Kelulut Honey Improves Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profiles and Ovarian Histomorphology in Letrozole-Induced Polycystic Ovary Syndrome Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Sample
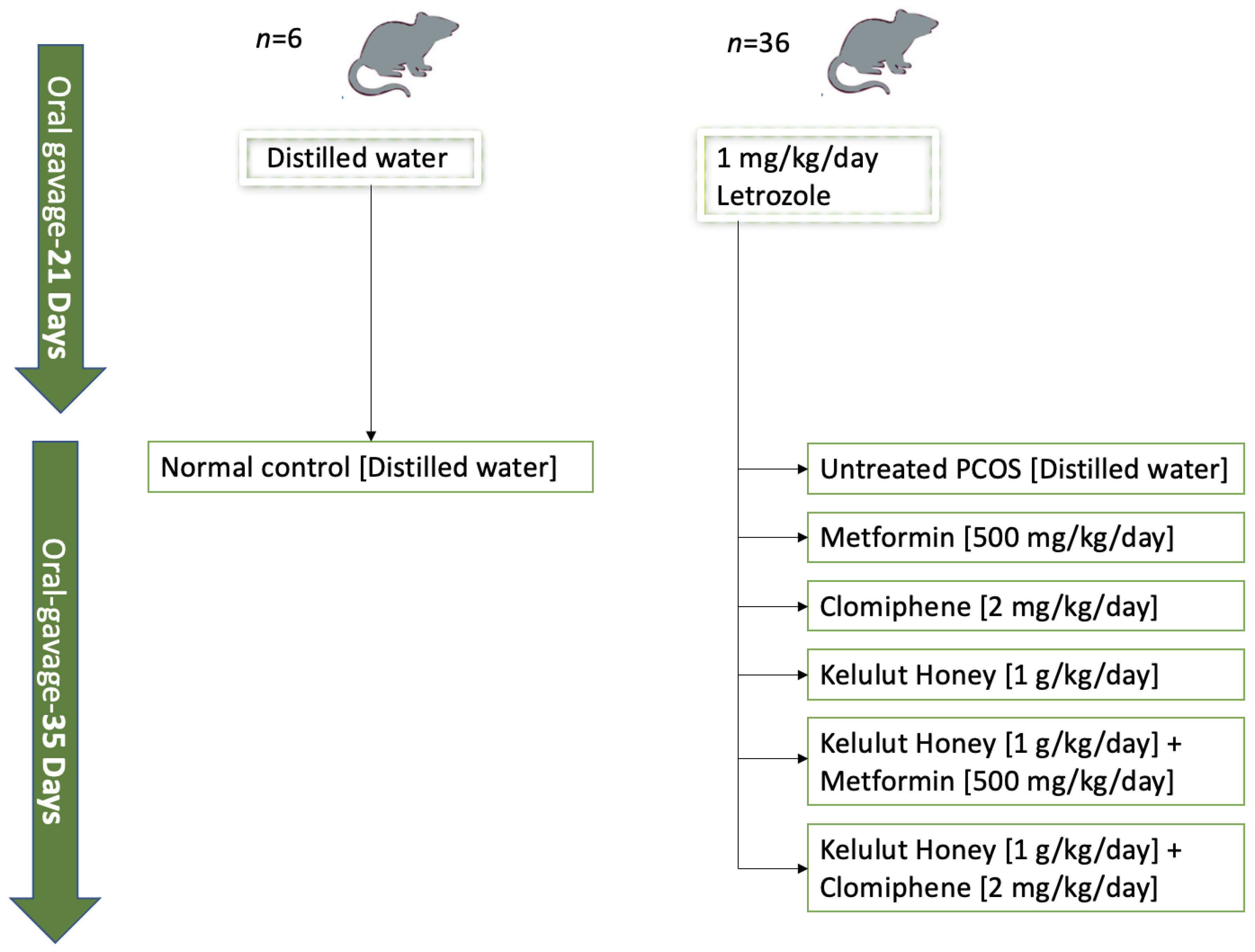
2.2. Animal Preparation
2.3. Animal Treatments
2.4. Histomorphological Analysis of the Ovaries
2.5. Protein Distribution Analysis by Immunohistochemistry (IHC)
2.6. mRNA Expression Analysis by Real-Time Polymerase Chain Reaction (qPCR)
2.7. Statistical Analysis
3. Results
3.1. Effects of KH on the Ovarian Histomorphological Changes
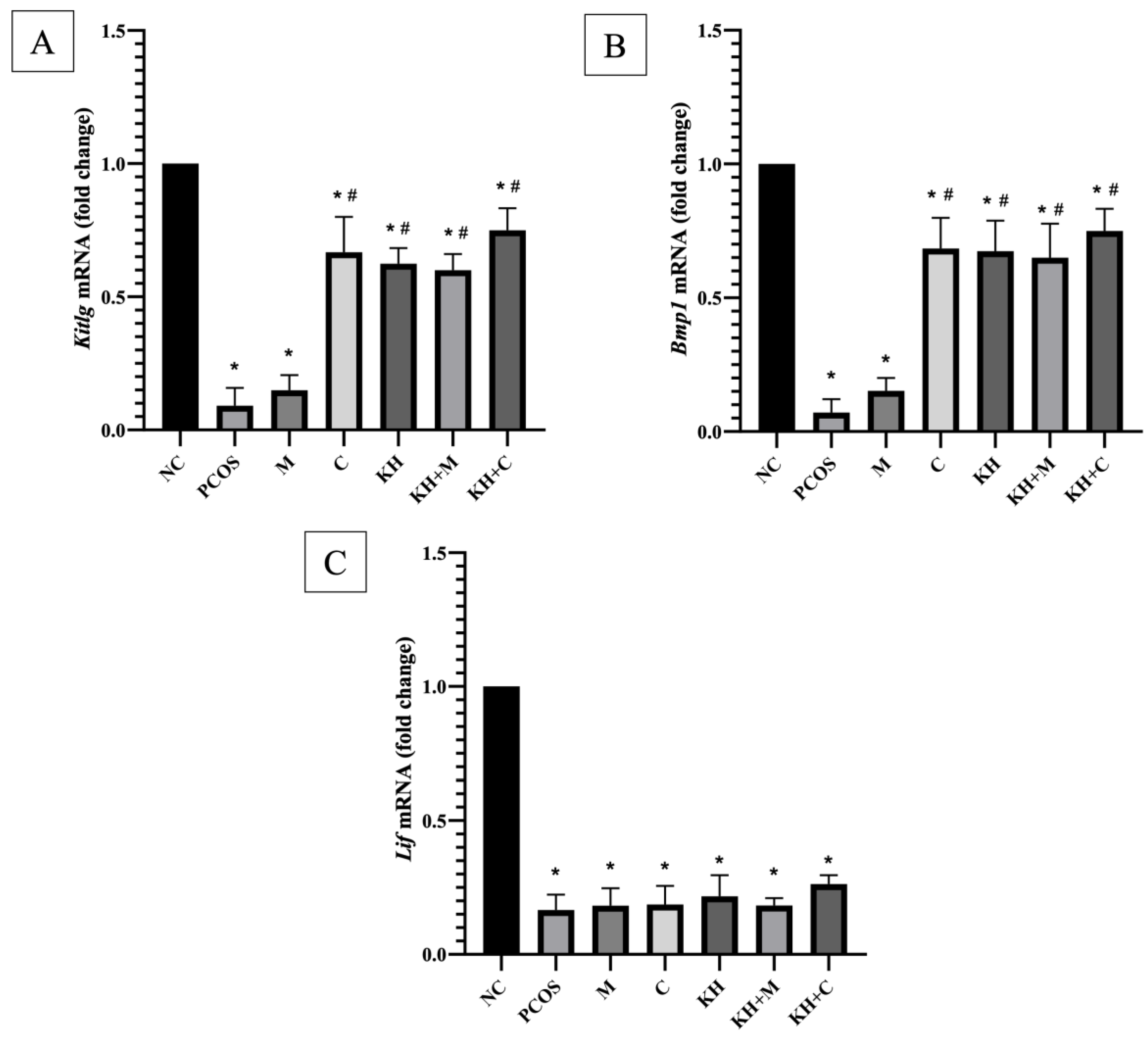
3.2. Effects of KH on Folliculogenesis-Related Genes
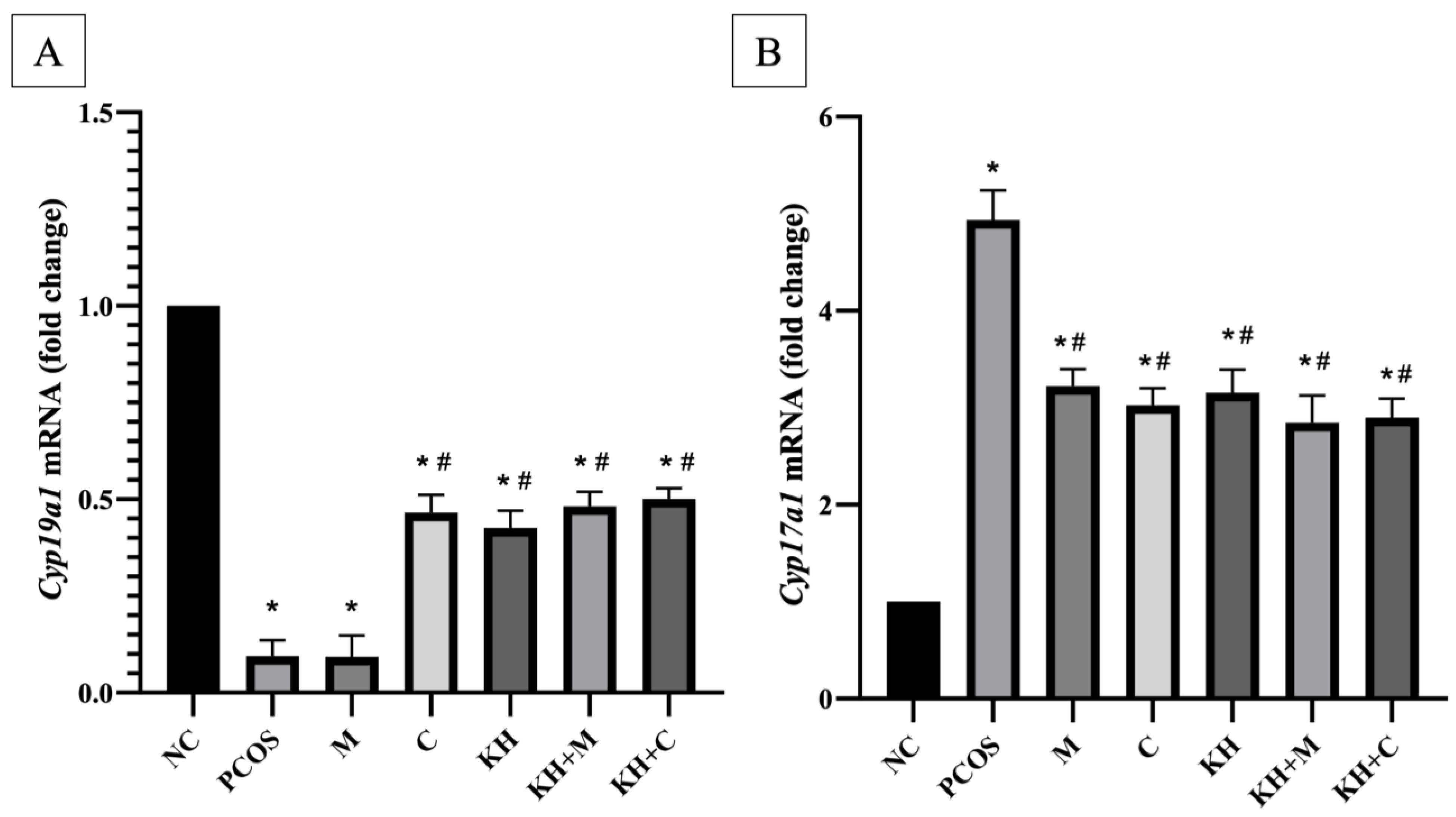
3.3. Effects of KH on Cyp17a1 and Cyp19a1 mRNA Expression
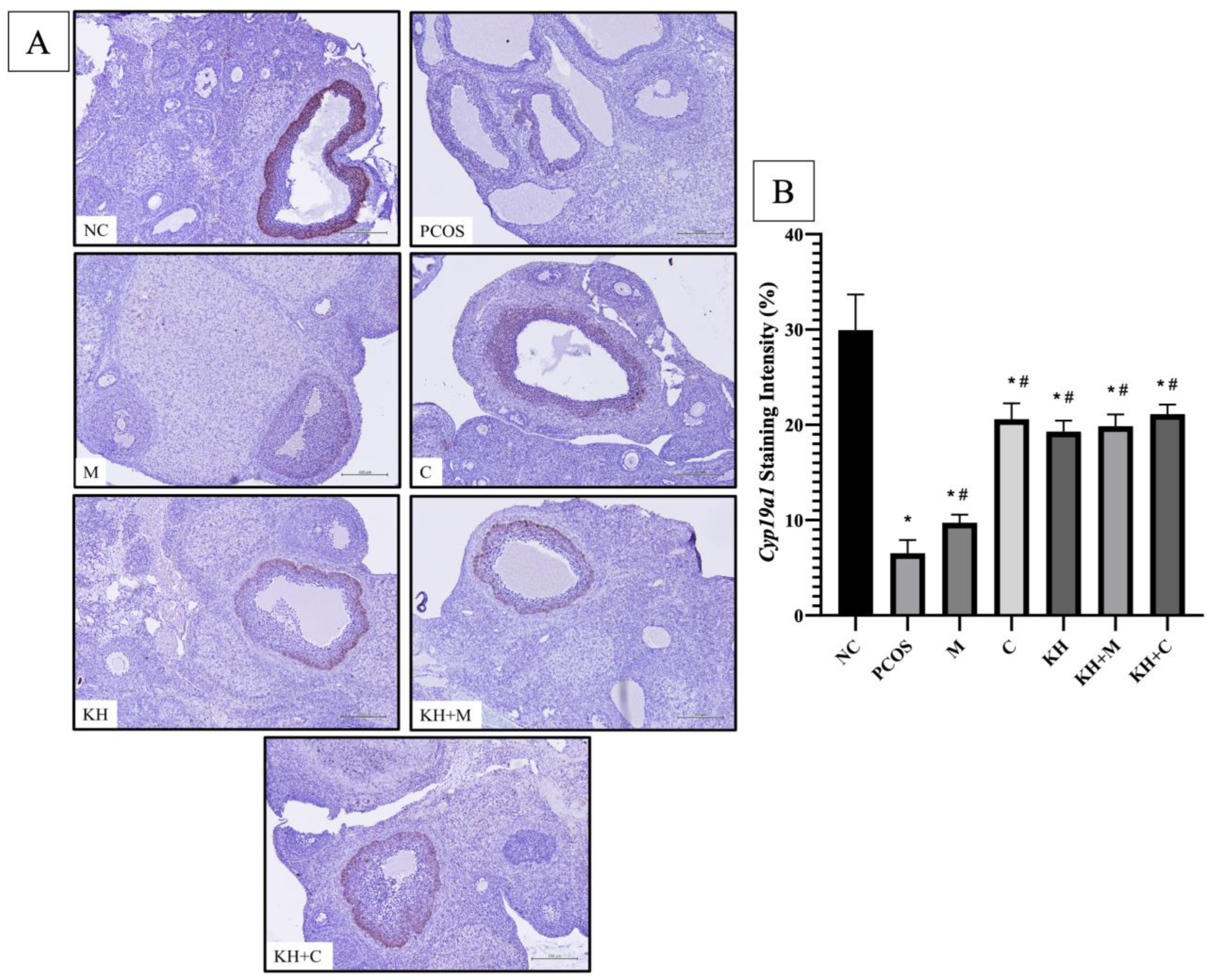
3.4. Effects of KH on Cyp17a1 and Cyp19a1 Protein Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270. [Google Scholar] [CrossRef]
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Dennett, C.C.; Simon, J. The role of polycystic ovary syndrome in reproductive and metabolic health: Overview and approaches for treatment. Diabetes Spectr. 2015, 28, 116–120. [Google Scholar] [CrossRef]
- Kyrou, I.; Karteris, E.; Robbins, T.; Chatha, K.; Drenos, F.; Randeva, H.S. Polycystic ovary syndrome (PCOS) and COVID-19: An overlooked female patient population at potentially higher risk during the COVID-19 pandemic. BMC Med. 2020, 18, 220. [Google Scholar] [CrossRef]
- Rosenfield, R.L. Current concepts of polycystic ovary syndrome pathogenesis. Curr. Opin. Pediatr. 2020, 32, 698–706. [Google Scholar] [CrossRef]
- Franks, S.; Hardy, K. Androgen Action in the Ovary. Front. Endocrinol. 2018, 9, 452. [Google Scholar] [CrossRef]
- Magoffin, D.A.; Agarwal, S.K.; Jakimiuk, A.J. Suppression of Aromatase Activity in Polycystic Ovary Syndrome. In Polycystic Ovary Syndrome; Chang, R.J., Ed.; Springer: New York, NY, USA, 1996; pp. 208–222. [Google Scholar]
- Yang, F.; Ruan, Y.-C.; Yang, Y.-j.; Wang, K.; Liang, S.-s.; Han, Y.-b.; Teng, X.-M.; Yang, J.-Z. Follicular hyperandrogenism downregulates aromatase in luteinized granulosa cells in polycystic ovary syndrome women. Reproduction 2015, 150, 289–296. [Google Scholar] [CrossRef]
- Ashraf, S.; Nabi, M.; Rasool, S.u.A.; Rashid, F.; Amin, S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: A review. Egypt. J. Med. Hum. Genet. 2019, 20, 25. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome: Pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, e3. [Google Scholar] [CrossRef]
- Nelson, V.L.; Legro, R.S.; Strauss, J.F., 3rd; McAllister, J.M. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol. Endocrinol. 1999, 13, 946–957. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Lin, H. Estrogen disorders: Interpreting the abnormal regulation of aromatase in granulosa cells (Review). Int. J. Mol. Med. 2021, 47, 73. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Tan, Y.; Xia, D.; Xia, Y.; Cao, Y.; Wang, W.; Wu, X.; Wang, H.; Yi, L.; et al. The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 11. [Google Scholar] [CrossRef]
- Panghiyangani, R.; Soeharso, P.; Andrijono; Suryandari, D.A.; Wiweko, B.; Kurniati, M.; Pujianto, D.A. CYP19A1 Gene Expression in Patients with Polycystic Ovarian Syndrome. J. Hum. Reprod. Sci. 2020, 13, 100–103. [Google Scholar] [CrossRef]
- Mostafa, R.; Ali, M.; Abdelazim, I.; Fahmy, A.; Farghali, M.; Abdel-Fatah, M.; Mahran, M. Relation between aromatase gene CYP19 variation and hyperandrogenism in Polycystic Ovary Syndrome Egyptian women. J. Infertil. Reprod. Biol. 2016, 4, 1–5. [Google Scholar]
- Sander, V.A.; Hapon, M.B.; Sícaro, L.; Lombardi, E.P.; Jahn, G.A.; Motta, A.B. Alterations of folliculogenesis in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 2008, 14, 367–378. [Google Scholar] [CrossRef]
- Franks, S.; Hardy, K. Folliculogenesis in Polycystic Ovaries. In Polycystic Ovary Syndrome: Current Controversies, from the Ovary to the Pancreas; Dunaif, A., Chang, R.J., Franks, S., Legro, R.S., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 1–7. [Google Scholar]
- Palma, G.A.; Argañaraz, M.E.; Barrera, A.D.; Rodler, D.; Mutto, A.; Sinowatz, F. Biology and biotechnology of follicle development. Sci. World J. 2012, 2012, 938138. [Google Scholar] [CrossRef]
- Bonnet, A.; Cabau, C.; Bouchez, O.; Sarry, J.; Marsaud, N.; Foissac, S.; Woloszyn, F.; Mulsant, P.; Mandon-Pepin, B. An overview of gene expression dynamics during early ovarian folliculogenesis: Specificity of follicular compartments and bi-directional dialog. BMC Genom. 2013, 14, 904. [Google Scholar] [CrossRef]
- Dunlop, C.E.; Anderson, R.A. The regulation and assessment of follicular growth. Scand. J. Clin. Lab. Investig. Suppl. 2014, 244, 13–17; discussion 17. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yang, H.; Lee, S.R.; Kwon, S.W.; Hong, E.J.; Lee, H.W. Welsh Onion Root (Allium fistulosum) Restores Ovarian Functions from Letrozole Induced-Polycystic Ovary Syndrome. Nutrients 2018, 10, 1430. [Google Scholar] [CrossRef]
- Morgante, G.; Massaro, M.G.; Di Sabatino, A.; Cappelli, V.; De Leo, V. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol. Endocrinol. 2018, 34, 4–9. [Google Scholar] [CrossRef]
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 12, Cd013505. [Google Scholar] [CrossRef]
- Domecq, J.P.; Prutsky, G.; Mullan, R.J.; Sundaresh, V.; Wang, A.T.; Erwin, P.J.; Welt, C.; Ehrmann, D.; Montori, V.M.; Murad, M.H. Adverse effects of the common treatments for polycystic ovary syndrome: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4646–4654. [Google Scholar] [CrossRef][Green Version]
- Moll, E.; Bossuyt, P.M.; Korevaar, J.C.; Lambalk, C.B.; van der Veen, F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: Randomised double blind clinical trial. Bmj 2006, 332, 1485. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ibrahim, S.F.; Jaffar, F.H.F.; Mokhtar, M.H.; Chin, K.Y.; Osman, K. Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review. Molecules 2021, 26, 649. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Ruslee, S.S.; Mokhtar, M.H. Protective Roles of Honey in Reproductive Health: A Review. Molecules 2021, 26, 3322. [Google Scholar] [CrossRef]
- Tam, C.; Erebara, A.; Einarson, A. Food-borne illnesses during pregnancy. Can. Fam. Physician 2010, 56, 341. [Google Scholar]
- Mohd Kamal, D.A.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Hungerford, N.L.; Webber, D.; Carpinelli de Jesus, M.; Zhang, J.; Stone, I.S.J.; Blanchfield, J.T.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 12128. [Google Scholar] [CrossRef]
- Zawawi, N.; Zhang, J.; Hungerford, N.L.; Yates, H.S.A.; Webber, D.C.; Farrell, M.; Tinggi, U.; Bhandari, B.; Fletcher, M.T. Unique physicochemical properties and rare reducing sugar trehalulose mandate new international regulation for stingless bee honey. Food Chem. 2022, 373, 131566. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Kelulut Honey Ameliorates Oestrus Cycle, Hormonal Profiles, and Oxidative Stress in Letrozole-Induced Polycystic Ovary Syndrome Rats. Antioxidants 2022, 11, 1879. [Google Scholar]
- Budin, S.; Jubaidi, F.; Azam, S.; Mohammed Yusof, N.; Taib, I.S.; Mohamed, J. Kelulut honey supplementation prevents sperm and testicular oxidative damage in streptozotocin-induced diabetic rats. J. Teknol. 2017, 79, 89–95. [Google Scholar] [CrossRef]
- Dahliansyah, D.; Petrika, Y. Pemberian Madu Trigona Sp. (Kelulut) Dan Sari Jeruk Siam Sambas Terhadap Kadar Hemoglobin Darah (Hb) Ibu Hamil. J. Surya Med. (JSM) 2020, 6, 157–162. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study. Life 2022, 12, 890. [Google Scholar] [CrossRef]
- Kafali, H.; Iriadam, M.; Ozardali, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef]
- Ndeingang, E.C.; Defo Deeh, P.B.; Watcho, P.; Kamanyi, A. Phyllanthus muellerianus (Euphorbiaceae) Restores Ovarian Functions in Letrozole-Induced Polycystic Ovarian Syndrome in Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 2965821. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Mokhtar, M.H. Effects of Testosterone on the Expression of Connexin 26 and Connexin 43 in the Uterus of Rats During Early Pregnancy. Vivo 2020, 34, 1863–1870. [Google Scholar] [CrossRef]
- Gozukara, I.O.; Pınar, N.; Ozcan, O.; Ozgur, T.; Dokuyucu, R.; Kurt, R.K.; Kucur, S.K.; Aksoy, A.N. Effect of colchicine on polycystic ovary syndrome: An experimental study. Arch. Gynecol. Obstet. 2016, 293, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Atis, A.; Aydin, Y.; Ciftci, F.; Sakız, D.; Arslan, A.; Toklu, A.S.; Donmez, M.; Goker, N. Hyberbaric oxygen increases atresia in normal & steroid induced PCO rat ovaries. Reprod. Biol. Endocrinol. 2012, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protocol 2019, 9, e3465. [Google Scholar] [CrossRef]
- Yoshino, O.; Shi, J.; Osuga, Y.; Harada, M.; Nishii, O.; Yano, T.; Taketani, Y. The function of bone morphogenetic proteins in the human ovary. Reprod. Med. Biol. 2011, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Canty-Laird, E.; Carré, G.A.; Mandon-Pépin, B.; Kadler, K.E.; Fabre, S. First evidence of bone morphogenetic protein 1 expression and activity in sheep ovarian follicles. Biol. Reprod. 2010, 83, 138–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilsson, E.E.; Kezele, P.; Skinner, M.K. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol. Cell. Endocrinol. 2002, 188, 65–73. [Google Scholar] [CrossRef]
- da Nóbrega, J.E.; Gonçalves, P.B.D.; Chaves, R.N.; Magalhães, D.d.M.; Rossetto, R.; Lima-Verde, I.B.; Pereira, G.R.; Campello, C.C.; Figueiredo, J.R.; de Oliveira, J.F.C. Leukemia inhibitory factor stimulates the transition of primordial to primary follicle and supports the goat primordial follicle viability in vitro. Zygote 2012, 20, 73–78. [Google Scholar] [CrossRef]
- Cadoret, V.; Jarrier-Gaillard, P.; Papillier, P.; Monniaux, D.; Guérif, F.; Dalbies-Tran, R. Leukemia inhibitory factor modulates the differentiation of granulosa cells during sheep in vitro preantral to antral follicle development and improves oocyte meiotic competence. Mol. Hum. Reprod. 2021, 27, gaab051. [Google Scholar] [CrossRef]
- Hutt, K.J.; McLaughlin, E.A.; Holland, M.K. KIT/KIT Ligand in Mammalian Oogenesis and Folliculogenesis: Roles in Rabbit and Murine Ovarian Follicle Activation and Oocyte Growth1. Biol. Reprod. 2006, 75, 421–433. [Google Scholar] [CrossRef]
- Triantafyllidou, O.; Sigalos, G.; Gkoles, L.; Kastora, S.; Vakas, P.; Batsiou, E.; Vlahos, N. The addition of clomiphene citrate to ovarian stimulation protocols for poor responders. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 136–140. [Google Scholar] [CrossRef]
- Dickey, R.P.; Holtkamp, D.E. Development, pharmacology and clinical experience with clomiphene citrate. Hum. Reprod. Update 1996, 2, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Fossum, G.; Gitlin, S.; Gracia, C.; Hansen, K.; La Barbera, A.; et al. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): A guideline. Fertil. Steril. 2017, 108, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Metformin Therapy for the Management of Infertility in Women with Polycystic Ovary Syndrome. BJOG Int. J. Obstet. Gynaecol. 2017, 124, e306–e313. [CrossRef] [PubMed]
- Azevedo, A.R.; Pais, A.S.; Almeida-Santos, T.; Pires, V.M.R.; Pessa, P.; Marques, C.C.; Nolasco, S.; Castelo-Branco, P.; Prates, J.A.M.; Lopes-da-Costa, L.; et al. Medical Grade Honey as a Promising Treatment to Improve Ovarian Tissue Transplantation. Bioengineering 2022, 9, 357. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid. Med. Cell. Longev. 2017, 2017, 4371714. [Google Scholar] [CrossRef]
- Suzuki, T.; Sugino, N.; Fukaya, T.; Sugiyama, S.; Uda, T.; Takaya, R.; Yajima, A.; Sasano, H. Superoxide dismutase in normal cycling human ovaries: Immunohistochemical localization and characterization. Fertil. Steril. 1999, 72, 720–726. [Google Scholar] [CrossRef]
- Karim, N. Antioxidant Properties of Stingless Bee Honey and Its Effect on the Viability of Lymphoblastoid Cell Line. Med. Health 2019, 14, 91–105. [Google Scholar] [CrossRef]
- Haron, H.; Talib, R.A.; Subramaniam, P.; Arifen, Z.N.Z.; Ibrahim, M. A Comparison of Chemical Compositions In Kelulut Honey from Different Regions. Malays. J. Anal. Sci. 2022, 26, 447–456. [Google Scholar]
- Ruslee, S.S.; Zaid, S.S.M.; Bakrin, I.H.; Goh, Y.M.; Mustapha, N.M. Protective effect of Tualang honey against cadmium-induced morphological abnormalities and oxidative stress in the ovary of rats. BMC Complement. Med. Ther. 2020, 20, 160. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Othman, S.; Kassim, N.M. Potential protective effect of Tualang honey on BPA-induced ovarian toxicity in prepubertal rat. BMC Complement. Altern. Med. 2014, 14, 509. [Google Scholar] [CrossRef]
- Emam, S.R.; Abd-Elsalam, R.M.; Azouz, A.A.; Ali, S.E.; El Badawy, S.A.; Ibrahim, M.A.; Hassan, B.B.; Issa, M.Y.; Elmosalamy, S.H. Linum usitatissimum seeds oil down-regulates mRNA expression for the steroidogenic acute regulatory protein and Cyp11A1 genes, ameliorating letrezole-induced polycystic ovarian syndrome in a rat model. J. Physiol. Pharmacol. 2021, 72, 55–67. [Google Scholar] [CrossRef]
- Rudic, J.; Jakovljevic, V.; Jovic, N.; Nikolic, M.; Sretenovic, J.; Mitrovic, S.; Bolevich, S.; Bolevich, S.; Mitrovic, M.; Raicevic, S.; et al. Antioxidative Effects of Standardized Aronia melanocarpa Extract on Reproductive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome. Antioxidants 2022, 11, 1099. [Google Scholar] [CrossRef]
- Kakuta, H.; Iguchi, T.; Sato, T. The Involvement of Granulosa Cells in the Regulation by Gonadotropins of Cyp17a1 in Theca Cells. Vivo 2018, 32, 1387–1401. [Google Scholar] [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Enhancement of BDNF Concentration and Restoration of the Hypothalamic-Pituitary-Adrenal Axis Accompany Reduced Depressive-Like Behaviour in Stressed Ovariectomised Rats Treated with Either Tualang Honey or Estrogen. Sci. World J. 2014, 2014, 310821. [Google Scholar] [CrossRef]
- Hirsch, A.; Hahn, D.; Kempná, P.; Hofer, G.; Nuoffer, J.M.; Mullis, P.E.; Flück, C.E. Metformin inhibits human androgen production by regulating steroidogenic enzymes HSD3B2 and CYP17A1 and complex I activity of the respiratory chain. Endocrinology 2012, 153, 4354–4366. [Google Scholar] [CrossRef]
- Attia, G.R.; Rainey, W.E.; Carr, B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001, 76, 517–524. [Google Scholar] [CrossRef]
- Stocco, C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids 2008, 73, 473–487. [Google Scholar] [CrossRef]
- Stocco, C. Tissue physiology and pathology of aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef]
- Guigon, C.l.J.; Mazaud, S.v.; Forest, M.G.; Brailly-Tabard, S.; Coudouel, N.l.; Magre, S. Unaltered Development of the Initial Follicular Waves and Normal Pubertal Onset in Female Rats after Neonatal Deletion of the Follicular Reserve. Endocrinology 2003, 144, 3651–3662. [Google Scholar] [CrossRef]
- Turner, K.J.; Macpherson, S.; Millar, M.R.; McNeilly, A.S.; Williams, K.; Cranfield, M.; Groome, N.P.; Sharpe, R.M.; Fraser, H.M.; Saunders, P.T. Development and validation of a new monoclonal antibody to mammalian aromatase. J. Endocrinol. 2002, 172, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, S.Y.; Lee, S.R.; Pyun, B.-J.; Kim, H.J.; Lee, Y.H.; Kwon, S.W.; Suh, D.H.; Lee, C.H.; Hong, E.-J.; et al. Therapeutic Effect of Ecklonia cava Extract in Letrozole-Induced Polycystic Ovary Syndrome Rats. Front. Pharmacol. 2018, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Pellatt, L.; Ramanathan, K.; Whitehead, S.A.; Mason, H.D. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 2009, 150, 4794–4801. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L. Preliminary study of Yulin mixture affecting the miR-320/SF-1/Cyp19a1 on mouse polycystic ovary syndrome model. Gynecol. Endocrinol. 2021, 37, 546–553. [Google Scholar] [CrossRef]



| Gene | Forward (F) and Reverse (R) Primer Sequence |
|---|---|
| Cyp17a1 | F→ATCCTGAGGTGAAGAAGAAG R→CAGTAAACTCTCCAATGCTG |
| Cyp19a1 | F→CTAACATCATTCTGAACATCGG R→CTGAAAATACCTGTAGGGAAC |
| KITLG | F→GTGCTCTCTTCAACATTAGG R→CTTGACTGTTTCTTCTTCCAG |
| LIF | F→AAACTCAATGCGACTACAG R→AATGACTTGCTTGTATGTCC |
| BMP1 | F→GGGGAGAAGATATTCTGAAC R→CCTCCACATAGTCATACCAG |
| GAPDH | F→CTCAATGGGAACTTAACAGG R→CTCTGTATAAGCAAGGATGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Zaid, S.S.M.; Mokhtar, M.H. Kelulut Honey Improves Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profiles and Ovarian Histomorphology in Letrozole-Induced Polycystic Ovary Syndrome Rats. Nutrients 2022, 14, 4364. https://doi.org/10.3390/nu14204364
Kamal DAM, Ibrahim SF, Ugusman A, Zaid SSM, Mokhtar MH. Kelulut Honey Improves Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profiles and Ovarian Histomorphology in Letrozole-Induced Polycystic Ovary Syndrome Rats. Nutrients. 2022; 14(20):4364. https://doi.org/10.3390/nu14204364
Chicago/Turabian StyleKamal, Datu Agasi Mohd, Siti Fatimah Ibrahim, Azizah Ugusman, Siti Sarah Mohamad Zaid, and Mohd Helmy Mokhtar. 2022. "Kelulut Honey Improves Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profiles and Ovarian Histomorphology in Letrozole-Induced Polycystic Ovary Syndrome Rats" Nutrients 14, no. 20: 4364. https://doi.org/10.3390/nu14204364
APA StyleKamal, D. A. M., Ibrahim, S. F., Ugusman, A., Zaid, S. S. M., & Mokhtar, M. H. (2022). Kelulut Honey Improves Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profiles and Ovarian Histomorphology in Letrozole-Induced Polycystic Ovary Syndrome Rats. Nutrients, 14(20), 4364. https://doi.org/10.3390/nu14204364






