Soy Isoflavones Protect Neuronal PC12 Cells against Hypoxic Damage through Nrf2 Activation and Suppression of p38 MAPK and AKT–mTOR Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
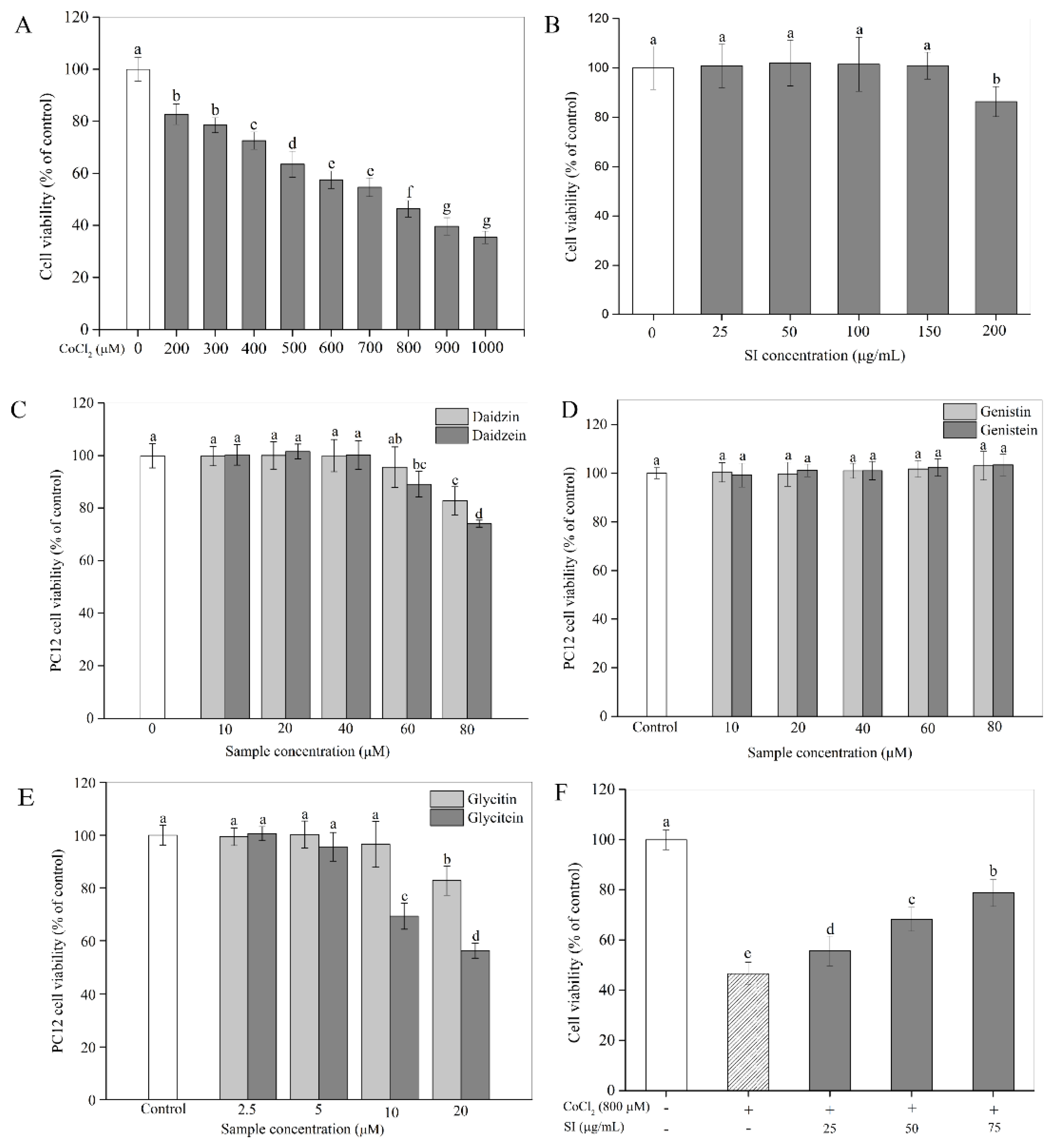
2.2. Cytotoxicity
2.3. Influence of SIs on Cell Viability Loss
2.4. Influence of SIs on ROS Production
2.5. Influence of SIs on Cell Cycle
2.6. Influence of SIs on Cell Apoptosis
2.7. Western Blot Analysis
2.8. Model Assessment and Molecular Docking
2.9. Statistical Analysis
3. Results
3.1. Cytotoxicity and Inhibition of Isoflavones on Cell Viability Loss
3.2. Attenuation of ROS Production by SIs
3.3. Improvement of Cell Cycle by SIs
3.4. Inhibition of Cell Apoptosis by SIs
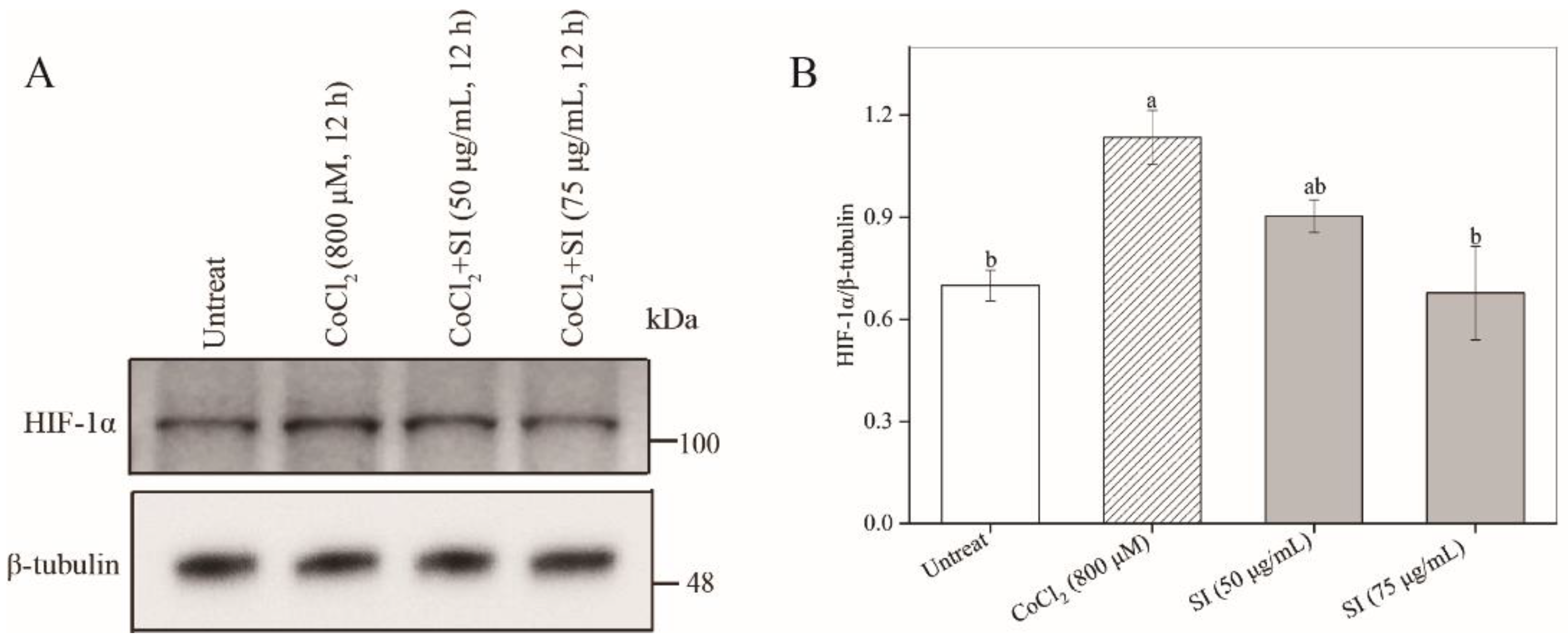
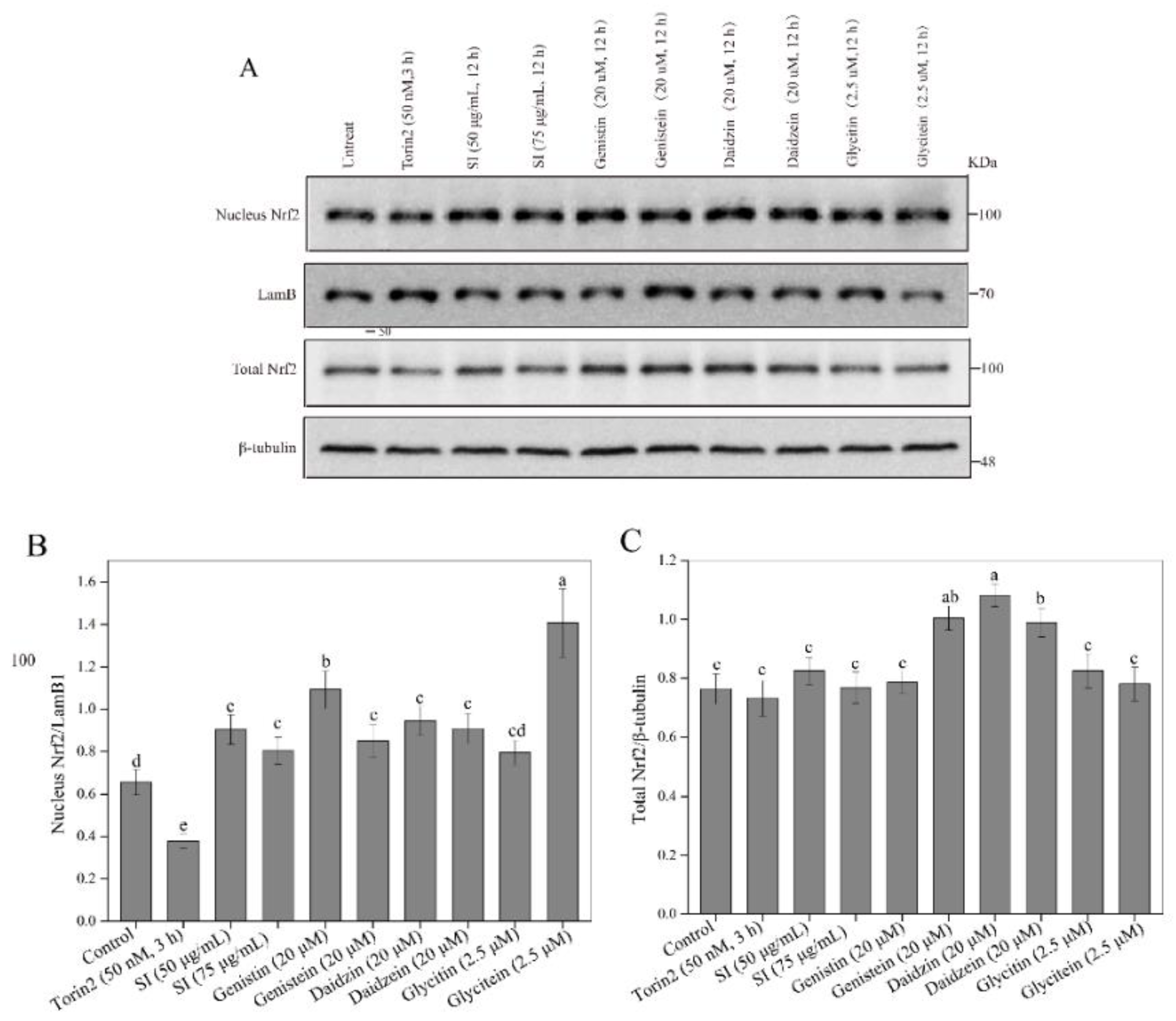
3.5. Facilitation of Nrf2 Activation by SIs
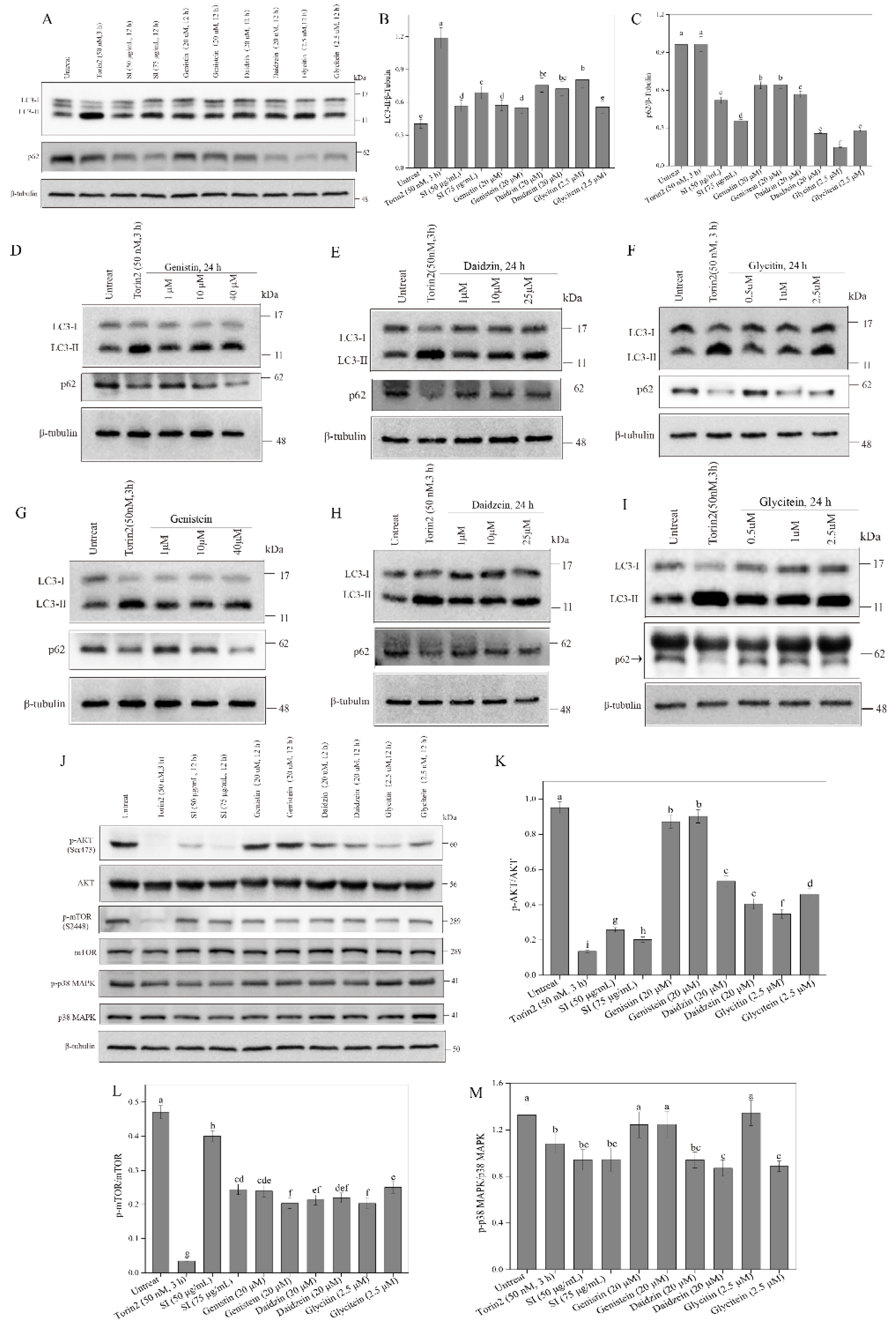
3.6. Activation of Autophagy by Isoflavones
3.7. Inhibition of p38 MAPK Signaling Pathway by Isoflavones
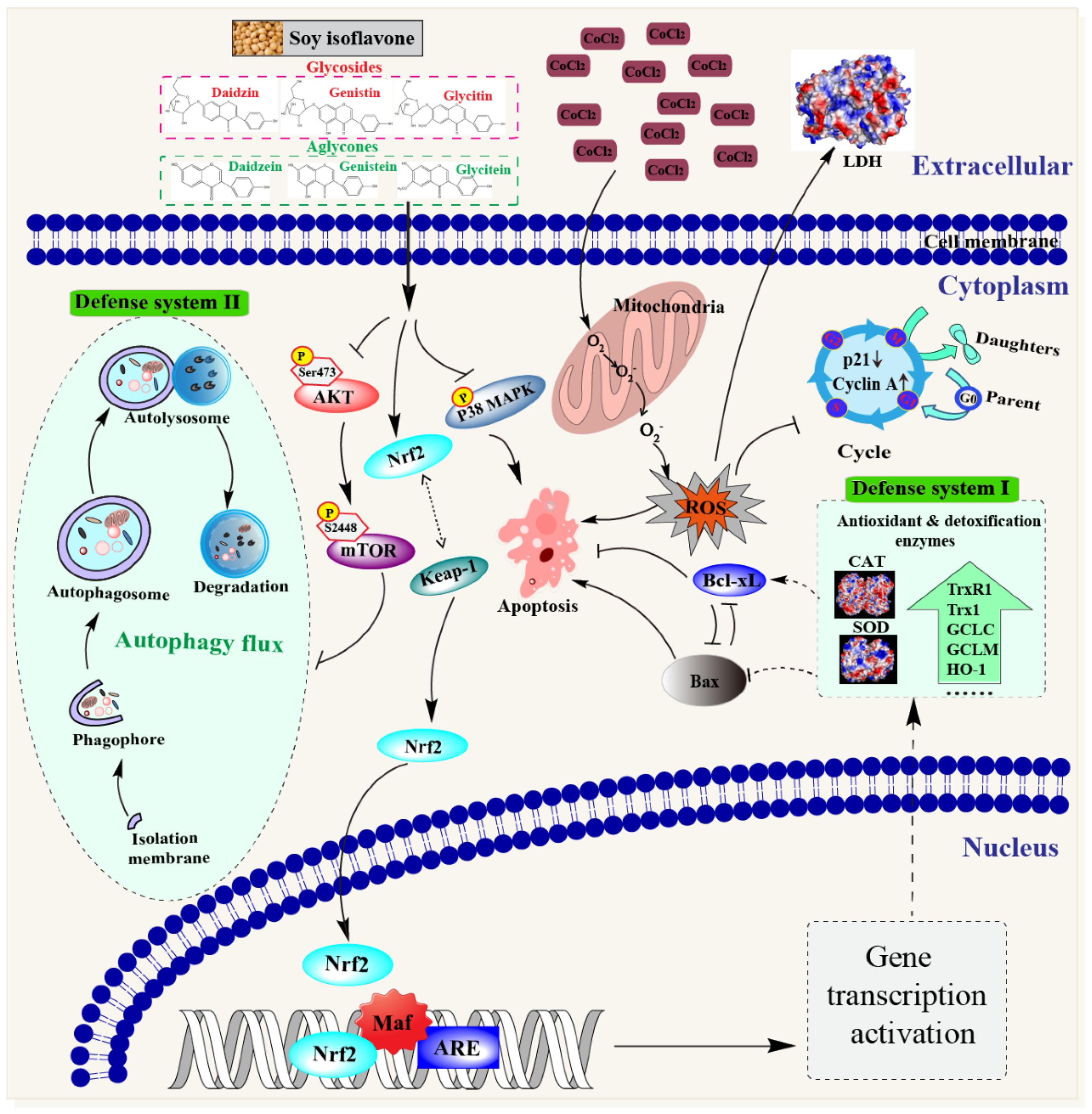
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of prenatal hypoxia in brain development, cognitive functions, and neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef] [PubMed]
- Oechmichen, M.; Meissner, C. Cerebral hypoxia and ischemia: The forensic point of view: A review. J. Forensic Sci. 2006, 51, 880–887. [Google Scholar] [CrossRef]
- Xu, L.; Li, Q.; Ke, Y.; Yung, W.H. Chronic intermittent hypoxia-Induced aberrant neural activities in the hippocampus of male rats revealed by long-term in vivo recording. Front. Cell. Neurosci. 2022, 15, 784045. [Google Scholar] [CrossRef] [PubMed]
- Koehler, R.C.; Yang, Z.J.; Lee, J.K.; Martin, L.J. Perinatal hypoxic-ischemic brain injury in large animal models: Relevance to human neonatal encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Peng, J.; Jiang, H.; Le, K. Resveratrol Improves Synaptic Plasticity in Hypoxic-Ischemic Brain Injury in Neonatal Mice via Alleviating SIRT1/NF-κB Signaling-Mediated Neuroinflammation. J. Mol. Neurosci. 2022, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Z.; Zhou, W.; Lu, C.; Ji, T.; Yang, W.; Jin, Z.; Tian, Y.; Lei, W.; Wu, S.; et al. SIRT1/PGC-1α signaling activation by mangiferin attenuates cerebral hypoxia/reoxygenation injury in neuroblastoma cells. Eur. J. Pharmacol. 2021, 907, 174236. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jiang, C.; Kang, Y.; Dai, Y.; Fang, W.; Huang, P. Curcumin exerts protective effects against hypoxia-reoxygenation injury via the enhancement of apurinic/apyrimidinic endonuclease 1 in SH-SY5Y cells: Involvement of the PI3K/AKT pathway. Int. J. Mol. Med. 2020, 45, 993–1004. [Google Scholar] [CrossRef]
- Lee, Y.B.; Lee, H.J.; Sohn, H.S. Soy isoflavones and cognitive function. J. Nutr. Biochem. 2005, 16, 641–649. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xia, Z.H.; Jiang, X.; Li, L.X.; Wang, H.G.; An, D.; Liu, Y.Q. Genistein inhibits amyloid peptide 25-35-induced neuronal death by modulating estrogen receptors, choline acetyltransferase and glutamate receptors. Arch. Biochem. Biophys. 2020, 693, 108561. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of blood-brain barrier permeability of polyphenols, anthocyanins, and their metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; He, J.B.; Yu, L.H.; Li, L.; Long, M.; Liu, M.D.; Li, P. Protective role of curcumin in Cadmium-induced esticular injury in mice by attenuating oxidative stress via Nrf2/ARE pathway. Environ. Sci. Pollut. Res. 2019, 26, 34575–34583. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, F.; Lu, S.; Ren, L.; Bian, S.; Liu, M.; Zhao, D.; Wang, S.; Wang, J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 2022, 283, 114739. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 6, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhu, X.; Du, L. P38 MAP kinase is involved in oleuropein-induced apoptosis in A549 cells by a mitochondrial apoptotic cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, Y.; Azi, F.; Zhou, J.; Liu, X.; Dai, Y.; Wang, Z.; Dong, M.; Xia, X. Inhibition of biofilm formation and quorum sensing by soy isoflavones in Pseudomonas aeruginosa. Food Control 2022, 133, 108629. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, B.B.; Yang, C.; Ou, Y.; Feng, X.; Muhoza, D.; Holmes, A.F.; Theus, S.; Deshmukh, S.; Haun, R.S.; Kaushal, G.P. Endoplasmic reticulum stress-induced autophagy provides cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLoS ONE 2015, 10, e0140025. [Google Scholar] [CrossRef]
- Alexandra, T.; Marina, I.M.; Daniela, M.; Ioana, S.I.; Maria, B.; Radu, R.; Maria, T.A.; Tudor, S.; Maria, G. Autophagy-a hidden but important actor on oral cancer scene. Int. J. Mol. Sci. 2020, 21, 9325. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamori, Y. Potential effects of soy isoflavones on the prevention of metabolic syndrome. Molecules 2021, 26, 5863. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkison’s disease, and Huntington’s disease: A mini review. Oxidative Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Mo, H.C.; Yang, K.H.; Jeong, Y.J.; Yoo, H.G.; Choi, N.K.; Oh, W.M.; Oh, H.K.; Kim, S.H.; Lee, J.H.; et al. Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12 cells. Life Sci. 2007, 80, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Guan, T.; Huang MCao, L.; Li, Y.; Cheng, H.; Jin, H.; Yu, D. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria- dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int. 2012, 60, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Amit, T.; Youdim, M.B.H.; Mandel, S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002, 277, 30574–30580. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, Y.; Wang, L.; Wu, H.; Azi, F.; Tekliye, M.; Zhou, J.; Liu, X.; Dong, M.; Xia, X. Neuroprotective potency of a soy whey fermented by Cordyceps militaris SN-18 against hydrogen peroxide-induced oxidative injury in PC12 cells. Eur. J. Nutr. 2022, 61, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yang, L.; Zhou, J.; Lin, R.; Zhang, J.; Lin, Q.; Wang, W.; Zhang, K. Protective effects of paeoniflorin against cobalt chloride-induced apoptosis of endothelial cells via HIF-1α pathway. Toxicol. Vitr. 2012, 26, 455–461. [Google Scholar] [CrossRef]
- Tong, Y.; Tong, K.; Zhu, Q.; Wu, Y.; Yang, Y.; Zhang, J.; Hu, P.; Yan, S. Cobalt chloride induced apoptosis by inhibiting GPC3 expression via the HIF-1α/c-Myc axis in HepG2 cells. Onco Targets Ther. 2019, 12, 10663–10670. [Google Scholar] [CrossRef]
- Jenssen, C.; Schick, B.; Wagner, S. Genistein prevents cadmium-induced neurotoxic effects through its antioxidant mechanisms. Drug Res. 2014, 65, 65–69. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Y.; Azi, F.; Tekliye, M.; Zhou, J.; Li, X.; Xu, Z.; Dong, M.; Xia, X. Soybean whey bio-processed using Weissella hellenica D1501 protects neuronal PC12 cells against oxidative damage. Front. Nutr. 2022, 9, 833555. [Google Scholar] [CrossRef]
- Li, M.; Huang, W.; Jie, F.; Wang, M.; Zhong, Y.; Chen, Q.; Lu, B. Discovery of Keap1-Nrf2 small-molecule inhibitors from phytochemicals based on molecular docking. Food Chem. Toxicol. 2019, 133, 110758. [Google Scholar] [CrossRef]
- Ji, W.; Wang, L.; He, S.; Yan, L.; Li, T.; Wang, J.; Kong, A.T.; Yu, S.; Zhang, Y. Effects of acute hypoxia exposure with different durations on activation of Nrf2-ARE pathway in mouse skeletal muscle. PLoS ONE 2018, 13, e0208474. [Google Scholar] [CrossRef]
- Baba, K.; Morimoto, H.; Imaoka, S. Seven in absentia homolog 2 (Siah2) protein is a regulator of NF-E2-related factor 2 (Nrf2). J. Biol. Chem. 2013, 288, 18393–18405. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Zhang, Q.; Li, X.; Zhu, X.; Wang, Q.; Cao, S.; Du, L. Mitochondria-mediated apoptosis was induced by oleuropein in H1299 cells involving activation of p38 MAP kinase. J. Cell. Biochem. 2019, 120, 5480–5494. [Google Scholar] [CrossRef]
- Ding, B.; Yuan, L.; Yu, H.; Li, L.; Ma, W.; Bi, Y.; Feng, J.; Xiao, R. Genistein and folic acid prevent oxidative injury induced by β-amyloid peptide. Basic Clin. Pharmacol. Toxicol. 2011, 108, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xiang, X.; Huang, F.; Zheng, M.; Cong, R.; Han, L.; Zhang, Z. Dietary polyphenol canolol from rapeseed oil attenuates oxidative stress-induced cell damage through the modulation of the p38 signaling pathway. RSC Adv. 2018, 8, 24338–24345. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Y.; Amrán, D.; Fernández, C.; de Blas, E.; Aller, P. Genistein selectively potentiates arsenic trioxide-induced apoptosis in human leukemia cells via reactive oxygen species generation and activation of reactive oxygen species-inducible protein kinases (p38-MAPK, AMPK). Int. J. Cancer 2008, 123, 1205–1214. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 2006, 281, 29776–29787. [Google Scholar] [CrossRef]
- Li, P.; Ma, K.; Wu, H.Y.; Wu, Y.P.; Li, B.X. Isoflavones induce BEX2-dependent autophagy to prevent ATR-induced neurotoxicity in SH-SY5Y cells. Cell. Physiol. Biochem. 2017, 43, 1866–1879. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Kao, S.T.; Lee, Y.C. Ferulic acid exerts anti-apoptotic effects against ischemic injury by activating HSP70/Bcl-2-and HSP70/autophagy-mediated signaling after permanent focal cerebral ischemia in rats. Am. J. Chin. Med. 2019, 47, 39–61. [Google Scholar] [CrossRef]


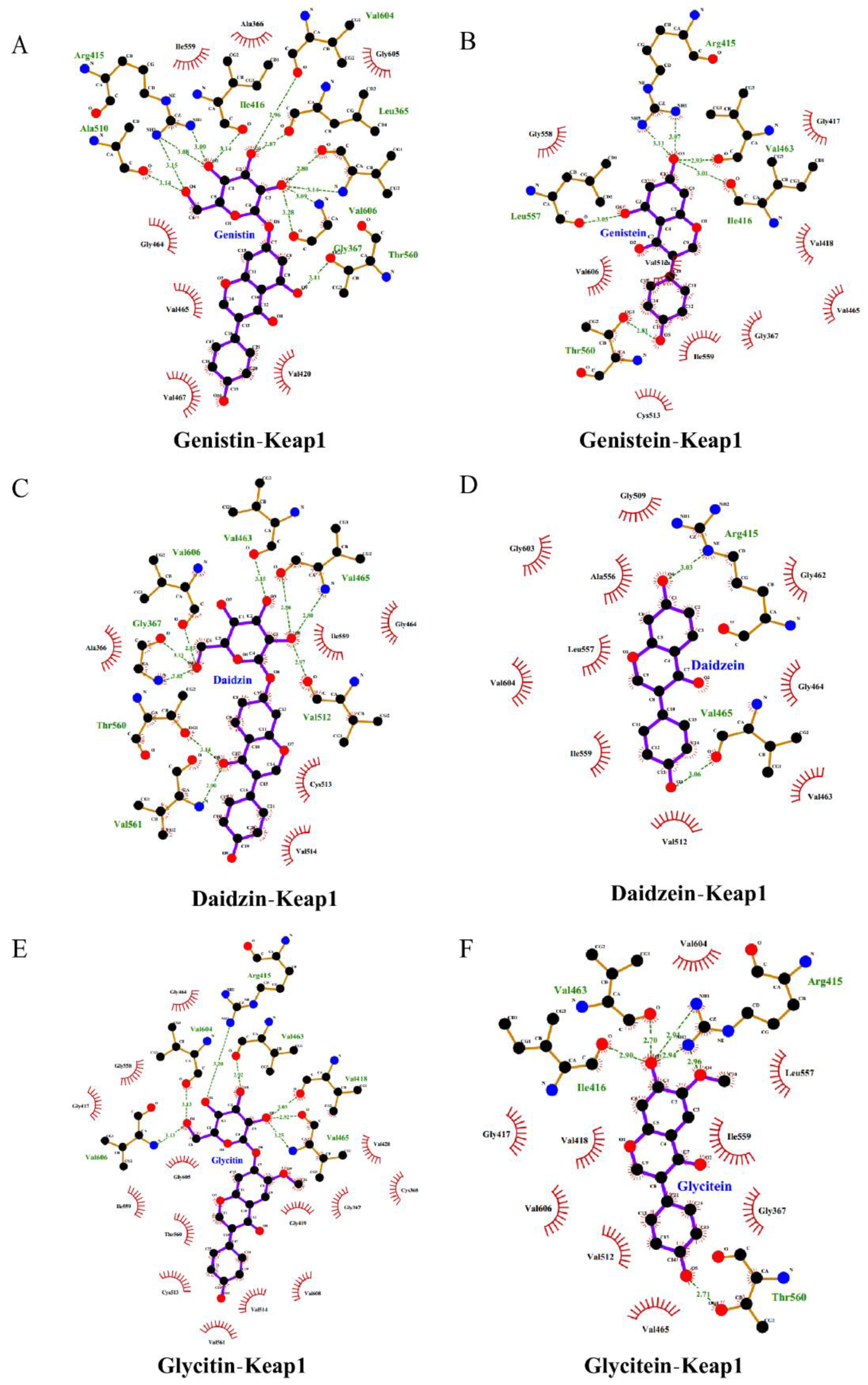
| Molecule | Docking Energy (kcal/mol) | Hydrogen-Bonding Interactions | Hydrogen Bond Distance | Key Hydrophobic Interactions |
|---|---|---|---|---|
| Daidzin | −10.7 | Val561 | 2.90 | Ala366, Gly513, Val465, Val514, Ile559, Gly464 |
| Thr560 | 3.14 | |||
| Gly367 | 3.13, 2.82 | |||
| Val606 | 2.83 | |||
| Val463 | 3.15 | |||
| Val465 | 2.86, 2.80 | |||
| Val512 | 2.97 | |||
| Glycitin | −10.4 | Val606 | 3.13 | Gly464, Gly558, Gly417, Gly605, Ile559, Thr560, Cys513, Val561, Val514, Val608, Gly419, Gly367, Cys368, Val420 |
| Val604 | 3.13 | |||
| Arg415 | 3.20 | |||
| Val463 | 2.92 | |||
| Val418 | 3.03 | |||
| Val465 | 2.92, 3.25 | |||
| Genistin | −10.7 | Ala510 | 3.14 | Ile559, Ala366, Gly605, Gly464, Val465, Gly467, Val420 |
| Arg415 | 3.15, 3.08, 3.09 | |||
| Ile416 | 3.14 | |||
| Val604 | 2.96 | |||
| Leu365 | 2.87 | |||
| Val606 | 2.80, 3.14 | |||
| Gly367 | 3.28, 3.09 | |||
| Thr560 | 3.11 | |||
| Daidzein | −9.7 | Arg415 | 3.03 | Gly509, Gly603, Ala556, Leu557, Val604, Ile559, Val512, Val463, Gly464, Gly462 |
| Val465 | 3.06 | |||
| Glycitein | −9.3 | Ile416 | 2.90 | Val604, Gly417, Val418, Val606, Val512, Val465, Leu557, Ile559, Gly367 |
| Arg415 | 2.91, 2.94, 2.96 | |||
| Val463 | 2.70 | |||
| Thr560 | 2.71 | |||
| Genistein | −9.2 | Arg415 | 3.11, 3.07 | Gly558, Val606, Cys513, Ile559, Gly367, Val465, Gly417, Val418 |
| Leu557 | 3.05 | |||
| Val463 | 2.93 | |||
| Ile416 | 3.01 | |||
| Thr560 | 2.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yin, L.; Dong, J.; Xia, X. Soy Isoflavones Protect Neuronal PC12 Cells against Hypoxic Damage through Nrf2 Activation and Suppression of p38 MAPK and AKT–mTOR Pathways. Antioxidants 2022, 11, 2037. https://doi.org/10.3390/antiox11102037
Zhang Y, Yin L, Dong J, Xia X. Soy Isoflavones Protect Neuronal PC12 Cells against Hypoxic Damage through Nrf2 Activation and Suppression of p38 MAPK and AKT–mTOR Pathways. Antioxidants. 2022; 11(10):2037. https://doi.org/10.3390/antiox11102037
Chicago/Turabian StyleZhang, Yongzhu, Liqing Yin, Jiajia Dong, and Xiudong Xia. 2022. "Soy Isoflavones Protect Neuronal PC12 Cells against Hypoxic Damage through Nrf2 Activation and Suppression of p38 MAPK and AKT–mTOR Pathways" Antioxidants 11, no. 10: 2037. https://doi.org/10.3390/antiox11102037
APA StyleZhang, Y., Yin, L., Dong, J., & Xia, X. (2022). Soy Isoflavones Protect Neuronal PC12 Cells against Hypoxic Damage through Nrf2 Activation and Suppression of p38 MAPK and AKT–mTOR Pathways. Antioxidants, 11(10), 2037. https://doi.org/10.3390/antiox11102037






