A Study of the Synthesis and Characterization of New Acrylamide Derivatives for Use as Corrosion Inhibitors in Nitric Acid Solutions of Copper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inhibitors Preparation
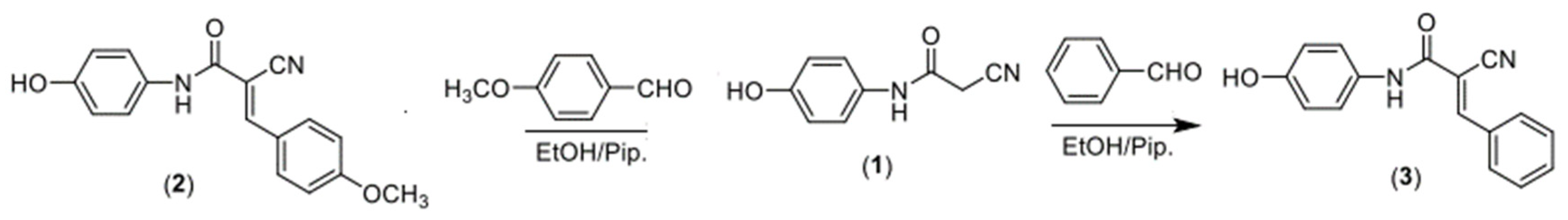
2.1.1. Synthesis of 2-Cyano-N-(4-hydroxyphenyl)-3-(4-methoxyphenyl)acrylamide
2.1.2. Synthesis of 2-Cyano-N-(4-hydroxyphenyl)-3-phenylacrylamide
2.2. Mass Loss Tests (ML)
2.3. Electrochemical Tests
2.4. Theoretical Calculations
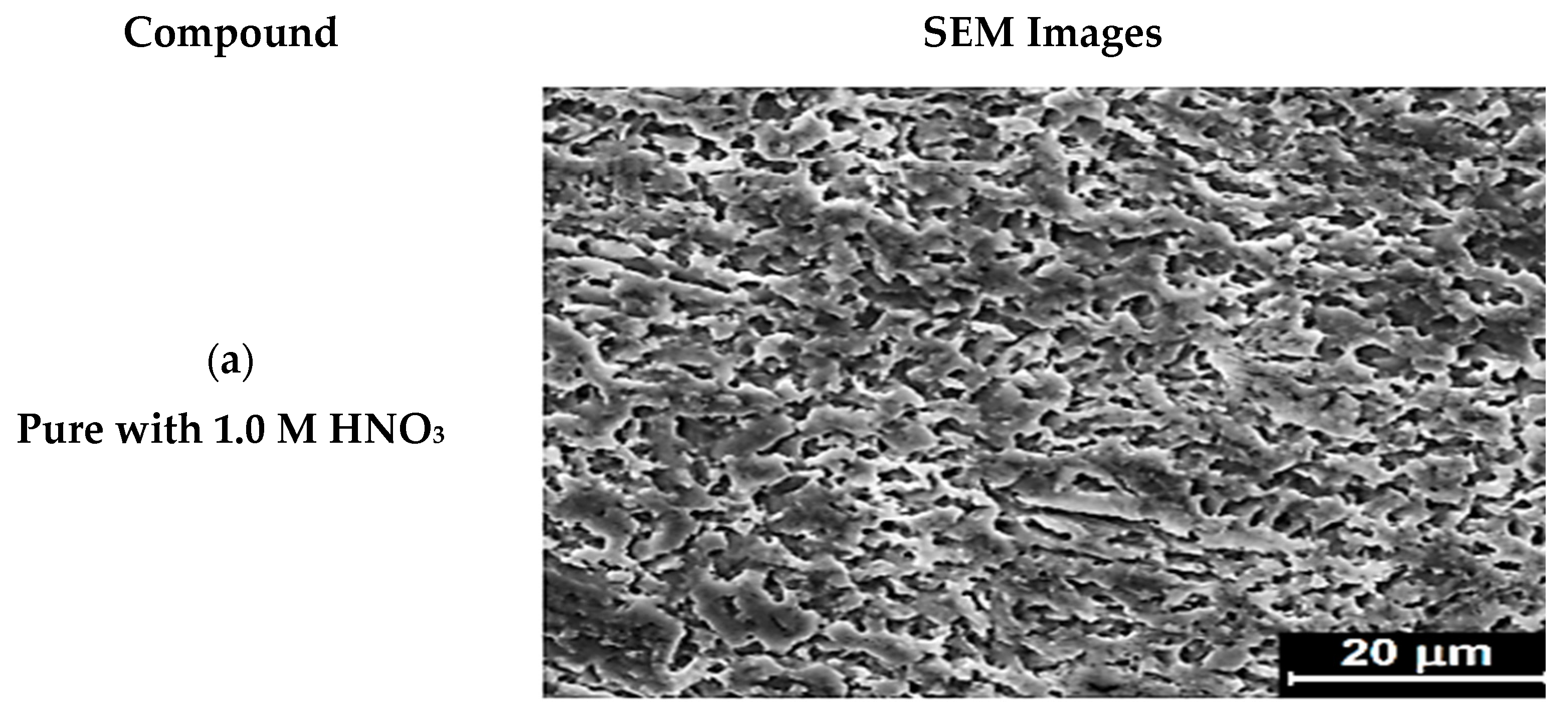
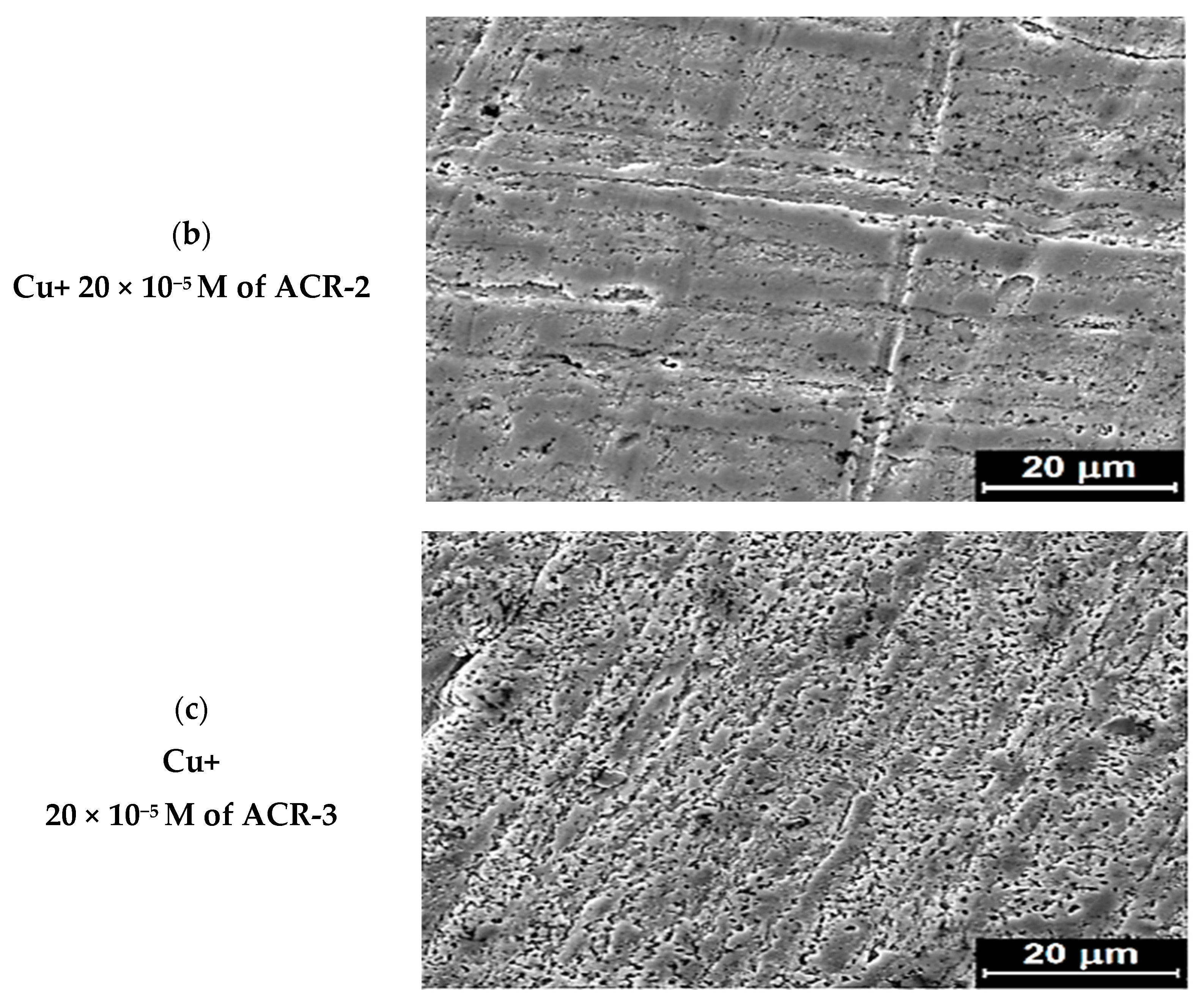
2.5. SEM
3. Results
3.1. 2-Cyano-N-(4-hydroxyphenyl)-3-(4-methoxyphenyl)acrylamide Characteristics
3.1.1. FTIR of Compound (ACR-2)
3.1.2. Proton Nuclear Magnetic Resonance (HNMR) of Compound (ACR-2)
3.1.3. Mass Spectroscopy of Compound (ACR-2)
3.2. 2-Cyano-N-(4-hydroxyphenyl)-3-phenylacrylamide Characteristics
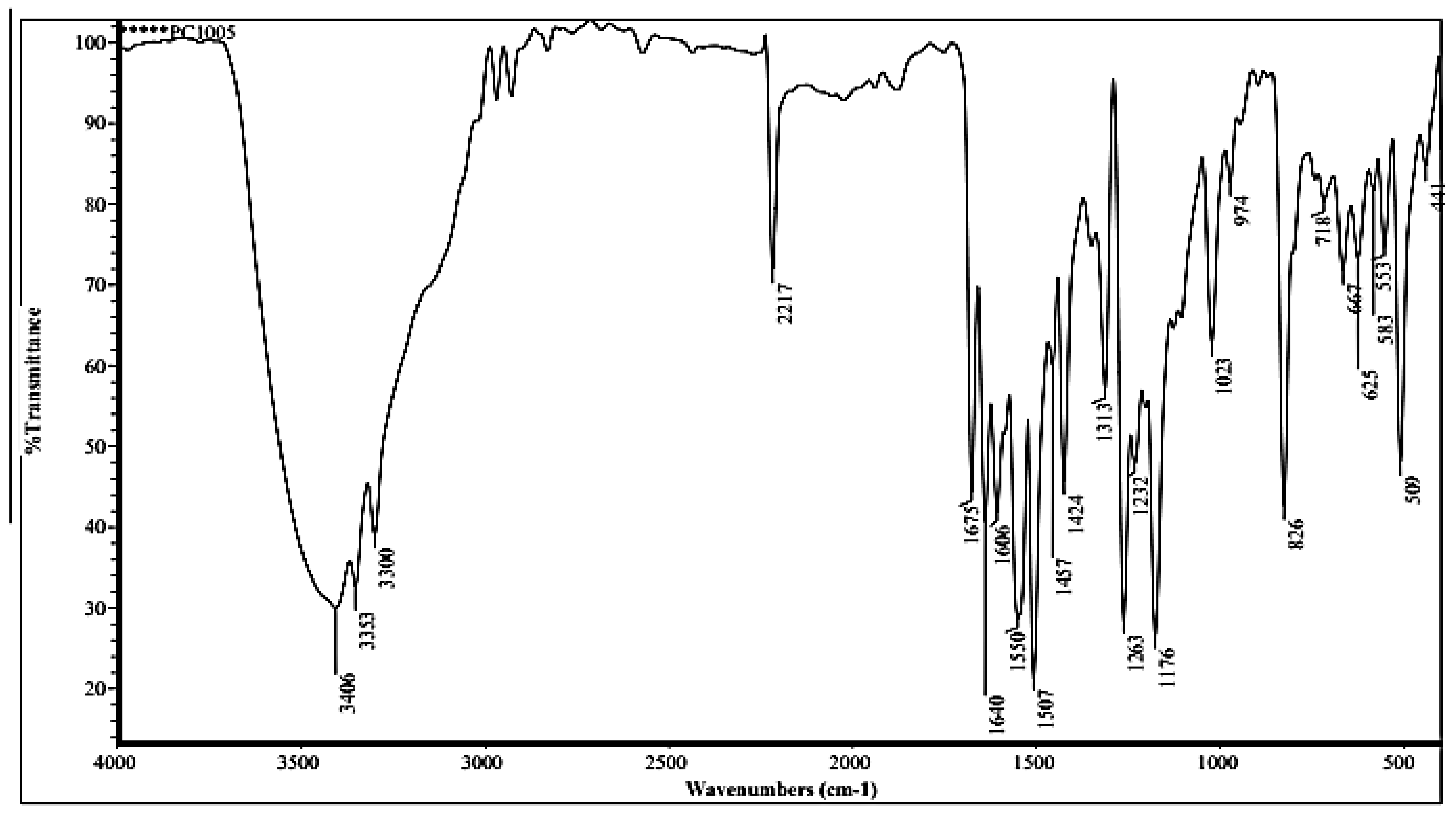
3.2.1. FTIR of Compound (ACR-3)
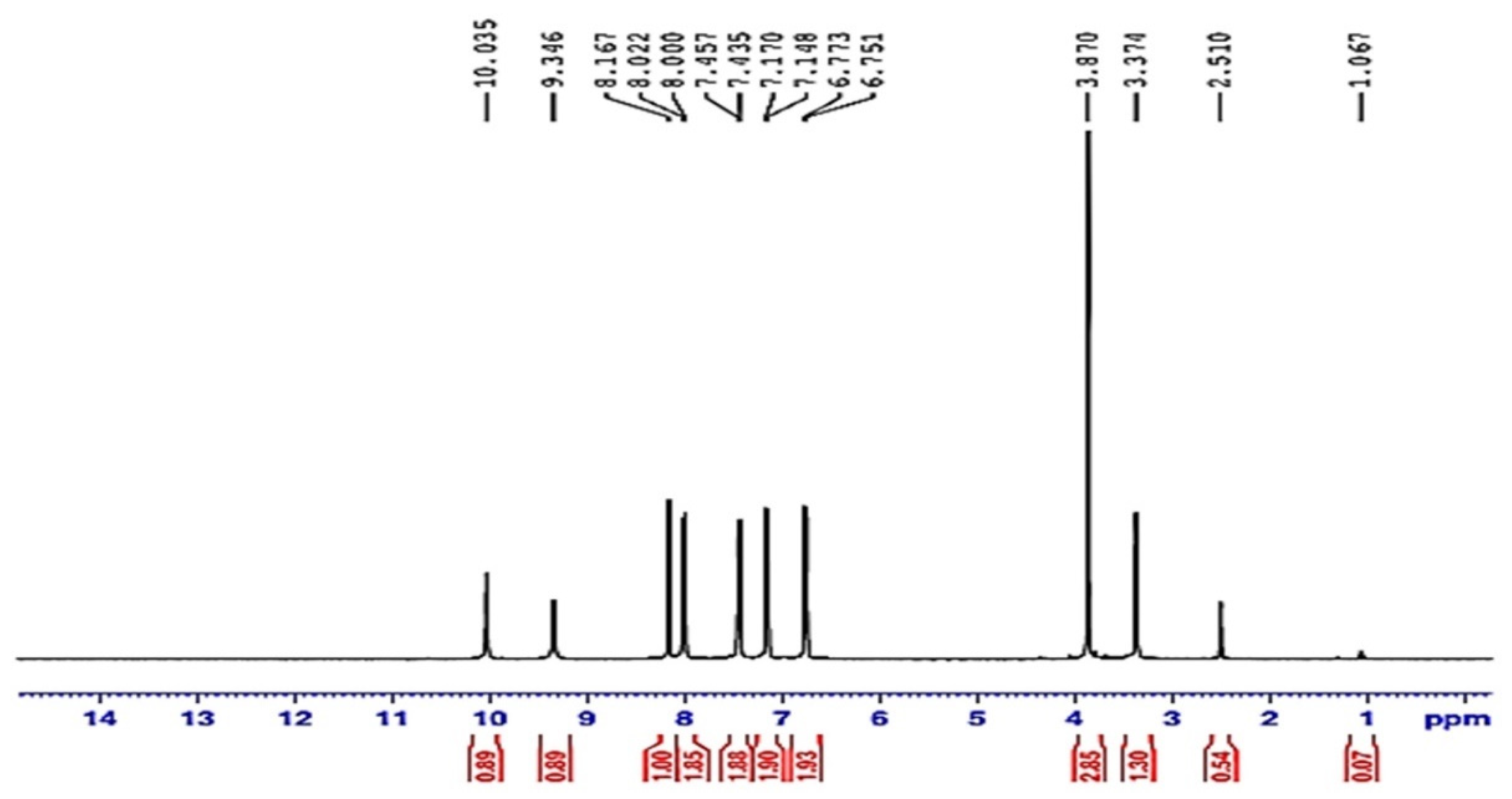
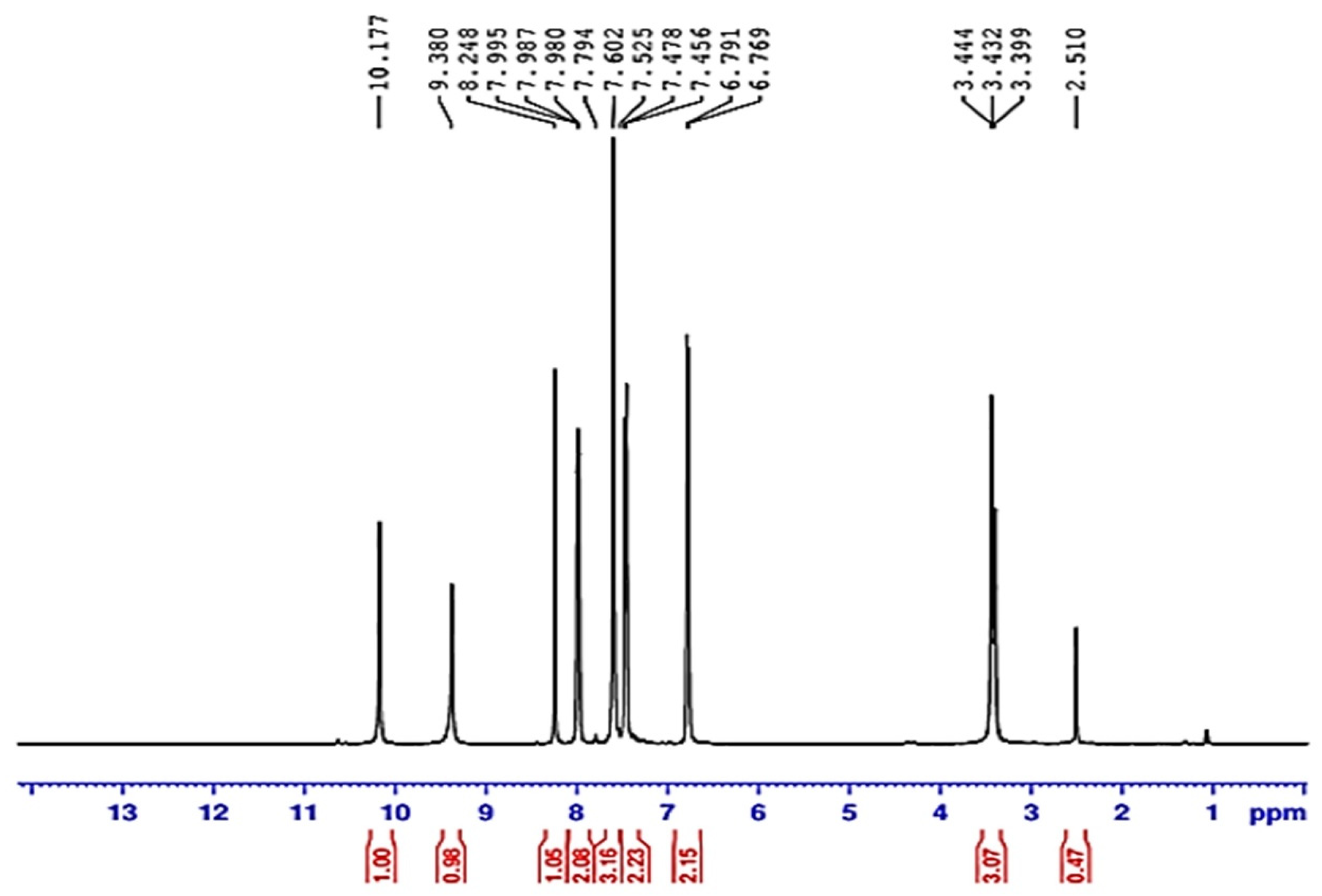
3.2.2. Proton Nuclear Magnetic Resonance (HNMR) of Compound (ACR-3)
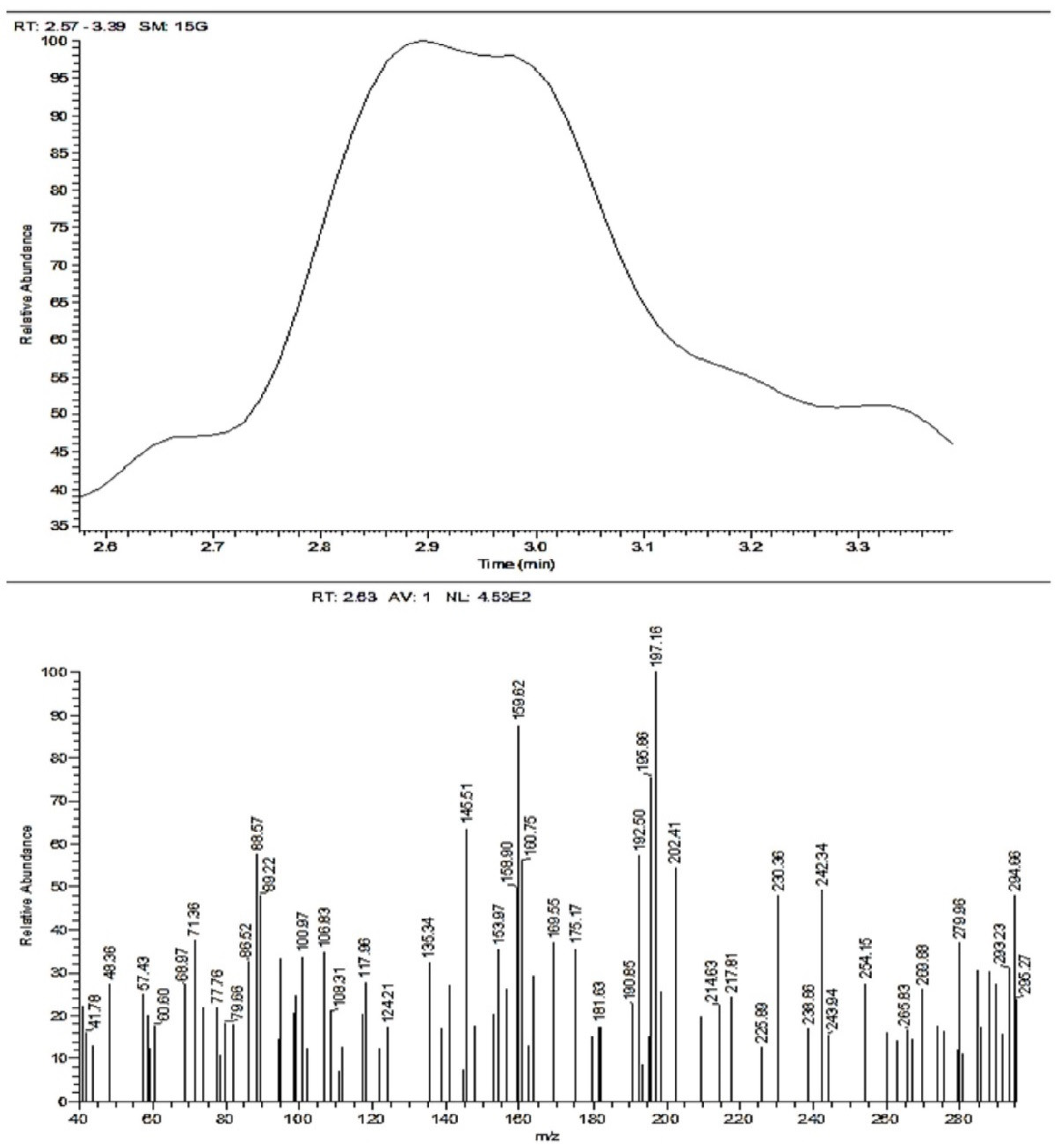
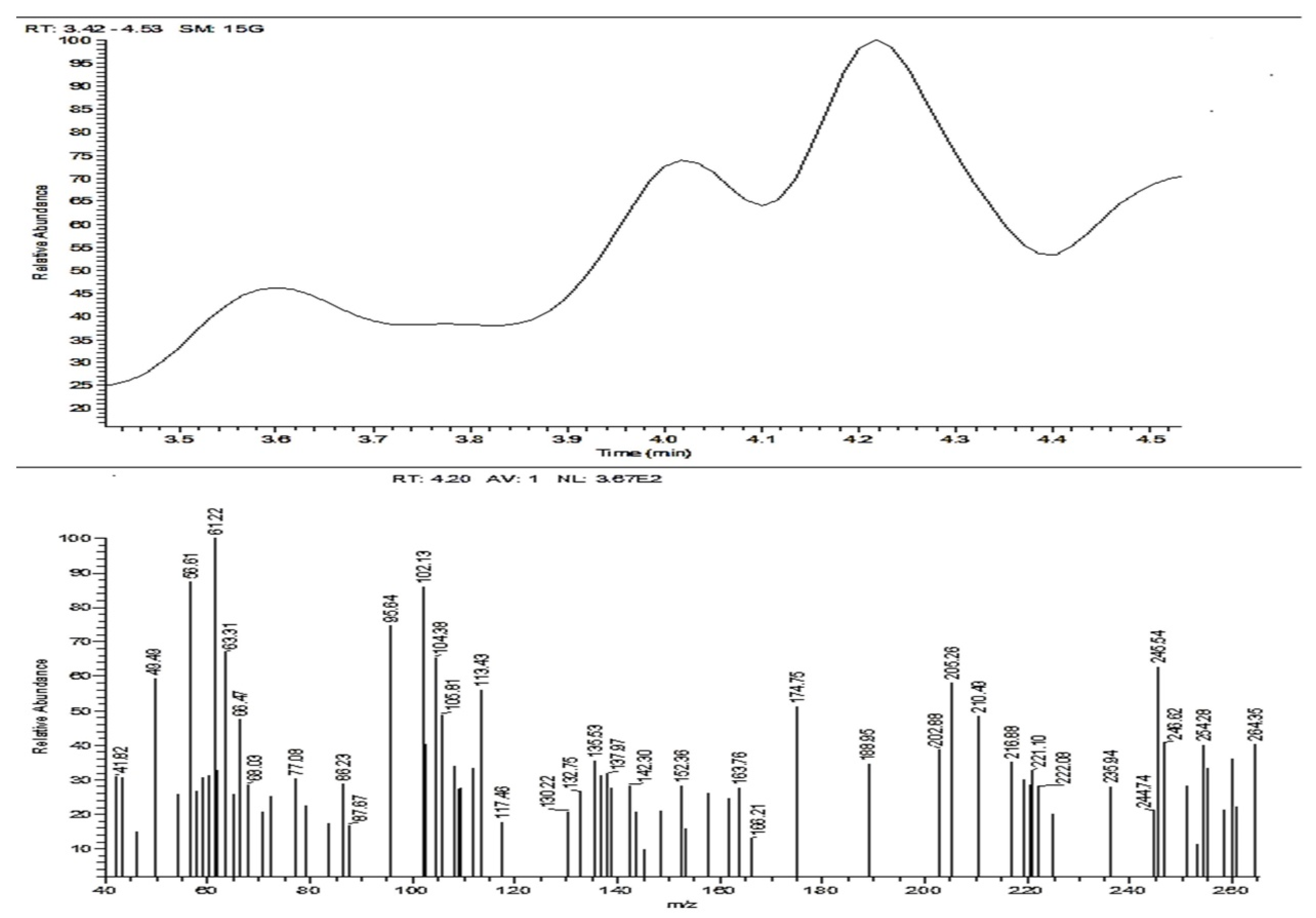
3.2.3. Mass Spectroscopy (ACR-3)
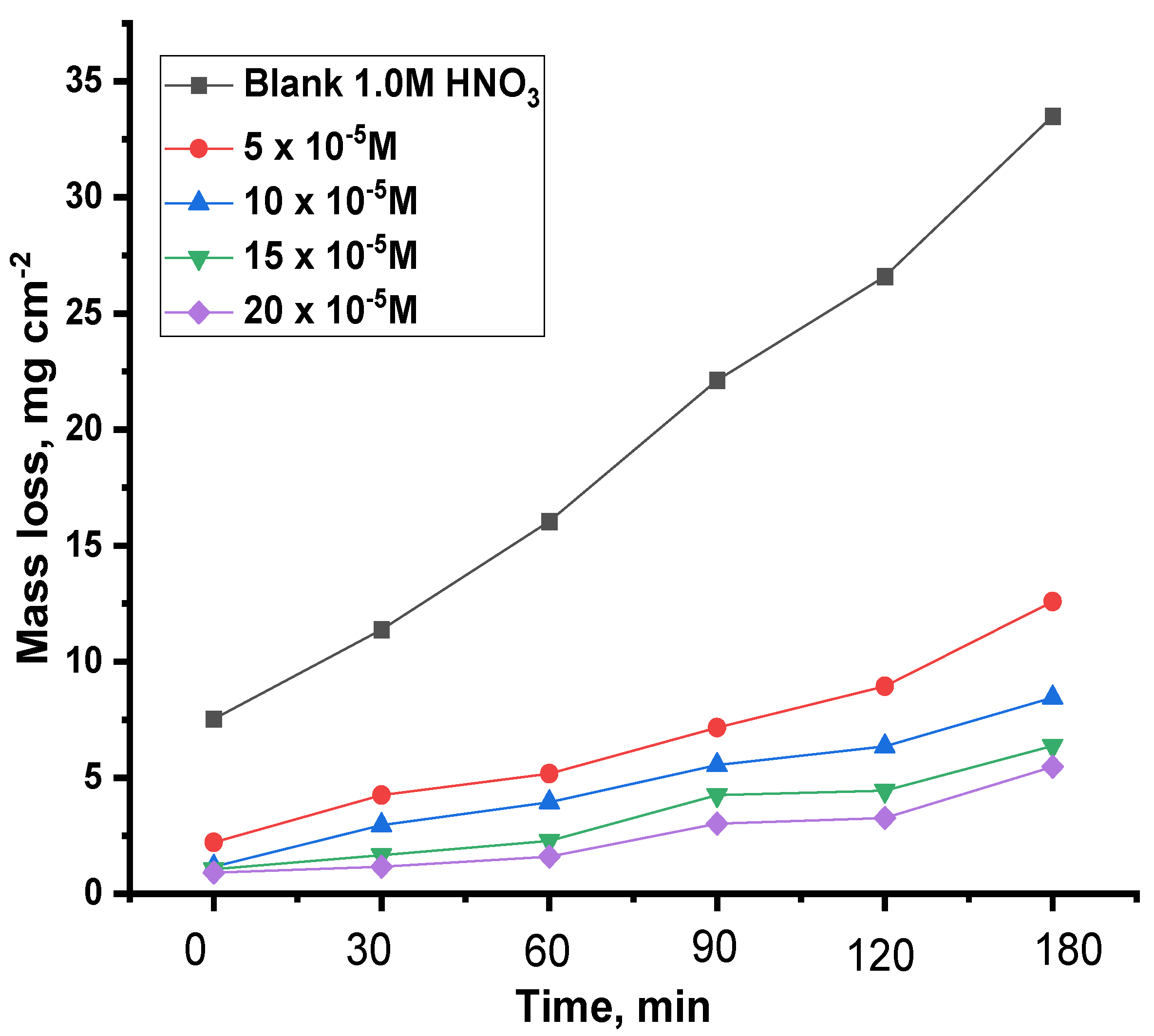
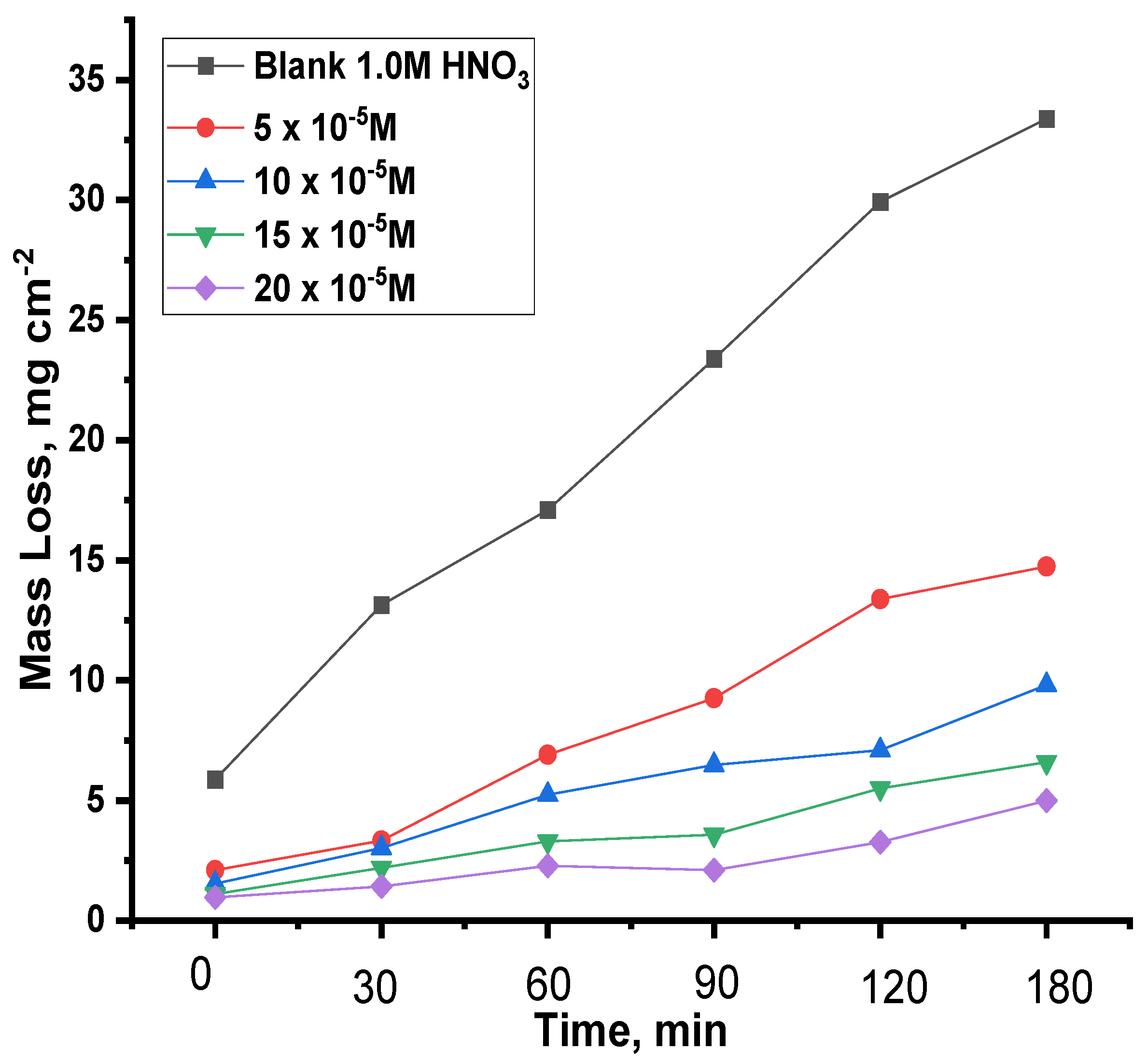
3.2.4. Mass Loss Test (ML)
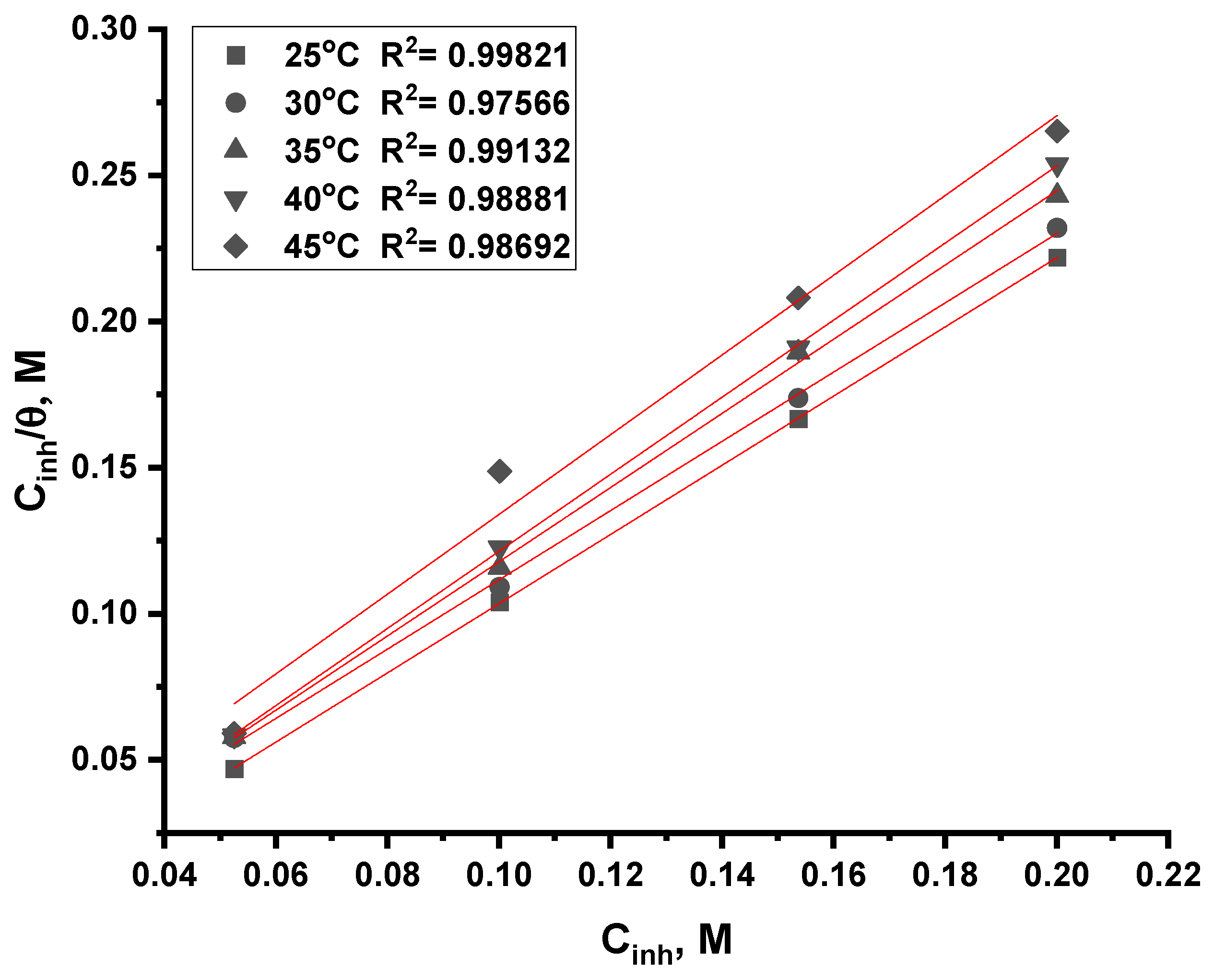
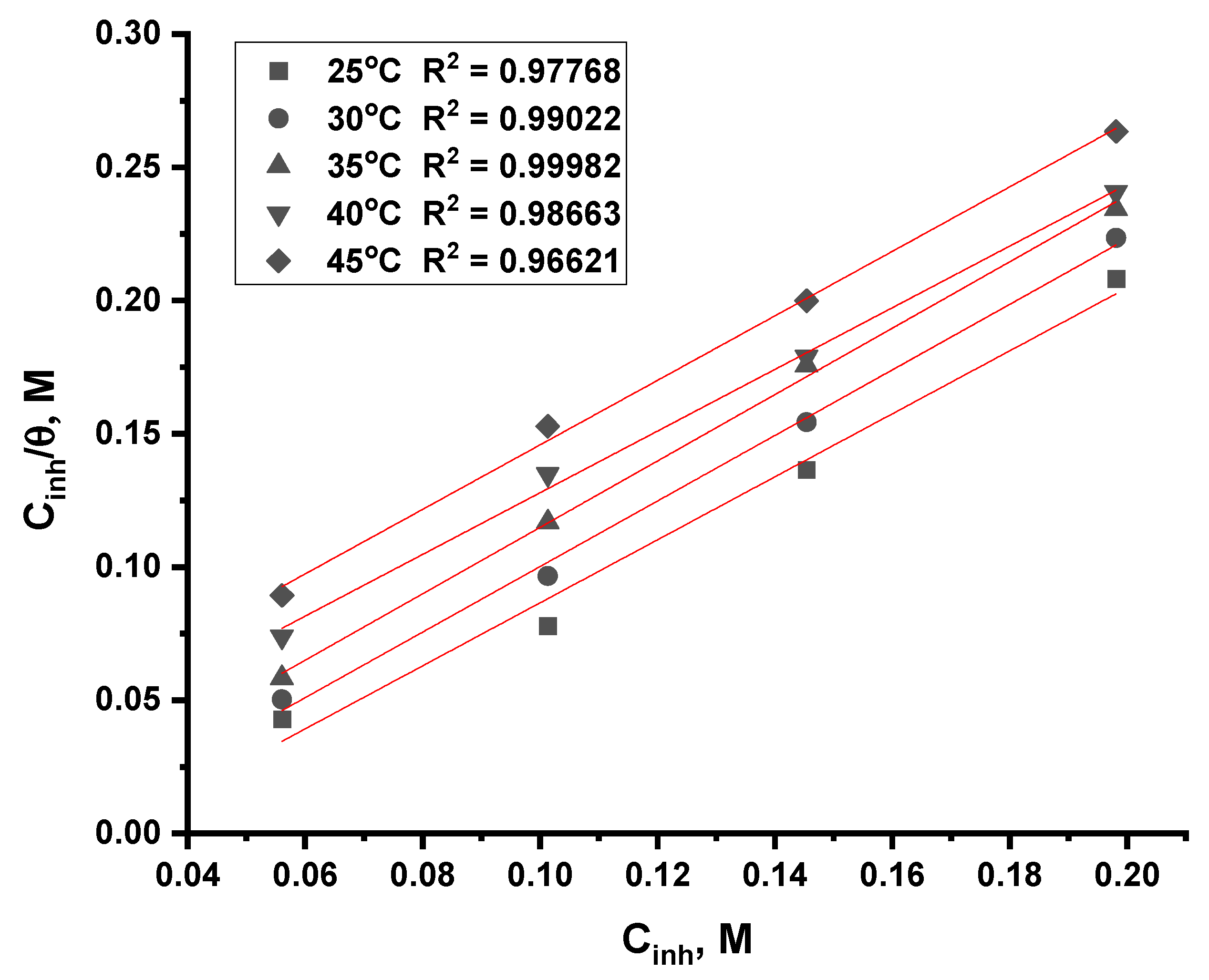
3.2.5. Adsorption Isotherm Analysis
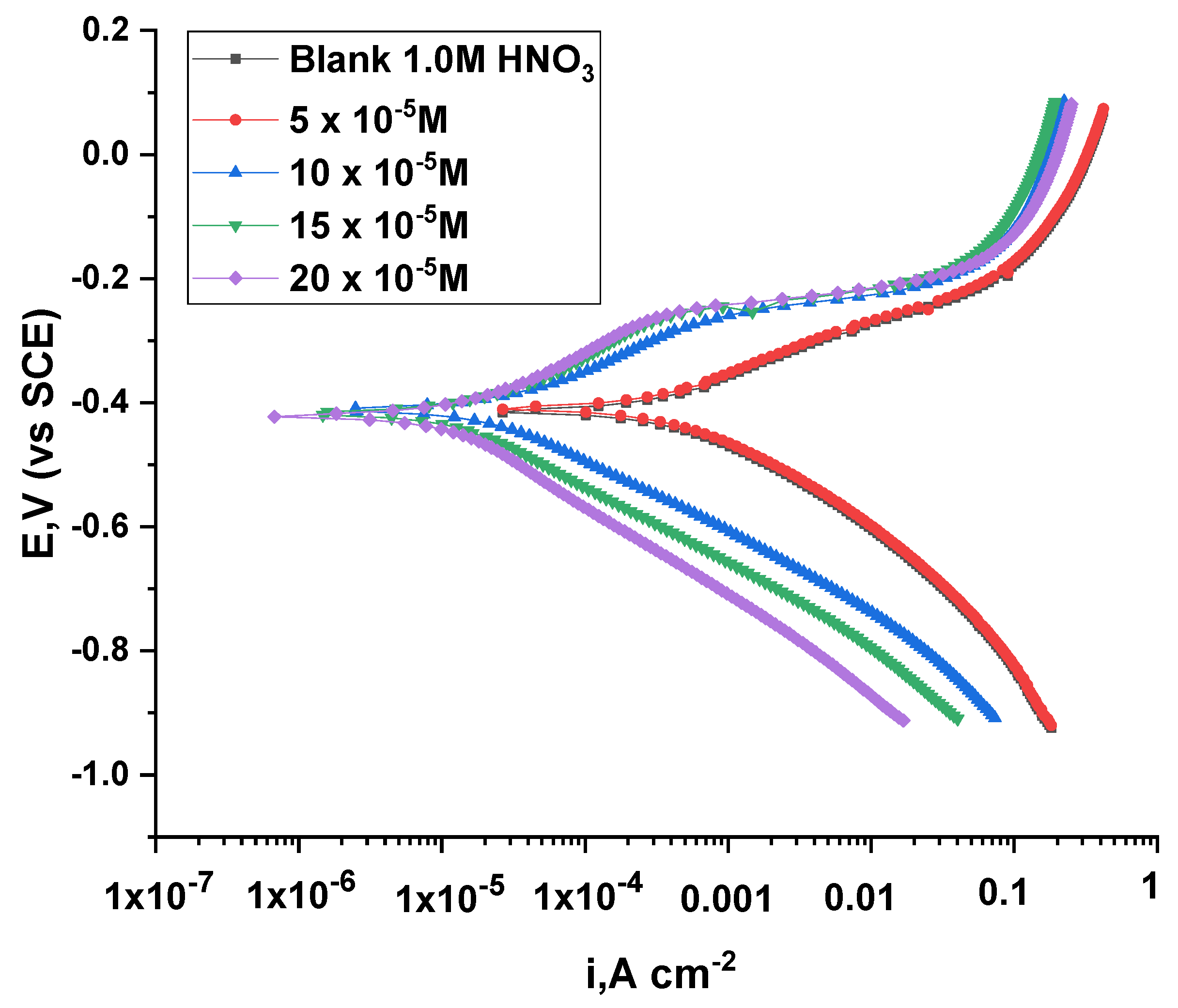
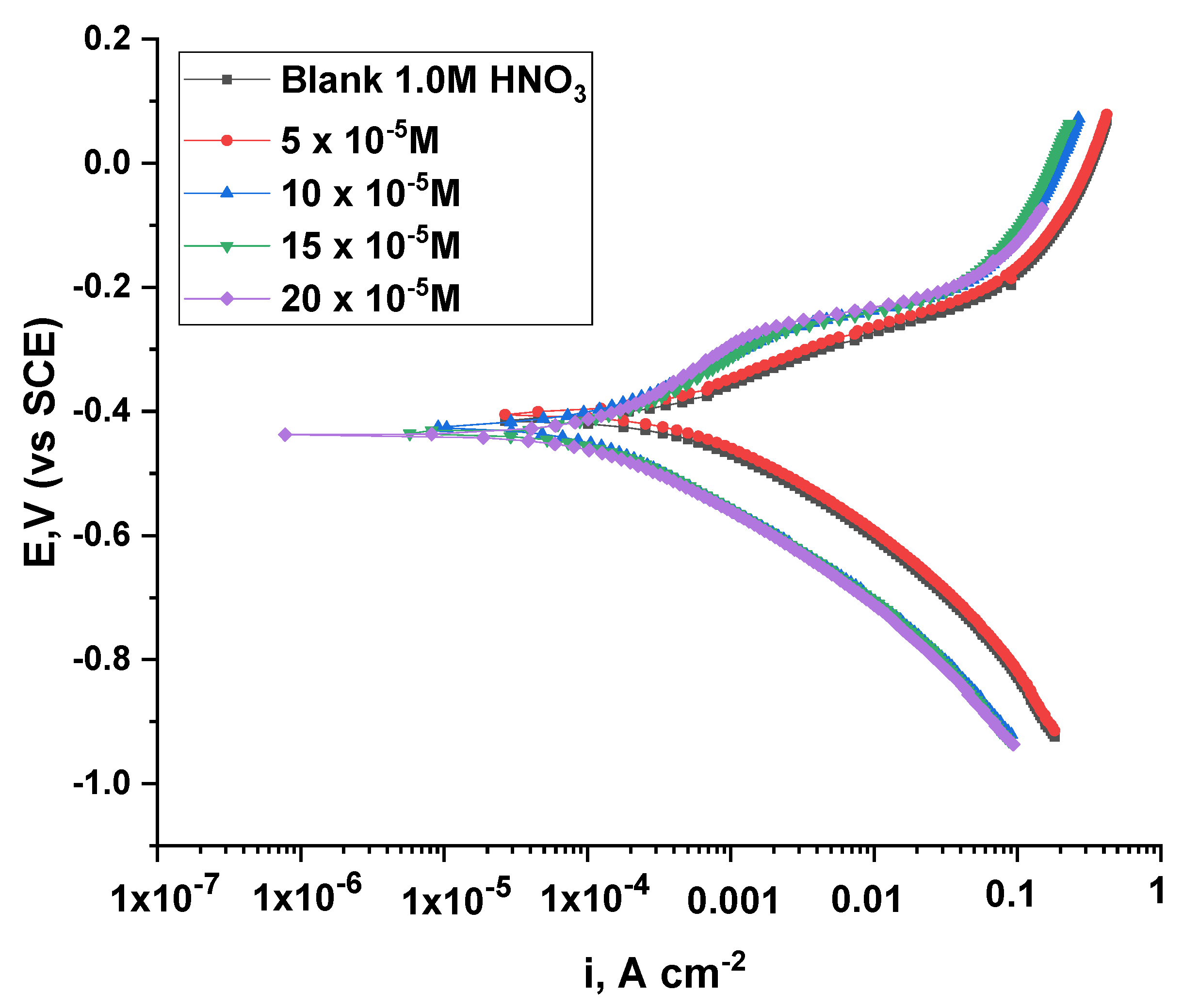
3.2.6. Potentiodynamic Polarization Test
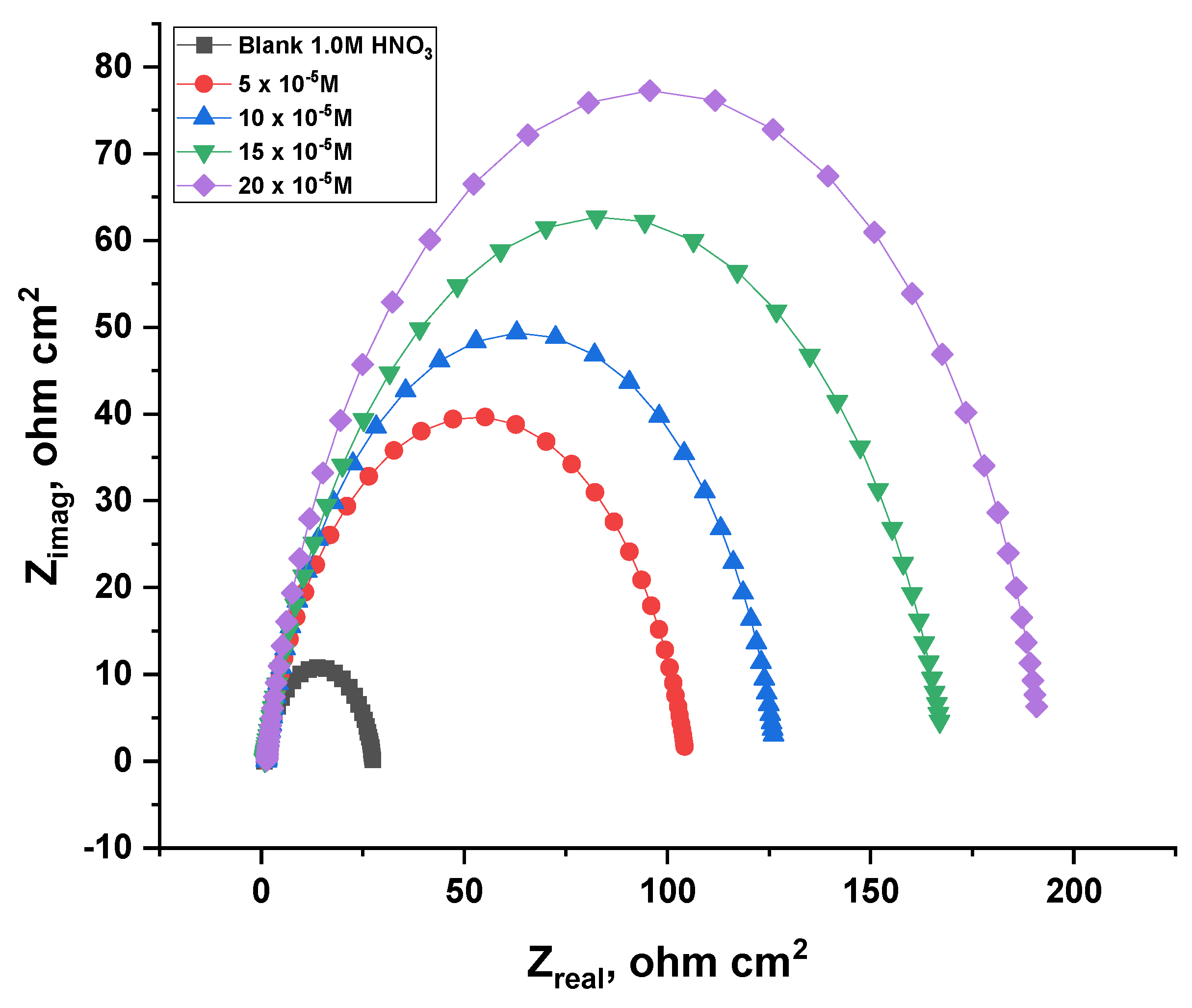
3.2.7. Electrochemical Impedance Spectroscopy (EIS) Tests
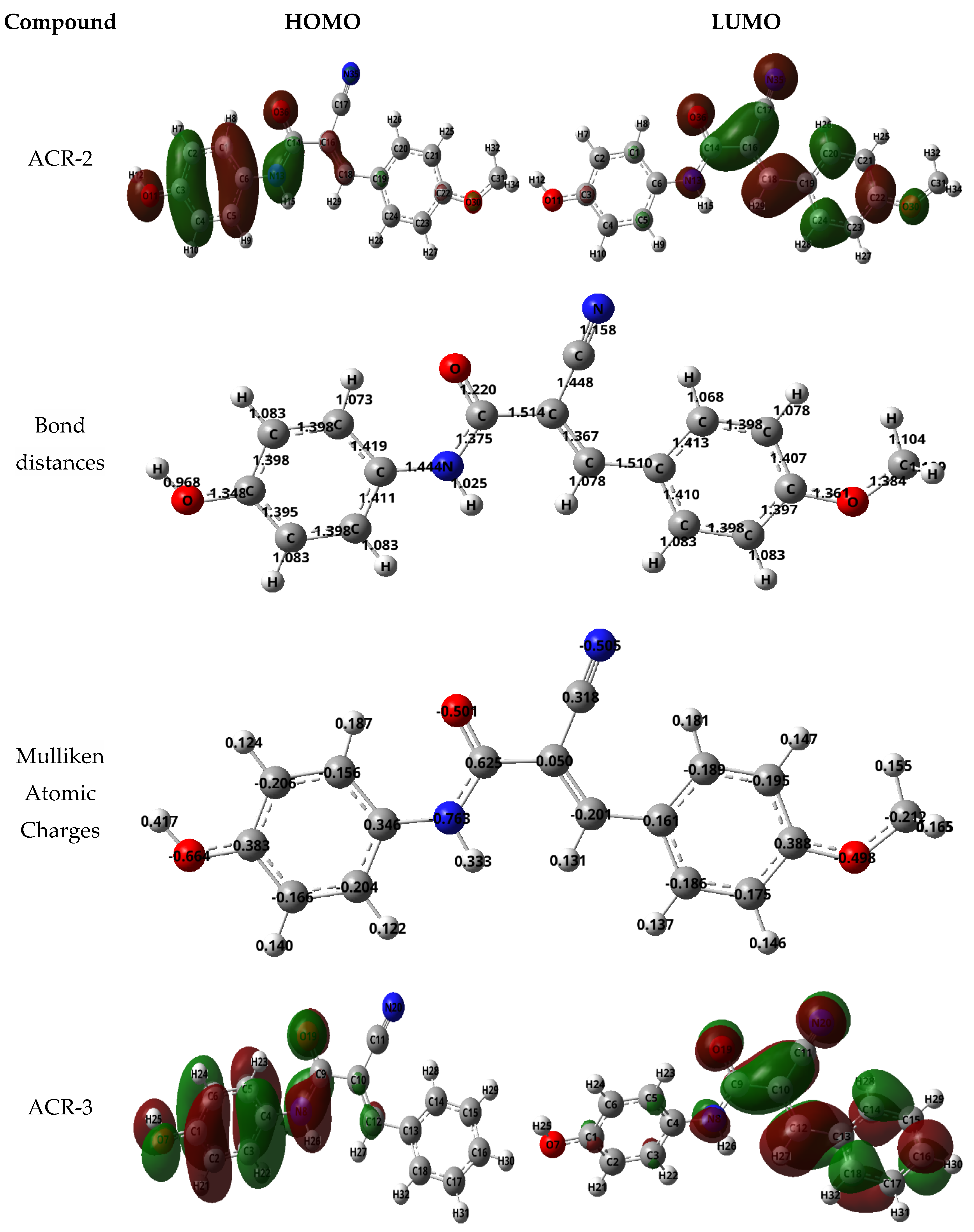
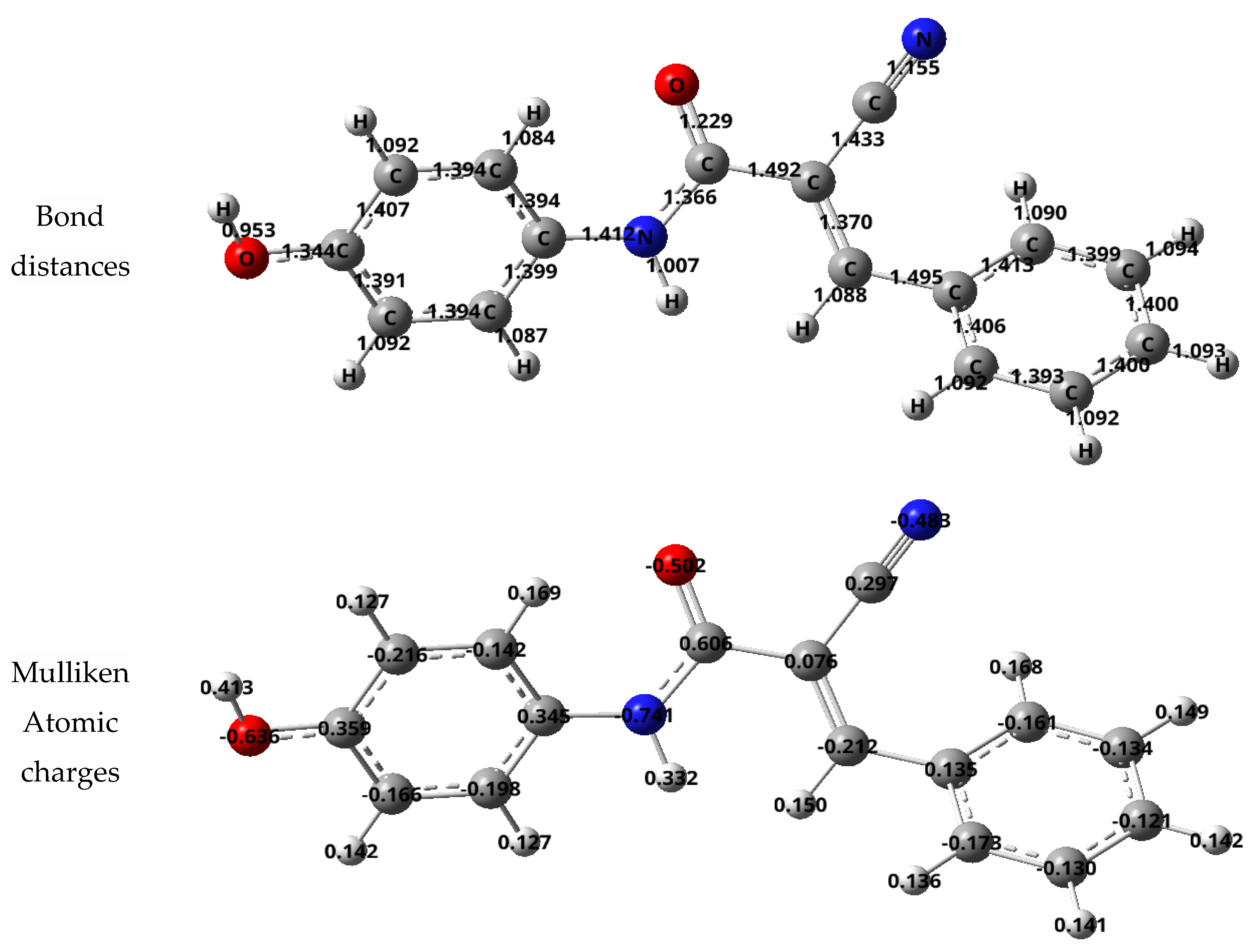
3.2.8. Molecular Modeling
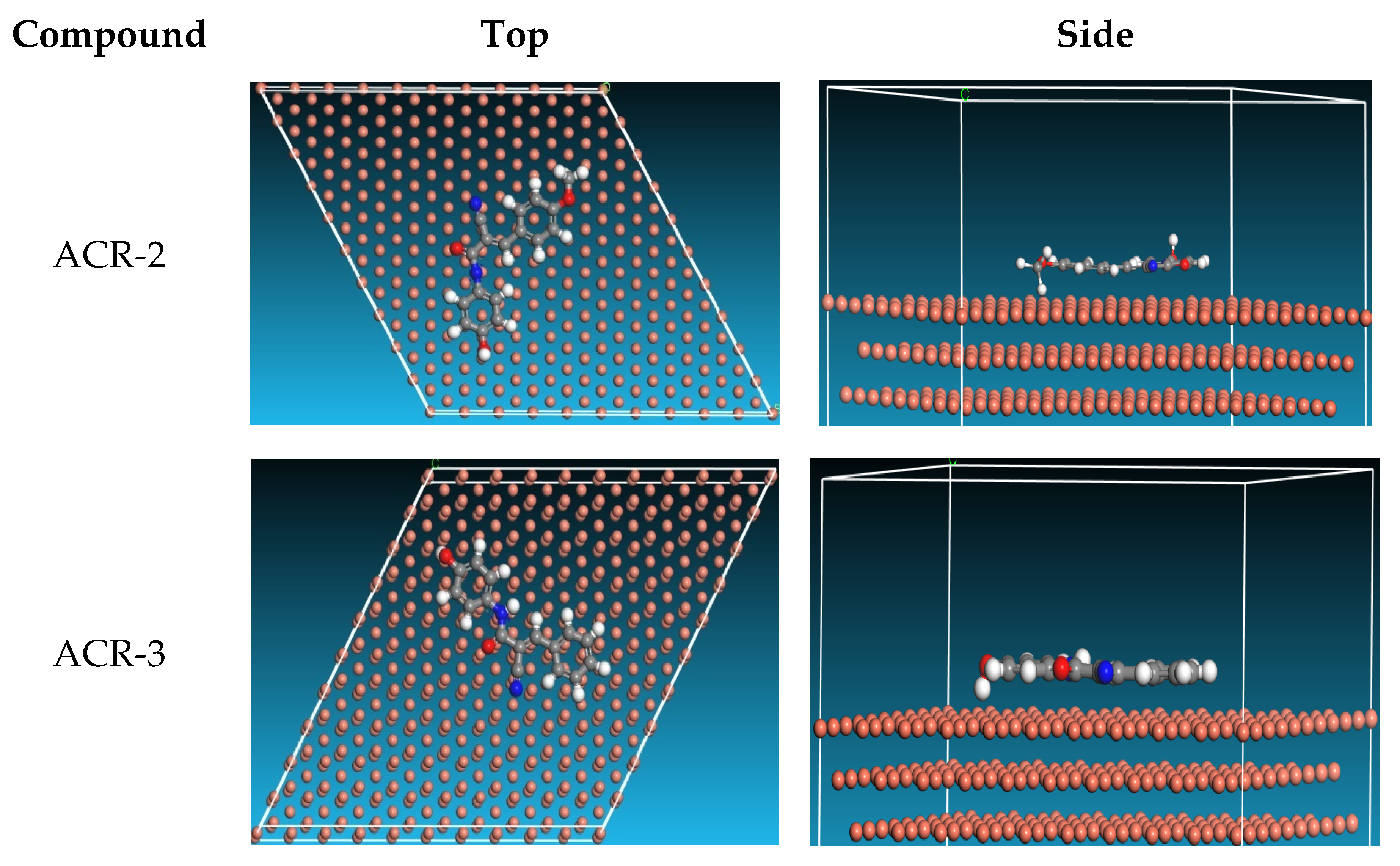
3.2.9. MD Simulation
3.2.10. Scanning Electron Microscope (SEM) Analysis
3.2.11. Mechanism of Inhibition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez, R.S.; da Silveira Rossi, R.A.; Vieira, L.G.M. Economic and financial consequences of process accidents in Brazil: Multiple case studies. Eng. Fail. Anal. 2022, 132, 105934. [Google Scholar] [CrossRef]
- Singh, A.K. Microbial induced corrosion and industrial economy. In Microbially Induced Corrosion and Its Mitigation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 45–55. [Google Scholar]
- Szymczak-Graczyk, A.; Laks, I.; Ksit, B.; Ratajczak, M. Analysis of the impact of omitted accidental actions and the method of land use on the number of construction disasters (a case study of Poland). Sustainability 2021, 13, 618. [Google Scholar] [CrossRef]
- Abdel-Karim, A.M.; El-Shamy, A.M. A review on green corrosion inhibitors for protection of archeological metal artifacts. J. Bio-Tribo-Corros. 2022, 8, 35. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kadhim, A.; Al-Adili, A.; Tawfiq, Z. Limits and developments in ecofriendly corrosion inhibitors of mild steel: A critical review. Part 1: Coumarins. Int. J. Corros. Scale Inhib. 2021, 10, 1355–1384. [Google Scholar]
- Kaban, A.P.S.; Mayangsari, W.; Anwar, M.S.; Maksum, A.; Riastuti, R.; Aditiyawarman, T.; Soedarsono, J.W. Experimental and modelling waste rice husk ash as a novel green corrosion inhibitor under acidic environment. Mater. Today Proc. 2022, 62, 4225–4234. [Google Scholar] [CrossRef]
- Kadhim, A.; Betti, N.; Al-Bahrani, H.; Al-Ghezi, M.; Gaaz, T.; Kadhum, A.; Alamiery, A. A mini review on corrosion, inhibitors and mechanism types of mild steel inhibition in an acidic environment. Int. J. Corros. Scale Inhib. 2021, 10, 861–884. [Google Scholar]
- Kadhim, A.; Al-Amiery, A.; Alazawi, R.; Al-Ghezi, M.; Abass, R. Corrosion inhibitors. A review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar]
- Hossain, N.; Asaduzzaman Chowdhury, M.; Kchaou, M. An overview of green corrosion inhibitors for sustainable and environment friendly industrial development. J. Adhes. Sci. Technol. 2021, 35, 673–690. [Google Scholar] [CrossRef]
- Fadda, A.A.; Ghanem, R.A.; Gaffer, H.E.; Waly, M.M.; Tawfik, E.H. Synthesis, Antimicrobial Evaluation, and Molecular Docking of New Azole, Azine, Thiazole, and Chromene Derivatives. Polycycl. Aromat. Compd. 2022, 42, 1–21. [Google Scholar] [CrossRef]
- El Basiony, N.; Tawfik, E.H.; El-raouf, M.A.; Fadda, A.A.; Waly, M.M. Synthesis, characterization, theoretical calculations (DFT and MC), and experimental of different substituted pyridine derivatives as corrosion mitigation for X-65 steel corrosion in 1 M HCl. J. Mol. Struct. 2021, 1231, 129999. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Jawad, Q.A.; Zinad, D.S.; Dawood Salim, R.; A Al-Amiery, A.; Sumer Gaaz, T.; Takriff, M.S.; Kadhum, A.A.H. Synthesis, characterization, and corrosion inhibition potential of novel thiosemicarbazone on mild steel in sulfuric acid environment. Coatings 2019, 9, 729. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; El-Azabawy, O.E.; Ismail, H.; Hegazy, M. Novel dispersed magnetite core–shell nanogel polymers as corrosion inhibitors for carbon steel in acidic medium. Corros. Sci. 2011, 53, 1680–1689. [Google Scholar] [CrossRef]
- Fazayel, A.; Khorasani, M.; Sarabi, A. The effect of functionalized polycarboxylate structures as corrosion inhibitors in a simulated concrete pore solution. Appl. Surf. Sci. 2018, 441, 895–913. [Google Scholar] [CrossRef]
- Das, T.K.; Poater, A. Review on the use of heavy metal deposits from water treatment waste towards catalytic chemical syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef]
- Rahmani, M.H.; Naderi, R.; Mahdavian, M. Pulse-reverse electrodeposition of a conversion coating based on zinc cation and 3-nitrobenzoic acid on carbon steel to enhance adhesion and protective function of epoxy coating. Prog. Org. Coat. 2022, 172, 107124. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, B.; Zhang, X.; Guo, L.; Zhang, S. Synthesized carbon dots with high N and S content as excellent corrosion inhibitors for copper in sulfuric acid solution. J. Mol. Liq. 2021, 338, 116702. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.; Qurashi, A. Recent trends in environmentally sustainable Sweet corrosion inhibitors. J. Mol. Liq. 2021, 326, 115117. [Google Scholar] [CrossRef]
- Fouda, A.; Abd El-Maksoud, S.; Belal, A.; El-Hossiany, A.; Ibrahium, A. Effectiveness of some organic compounds as corrosion inhibitors for stainless steel 201 in 1 M HCl: Experimental and theoretical studies. Int. J. Electrochem. Sci. 2018, 13, 9826–9846. [Google Scholar] [CrossRef]
- Fouda, A.; Wahba, A.; Eissa, M. Aluminum corrosion prevention in 1.0 M HCl solution by cystosiera myrica extract: An experimental and biological study. J. Indian Chem. Soc. 2022, 99, 100619. [Google Scholar] [CrossRef]
- Xue, Y.; Pan, D.; Zuo, F.; Xiao, S.; Li, X.; Lou, F.; Li, M.; Ouyang, Y. Improved performance of self-reactivated Pt–ThO2/C catalysts in a direct ethanol fuel cell. RSC Adv. 2022, 12, 17012–17019. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, K.; Chouchane, T.; Selatnia, I.; Sid, A.; Mosset, P. Evaluation of the effect of Tetramethylammonium hydroxide on the corrosion inhibition of A9M steel in industrial water: An experimental, morphological and MD simulation insights. Chem. Data Collect. 2020, 28, 100391. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Wu, D.; Wang, S.; Yu, R.; Zhang, F.; Kaya, S. Electrochemical and computational studies on the corrosion inhibition of mild steel by 1-hexadecyl-3-methylimidazolium bromide in HCl medium. Int. J. Electrochem. Sci. 2020, 15, 1893–1903. [Google Scholar] [CrossRef]
- Azad, I.; Akhter, Y.; Khan, T.; Azad, M.I.; Chandra, S.; Singh, P.; Kumar, D.; Nasibullah, M. Synthesis, quantum chemical study, AIM simulation, in silico ADMET profile analysis, molecular docking and antioxidant activity assessment of aminofuran derivatives. J. Mol. Struct. 2020, 1203, 127285. [Google Scholar] [CrossRef]
- Mumit, M.A.; Pal, T.K.; Alam, M.A.; Islam, M.A.-A.-A.-A.; Paul, S.; Sheikh, M.C. DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2,4,5-trimethoxyphenylmethylene) hydrazinecarbodithioate. J. Mol. Struct. 2020, 1220, 128715. [Google Scholar] [CrossRef]
- Arshad, M.N.; Shafiq, I.; Khalid, M.; Asiri, A.M. Exploration of the Intriguing Photovoltaic Behavior for Fused Indacenodithiophene-Based A–D–A Conjugated Systems: A DFT Model Study. ACS Omega 2022, 7, 11606–11617. [Google Scholar] [CrossRef]
- Zhou, J.; Niu, X.; Cui, Y.; Wang, Z.; Wang, J.; Wang, R. Study on the film forming mechanism, corrosion inhibition effect and synergistic action of two different inhibitors on copper surface chemical mechanical polishing for GLSI. Appl. Surf. Sci. 2020, 505, 144507. [Google Scholar] [CrossRef]
- Ayeni, F.; Madugu, I.; Sukop, P.; Ihom, A.; Alabi, O.; Okara, R.; Abdulwahab, M. Effect of aqueous extracts of bitter leaf powder on the corrosion inhibition of Al-Si alloy in 0.5 M caustic soda solution. J. Miner. Mater. Charact. Eng. 2012, 11, 667–670. [Google Scholar] [CrossRef]
- Sayed, E.M.; Hassanien, R.; Farhan, N.; Aly, H.F.; Mahmoud, K.; Bakhite, E.A. Nitrophenyl-group-containing Heterocycles. Part I. Synthesis, Characterization, Anticancer Activity and Antioxidant Properties of Some New 5,6,7,8-tetrahydro-isoquinolines Bearing 3 (4)-nitrophenyl Group. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- Jafari, H.; Rezaeivala, M.; Mokhtarian, N.; Berisha, A.; Ameri, E. Corrosion Inhibition of Carbon Steel in 0.5 M H2SO4 by New Reduced Schiff Base Ligand. J. Bio-Tribo-Corros. 2022, 8, 81. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Chauhan, D.S.; Quraishi, M.; Kaya, S. Anti-corrosion investigation of pyrimidine derivatives as green and sustainable corrosion inhibitor for N80 steel in highly corrosive environment: Experimental and AFM/XPS study. Sustain. Chem. Pharm. 2020, 16, 100257. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, Y. Corrosion inhibition of aluminum in hydrochloric acid solution by alkylimidazolium ionic liquids. Mater. Chem. Phys. 2010, 119, 57–64. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.; Peikari, M.; Shadizadeh, S.; Hashemi, S. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Fouda, A.; Wahba, A.; Al-Bonayan, A.M. Corrosion Inhibition of Stainless Steel in 1.0 M Hydrochloric Acid Solution Using Novel Nonionic Surfactant: Electrochemical and Density Functional Theory/B3LYP/6-31G* Analysis. Surf. Eng. Appl. Electrochem. 2021, 57, 689–702. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Damej, M.; Lgaz, H.; Salghi, R.; Ali, I.H.; Benmessaoud, M.; Masroor, S.; Chung, I.-M. Bolaamphiphile-class surfactants as corrosion inhibitor model compounds against acid corrosion of mild steel. J. Mol. Liq. 2020, 309, 113070. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Koudad, M.; Chelfi, T.; Erkan, S.; Azzouzi, M.; Aouniti, A.; Savaş, K.; Kaddouri, M.; Benchat, N.; Oussaid, A. Experimental and theoretical study of new Schiff bases based on imidazo (1, 2-a) pyridine as corrosion inhibitor of mild steel in 1 M HCl. J. Mol. Struct. 2021, 1226, 129372. [Google Scholar] [CrossRef]
- Council, N.R. The Limits of Organic Life in Planetary Systems; The National Academy of Press: Washington, DC, USA, 2019. [Google Scholar]
- Anadebe, V.C.; Chukwuike, V.I.; Selvaraj, V.; Pandikumar, A.; Barik, R.C. Sulfur-doped graphitic carbon nitride (Sg-C3N4) as an efficient corrosion inhibitor for X65 pipeline steel in CO2-saturated 3.5% NaCl solution: Electrochemical, XPS and Nanoindentation Studies. Process Saf. Environ. Prot. 2022, 164, 715–728. [Google Scholar] [CrossRef]
- Messikh, S.; Salhi, R.; Benali, O.; Ouici, H.; Gherraf, N. Synthesis and evaluation of 5-(Phenyl)-4H-1,2,4-triazole-3-thiol as Corrosion Inhibitor for Mild Steel in 0.5 M H2SO4 and its synergistic effect with potassium iodide. Inter. J. Chem. Biochem. Sci. 2020, 17, 14–38. [Google Scholar]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Zhao, D.; Xiao, C.; Subedi, B.; Shrestha, N.; Junda, M.M.; Wang, C.; Jiang, C.S.; Al-Jassim, M. Reducing saturation-current density to realize high-efficiency low-bandgap mixed tin–lead halide perovskite solar cells. Adv. Energy Mater. 2019, 9, 1803135. [Google Scholar] [CrossRef]
- Chaudhary, S.; Tak, R.K. Natural corrosion inhibition and adsorption characteristics of tribulus terrestris plant extract on aluminium in hydrochloric acid environment. Biointerface Res. Appl. Chem 2022, 12, 2603–2617. [Google Scholar]
- Rajamohan, N.; Al Shibli, F.S.Z.S.; Rajasimman, M.; Vasseghian, Y. Eco-friendly biomass from Ziziphus spina-christi for protection of carbon steel in acidic conditions–Parameter effects and corrosion mechanism studies. Chemosphere 2022, 291, 132756. [Google Scholar] [CrossRef] [PubMed]
- El Faydy, M.; Touir, R.; Touhami, M.E.; Zarrouk, A.; Jama, C.; Lakhrissi, B.; Olasunkanmi, L.; Ebenso, E.; Bentiss, F. Corrosion inhibition performance of newly synthesized 5-alkoxymethyl-8-hydroxyquinoline derivatives for carbon steel in 1 M HCl solution: Experimental, DFT and Monte Carlo simulation studies. Phys. Chem. Chem. Phys. 2018, 20, 20167–20187. [Google Scholar] [CrossRef]
- Sayed, A.R.; El-Lateef, H.M.A. Thiocarbohydrazones based on adamantane and ferrocene as efficient corrosion Inhibitors for hydrochloric acid pickling of C-steel. Coatings 2020, 10, 1068. [Google Scholar] [CrossRef]
- Hadisaputra, S.; Purwoko, A.A.; Savalas, L.R.T.; Prasetyo, N.; Yuanita, E.; Hamdiani, S. Quantum chemical and Monte Carlo simulation studies on inhibition performance of caffeine and its derivatives against corrosion of copper. Coatings 2020, 10, 1086. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.; Obot, I.; El-Khaiary, M.I. Quantum chemical investigation and statistical analysis of the relationship between corrosion inhibition efficiency and molecular structure of xanthene and its derivatives on mild steel in sulphuric acid. J. Mol. Struct. 2011, 1002, 86–96. [Google Scholar] [CrossRef]
- Niamien, P.; Essy, F.; Trokourey, A.; Yapi, A.; Aka, H.; Diabate, D. Correlation between the molecular structure and the inhibiting effect of some benzimidazole derivatives. Mater. Chem. Phys. 2012, 136, 59–65. [Google Scholar] [CrossRef]
- Rodríguez-Valdez, L.M.; Martínez-Villafañe, A.; Glossman-Mitnik, D. Computational simulation of the molecular structure and properties of heterocyclic organic compounds with possible corrosion inhibition properties. J. Mol. Struct. Theochem. 2005, 713, 65–70. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Yan, S.; Zou, X.; Chen, S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 2017, 126, 295–304. [Google Scholar] [CrossRef]
- Kovačević, N.; Milošev, I.; Kokalj, A. The roles of mercapto, benzene, and methyl groups in the corrosion inhibition of imidazoles on copper: II. Inhibitor–copper bonding. Corros. Sci. 2015, 98, 457–470. [Google Scholar] [CrossRef]
- Odey, J.O.; Ubana, E.I.; Eko, I.J.; Jones, O.O. Synthesis, characterization and theoretical studies of the photovoltaic properties of novel bifunctional reactive disperse dyes based on aminothiazole derivatives. J. Mol. Struct. 2022, 1269, 133749. [Google Scholar] [CrossRef]
- Patra, C.; Gupta, R.; Bedadeep, D.; Narayanasamy, S. Surface treated acid-activated carbon for adsorption of anionic azo dyes from single and binary adsorptive systems: A detail insight. Environ. Pollut. 2020, 266, 115102. [Google Scholar] [CrossRef]
- Beniken, M.; Salim, R.; Ech–chihbi, E.; Sfaira, M.; Hammouti, B.; Touhami, M.E.; Mohsin, M.; Taleb, M. Adsorption behavior and corrosion inhibition mechanism of a polyacrylamide on C–steel in 0.5 M H2SO4: Electrochemical assessments and molecular dynamic simulation. J. Mol. Liq. 2022, 348, 118022. [Google Scholar] [CrossRef]


| Element | Sn | Fe | Ni | Pb | As | Cu |
|---|---|---|---|---|---|---|
| Weight % | 1.44 | 0.89 | 0.27 | 2.5 | 37.5 | 56.8 |
| ACR-2 × 10−5 M | 5 | 10 | 15 | 20 |
|---|---|---|---|---|
| θ | 0.7694 | 0.7898 | 0.8079 | 0.8350 |
| 76.94 | 78.98 | 80.79 | 83.50 |
| ACR-3 × 10−5 M | 5 | 10 | 15 | 20 |
|---|---|---|---|---|
| θ | 0.271 | 0.448 | 0.626 | 0.72 |
| 27.1 | 44.8 | 62.6 | 72.0 |
| Temp., °C | Kads | logkads | ΔG | ΔH | ΔS |
|---|---|---|---|---|---|
| 25 | −1.15066 | 0.0609 | −10.3004 | −0.008848 | 0.034595 |
| 30 | −1.17425 | 0.0698 | −10.5244 | 0.034734 | |
| 35 | −1.16331 | 0.0657 | −10.674 | 0.034656 | |
| 40 | −1.1763 | 0.0705 | −10.8762 | 0.034748 | |
| 45 | −1.45069 | 0.1615 | −11.604 | 0.03649 |
| Temp., °C | Kads | logkads | ΔG | ΔH | ΔS |
|---|---|---|---|---|---|
| 25 | −1315.1 | 3.1189 | −27.7486 | −0.13403 | 0.092666 |
| 30 | −232.558 | 2.3665 | −23.8491 | 0.07871 | |
| 35 | −1996.57 | 3.3002 | −29.749 | 0.096588 | |
| 40 | −925.926 | 2.9665 | −28.232 | 0.090198 | |
| 45 | −34.4708 | 1.5374 | −19.9815 | 0.062835 |
| Comp. | Conc. × 10−5 M | icorr, μA cm−2 | −Ecorr, mV vs. SCE | βa mV dec−1 | βc mV dec−1 | C.R mpy | θ | |
|---|---|---|---|---|---|---|---|---|
| Blank | 0.0 | 494.0 | 413 | 123 | 181 | 131 | - | - |
| ACR-2 | 5 | 108.0 | 423 | 116 | 180 | 28 | 0.781 | 78.1 |
| 10 | 97.0 | 421 | 123 | 191 | 20 | 0.804 | 80.4 | |
| 15 | 71 | 428 | 122 | 186 | 13 | 0.856 | 85.6 | |
| 20 | 57.0 | 430 | 127 | 179 | 8 | 0.885 | 88.5 | |
| ACR-3 | 5 | 115.0 | 423 | 125 | 190 | 47 | 0.767 | 76.7 |
| 10 | 78 | 421 | 119 | 183 | 31 | 0.842 | 84.2 | |
| 15 | 62.0 | 423 | 120 | 189 | 28 | 0.874 | 87.4 | |
| 20 | 55.0 | 418 | 121 | 181 | 23 | 0.889 | 88.9 |
| Inhibitor A, M | Cdl, µF cm−2 | Rp, Ω cm2 | θ | |
|---|---|---|---|---|
| Blank | 165 | 27 | - | - |
| 5 | 155 | 104 | 0.744 | 74.4 |
| 10 | 144 | 126 | 0.788 | 78.8 |
| 15 | 125 | 168 | 0.841 | 84.1 |
| 20 | 106 | 192 | 0.861 | 86.1 |
| Inhibitor B, M | Cdl, µF cm−2 | Rct, Ω cm2 | θ | |
|---|---|---|---|---|
| Blank | 165 | 27 | - | - |
| 5 | 95 | 150 | 0.719 | 71.9 |
| 10 | 115 | 146 | 0.768 | 76.8 |
| 15 | 150 | 140 | 0.822 | 82.2 |
| 20 | 172 | 135 | 0.845 | 84.5 |
| Structure | Total Energy | Adsorption Energy | Rigid Adsorption Energy | Deformation Energy | Eads: Compound |
|---|---|---|---|---|---|
| Cu (1 1 1) −1(ACR-2) | −154.37 | −94.72 | −96.49 | 1.770 | −94.72 |
| Cu (1 1 1) −1(ACR-3) | −164.41 | −83.73 | −87.94 | 4.215 | −83.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Rayyan, A.; Al Jahdaly, B.A.; AlSalem, H.S.; Alhadhrami, N.A.; Hajri, A.K.; Bukhari, A.A.H.; Waly, M.M.; Salem, A.M. A Study of the Synthesis and Characterization of New Acrylamide Derivatives for Use as Corrosion Inhibitors in Nitric Acid Solutions of Copper. Nanomaterials 2022, 12, 3685. https://doi.org/10.3390/nano12203685
Abu-Rayyan A, Al Jahdaly BA, AlSalem HS, Alhadhrami NA, Hajri AK, Bukhari AAH, Waly MM, Salem AM. A Study of the Synthesis and Characterization of New Acrylamide Derivatives for Use as Corrosion Inhibitors in Nitric Acid Solutions of Copper. Nanomaterials. 2022; 12(20):3685. https://doi.org/10.3390/nano12203685
Chicago/Turabian StyleAbu-Rayyan, Ahmed, Badreah Ali Al Jahdaly, Huda S. AlSalem, Nahlah A. Alhadhrami, Amira K. Hajri, Abeer Abdulaziz H. Bukhari, Mohamed M. Waly, and Aya M. Salem. 2022. "A Study of the Synthesis and Characterization of New Acrylamide Derivatives for Use as Corrosion Inhibitors in Nitric Acid Solutions of Copper" Nanomaterials 12, no. 20: 3685. https://doi.org/10.3390/nano12203685





