Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
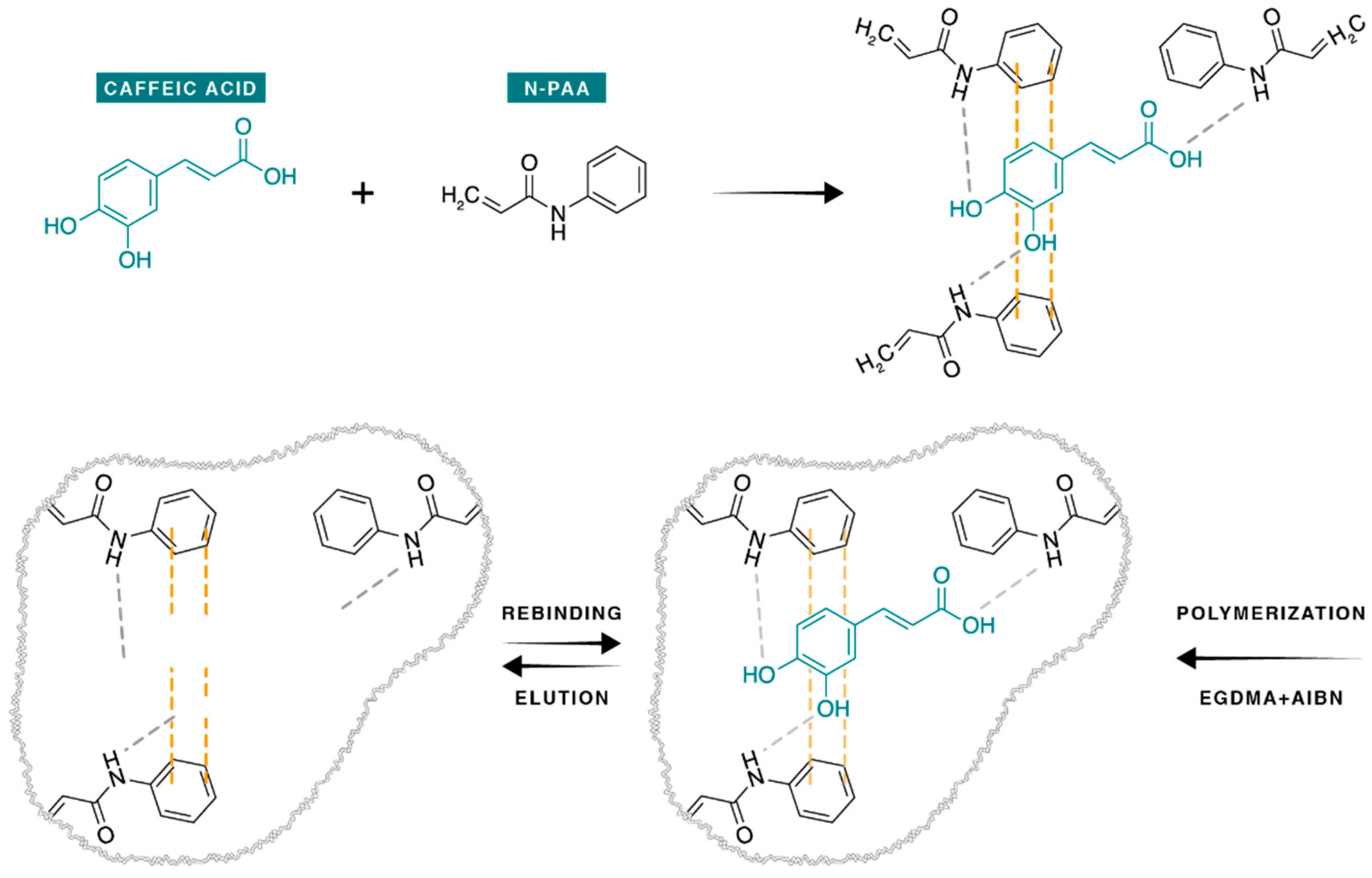
2.3. Synthesis of the Molecularly Imprinted and Non-Imprinted Polymers
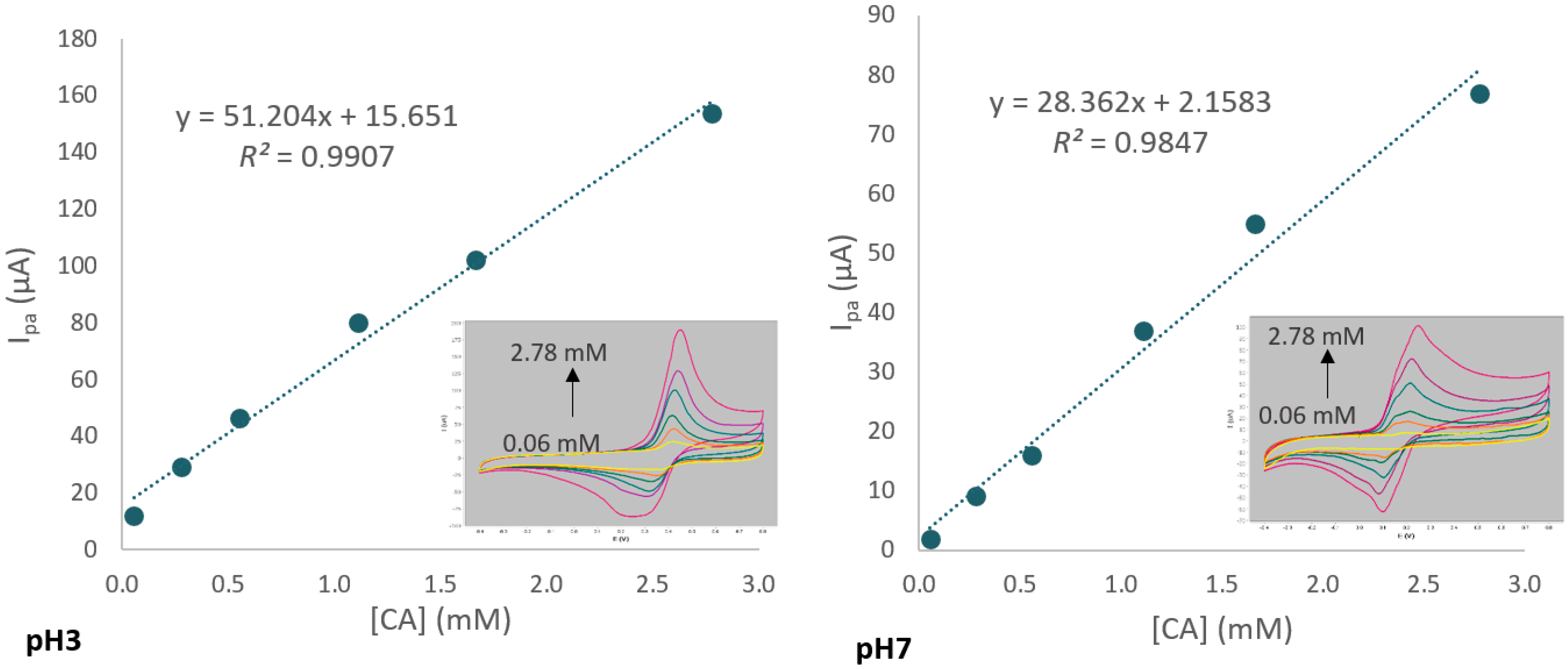
2.4. Effect of pH on Electrochemical Detection of CA in Hydroalcoholic Medium
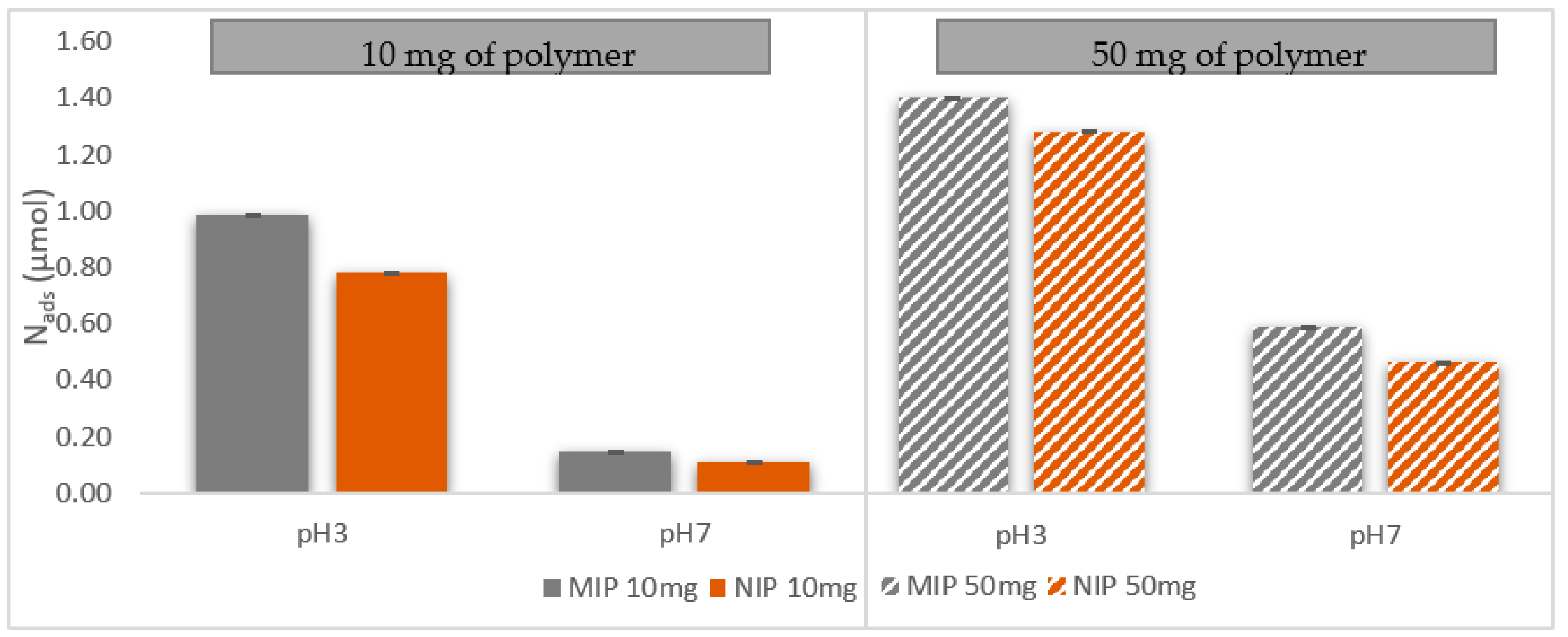
2.5. Rebinding Experiments of CA Using MIP and NIP in Hydroalcoholic Medium: Effect of pH and Polymer Mass
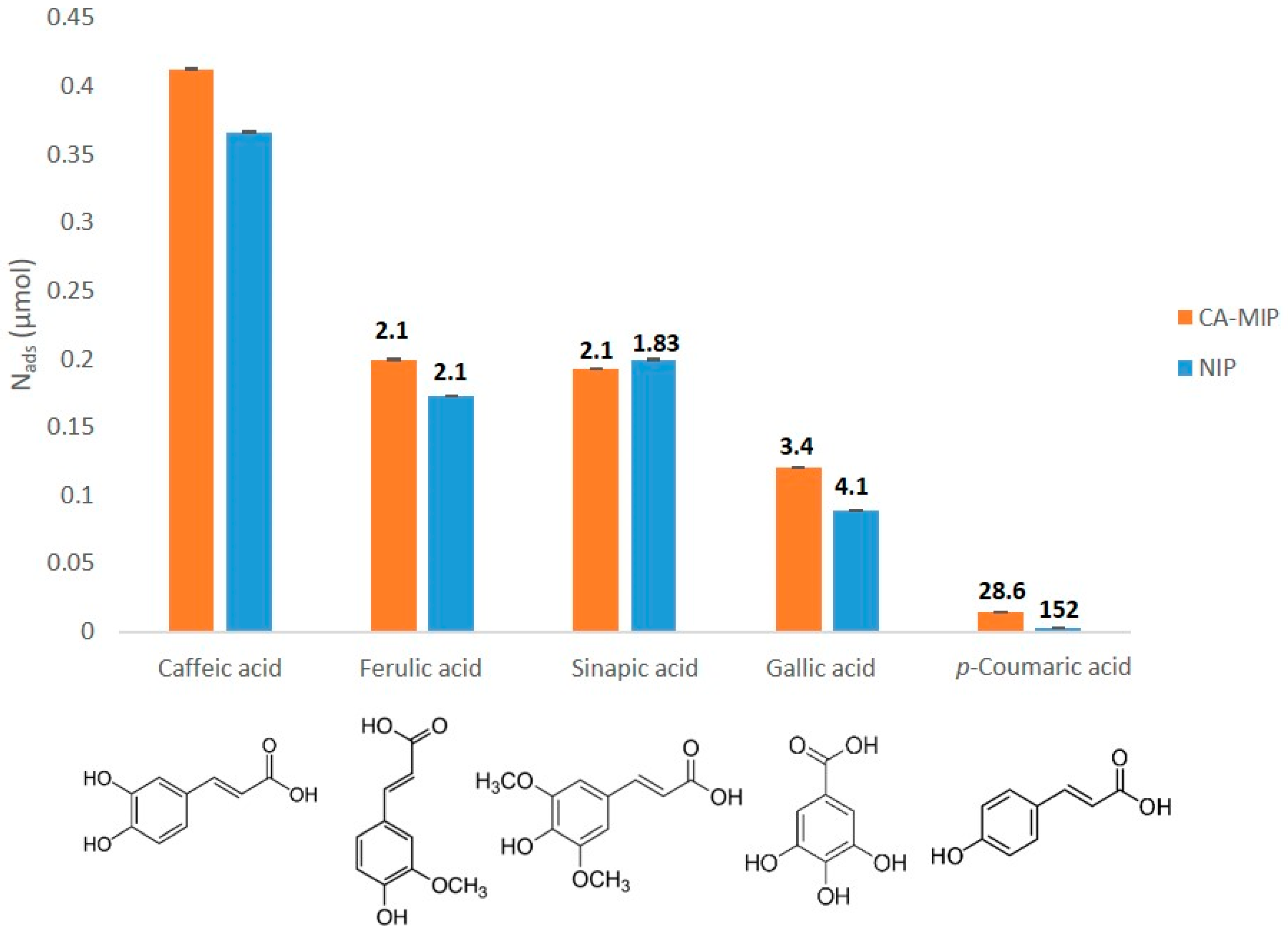
2.6. Selectivity Studies
2.7. Preparation and Electrochemical Analysis of CA in Wine Samples
2.8. HPLC Determination of CA in Wine Samples
3. Results and Discussion
3.1. Synthesis of the Molecularly Imprinted CA-MIP and Non-Imprinted Polymer
3.2. Morphological Characterization
3.3. Effect of pH on CA Detection by Voltammetry
3.4. Effect of pH on the Rebinding Properties of CA
3.5. Effect of the Polymer Mass on the Rebinding of CA
3.6. Selectivity Studies
3.7. Application to Wine, Validation, Recovery Tests, and Limit of Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, Q.; Lin, C.-L.G. Oxidative Damage to RNA: Mechanisms, Consequences, and Diseases. Cell. Mol. Life Sci. 2010, 67, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Jacobitz, A.W.; Liu, Q.; Suravajjala, S.; Agrawal, N.J. Tryptophan Oxidation of a Monoclonal Antibody Under Diverse Oxidative Stress Conditions: Distinct Oxidative Pathways Favor Specific Tryptophan Residues. J. Pharm. Sci. 2021, 110, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.M.; Chibane, M.; Monti, J.P.; Richard, T. Wine Polyphenols: Potential Agents in Neuroprotection. Oxid. Med. Cell. Longev. 2012, 2012, 805762. [Google Scholar] [CrossRef]
- Elhachem, M.; Cayot, P.; Abboud, M.; Louka, N.; Maroun, R.G.; Bou-maroun, E. The Importance of Developing Electrochemical Sensors Based on Molecularly Imprinted Polymers for a Rapid Detection of Antioxidants. Antioxidants 2021, 10, 382. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic Acid and Its Derivatives as Potential Modulators of Oncogenic Molecular Pathways: New Hope in the Fight against Cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Balasaheb, S.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Advances 2015, 27986–28006. [Google Scholar] [CrossRef]
- Debelo, H.; Li, M.; Ferruzzi, M.G. Processing Influences on Food Polyphenol Profiles and Biological Activity. Curr. Opin. Food Sci. 2020, 32, 90–102. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis Vinifera Var. Merlot, Tannat, and Syrah). Antioxidants 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Morozova, K.; Lawrence, N.; Ferrentino, G.; Scampicchio, M. Radical Scavenging Activity of Antioxidants by Cyclic Voltammetry. Electroanalysis 2021, 33, 23–28. [Google Scholar] [CrossRef]
- Schaumlöffel, L.d.S.; Dambros, J.W.V.; Bolognese Fernandes, P.R.; Gutterres, M.; Piatnicki, C.M.S. Direct and Simultaneous Determination of Four Phenolic Antioxidants in Biodiesel Using Differential Pulse Voltammetry Assisted by Artificial Neural Networks and Variable Selection by Decision Trees. Fuel 2019, 236, 803–810. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Lafarge, C.; Bitar, M.; El Hosry, L.; Cayot, P.; Bou-Maroun, E. Comparison of Molecularly Imprinted Polymers (MIP) and Sol–Gel Molecularly Imprinted Silica (MIS) for Fungicide in a Hydro Alcoholic Solution. Mater. Today Commun. 2020, 24, 101157. [Google Scholar] [CrossRef]
- Pichon, V. Selective Sample Treatment Using Molecularly Imprinted Polymers. J. Chromatogr. A 2007, 1152, 41–53. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Application of Molecularly Imprinted Polymers in Microextraction and Solventless Extraction Techniques, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 86, ISBN 9780444642660. [Google Scholar]
- Du, F.; Guo, L.; Qin, Q.; Zheng, X.; Ruan, G.; Li, J.; Li, G. Recent Advances in Aptamer-Functionalized Materials in Sample Preparation. TrAC Trends Anal. Chem. 2015, 67, 134–146. [Google Scholar] [CrossRef]
- Pichon, V. Aptamer-Based and Immunosorbents; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128169063. [Google Scholar]
- Kadhirvel, P.; Combès, A.; Bordron, L.; Pichon, V. Development and Application of Water-Compatible Molecularly Imprinted Polymers for the Selective Extraction of Carbamazepine from Environmental Waters. Anal. Bioanal. Chem. 2019, 411, 1525–1536. [Google Scholar] [CrossRef]
- Zhu, G.; Cheng, G.; Wang, P.; Li, W.; Wang, Y.; Fan, J. Water Compatible Imprinted Polymer Prepared in Water for Selective Solid Phase Extraction and Determination of Ciprofloxacin in Real Samples. Talanta 2019, 200, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qian, Z.; Hu, S.; Gong, T.; Xian, Q. Molecularly Imprinted Solid Phase Extraction Coupled with Gas Chromatography-Mass Spectrometry for Determination of N-Nitrosodiphenylamine in Water Samples. Chemosphere 2018, 212, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, M.; Peng, J.; Yang, L.; Wang, X.; Wang, F.; Zhang, X.; Wu, Q.; Chen, R.; Chen, J. Dummy Molecularly Imprinted Solid Phase Extraction of Climbazole from Environmental Water Samples. Talanta 2019, 196, 47–53. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Zhang, L.; Zuo, Y. Graphene Oxide Based Molecularly Imprinted Polymers Modified with β-Cyclodextrin for Selective Extraction of Di(2-Ethylhexyl) Phthalate in Environmental Waters. J. Sep. Sci. 2019, 42, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Curulli, A.; Pasquali, M.; Zane, D. Determination of Caffeic Acid in Wine Using PEDOT Film Modified Electrode. Food Chem. 2014, 156, 81–86. [Google Scholar] [CrossRef]
- Velmurugan, M.; Balasubramanian, P.; Chen, S.M. Determination of Caffeic Acid in Wine Samples Based on the Electrochemical Reduction of Graphene Oxide Modified Screen Printed Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 4173–4182. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Chen, H.A. A Voltammetric Determination of Caffeic Acid in Red Wines Based on the Nitrogen Doped Carbon Modified Glassy Carbon Electrode. Sci. Rep. 2017, 7, 45924. [Google Scholar] [CrossRef]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Exploring the Chemical Space of White Wine Antioxidant Capacity: A Combined DPPH, EPR and FT-ICR-MS Study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef]
- Lingua, M.; Fabani, M.; Wunderlin, D.; Baroni, V. In Vivo Antioxidant Activity of Grape, Pomace and Wine from Three Red Varieties Grown in Argentina: Its Relationship to Phenolic Profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V. Part I. Polyphenols Composition and Antioxidant Potential during ‘Blaufränkisch’ Grape Maceration and Red Wine Maturation, and the Effects of Trans-Resveratrol Addition. Food Chem. Toxicol. 2020, 137, 111122. [Google Scholar] [CrossRef]
- Bitar, M.; Maalouly, J.; Chebib, H.; Lerbret, A.; Cayot, P.; Bou-maroun, E. Experimental Design Approach in the Synthesis of Molecularly Imprinted Polymers Specific for Iprodione Fungicide Experimental Design Approach in the Synthesis of Molecularly Imprinted Polymers Specific for Iprodione Fungicide. REACT 2015, 94, 17–24. [Google Scholar] [CrossRef]
- Miura, C.; Matsunaga, H.; Haginaka, J. Molecularly Imprinted Polymer for Caffeic Acid by Precipitation Polymerization and Its Application to Extraction of Caffeic Acid and Chlorogenic Acid from Eucommia Ulmodies Leaves. J. Pharm. Biomed. Anal. 2016, 127, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]

| Cyclic Voltammetry | HPLC-UV | ||||
|---|---|---|---|---|---|
| Wine Sample | Added (CA) (mmol/L) | Found (CA) (mmol/L) | Recovery (%) | Found (CA) (mmol/L) | Recovery (%) |
| No CA added | 0 | 0.009 ± 0.004 | - | 0.010 ± 0.002 | - |
| 1st level | 0.167 | 0.149 ± 0.009 | 89 | 0.141 ± 0.005 | 88 |
| 2nd level | 0.222 | 0.187 ± 0.01 | 84 | 0.183 ± 0.008 | 89 |
| 3rd level | 0.278 | 0.221 ± 0.008 | 79 | 0.230 ± 0.019 | 83 |
| 4th level | 0.416 | 0.337 ± 0.014 | 81 | 0.343 ± 0.013 | 81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhachem, M.; Bou-Maroun, E.; Abboud, M.; Maroun, R.G.; Cayot, P. Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine. Antioxidants 2022, 11, 2036. https://doi.org/10.3390/antiox11102036
Elhachem M, Bou-Maroun E, Abboud M, Maroun RG, Cayot P. Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine. Antioxidants. 2022; 11(10):2036. https://doi.org/10.3390/antiox11102036
Chicago/Turabian StyleElhachem, Marie, Elias Bou-Maroun, Maher Abboud, Richard G. Maroun, and Philippe Cayot. 2022. "Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine" Antioxidants 11, no. 10: 2036. https://doi.org/10.3390/antiox11102036
APA StyleElhachem, M., Bou-Maroun, E., Abboud, M., Maroun, R. G., & Cayot, P. (2022). Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine. Antioxidants, 11(10), 2036. https://doi.org/10.3390/antiox11102036









