0.624 | |
| RV FAC (%) | 41.51 (4.66) | 43.49 (7.68) | 0.104 | |
| RV GLS (%) | −19.65 (3.75) | −21.15 (4.57) | 0.608 | |
| Mean PAP (mmHg) | 28.59 (7.08) | 29.62 (8.19) | 0.075 | |
| Before Treatment | 3 Months after CPAP Treatment | p-Value * | |
|---|---|---|---|
| Mean (Standard Deviation) | |||
| Galectin-3 (ng/mL) | 17.52 ± 1.19 | 11.64 ± 0,97 | 0.001 * |
| sST2 (ng/mL) | 0.56 ± 0.47 | 0.41 ± 0.56 | 0.047 * |
| ET-1 (ng/mL) | 3.82 ± 2.27 | 3.56 ± 2.27 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratavičienė, L.; Tamulėnaitė, E.; Vasylė, E.; Januškevičius, A.; Ereminienė, E.; Malakauskas, K.; Žemaitis, M.; Miliauskas, S. Changes in Left Heart Geometry, Function, and Blood Serum Biomarkers in Patients with Obstructive Sleep Apnea after Treatment with Continuous Positive Airway Pressure. Medicina 2022, 58, 1511. https://doi.org/10.3390/medicina58111511
Kondratavičienė L, Tamulėnaitė E, Vasylė E, Januškevičius A, Ereminienė E, Malakauskas K, Žemaitis M, Miliauskas S. Changes in Left Heart Geometry, Function, and Blood Serum Biomarkers in Patients with Obstructive Sleep Apnea after Treatment with Continuous Positive Airway Pressure. Medicina. 2022; 58(11):1511. https://doi.org/10.3390/medicina58111511
Chicago/Turabian StyleKondratavičienė, Laima, Eglė Tamulėnaitė, Eglė Vasylė, Andrius Januškevičius, Eglė Ereminienė, Kęstutis Malakauskas, Marius Žemaitis, and Skaidrius Miliauskas. 2022. "Changes in Left Heart Geometry, Function, and Blood Serum Biomarkers in Patients with Obstructive Sleep Apnea after Treatment with Continuous Positive Airway Pressure" Medicina 58, no. 11: 1511. https://doi.org/10.3390/medicina58111511
APA StyleKondratavičienė, L., Tamulėnaitė, E., Vasylė, E., Januškevičius, A., Ereminienė, E., Malakauskas, K., Žemaitis, M., & Miliauskas, S. (2022). Changes in Left Heart Geometry, Function, and Blood Serum Biomarkers in Patients with Obstructive Sleep Apnea after Treatment with Continuous Positive Airway Pressure. Medicina, 58(11), 1511. https://doi.org/10.3390/medicina58111511







