Differential Expression of α-Enolase in Clinical Gastric Tissues and Cultured Normal/Cancer Cells in Response to Helicobacter pylori Infection and cagA Transfection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Specimen Collection and Detection
2.2. Co-Cultivation of Gastric Cells and H. pylori
2.3. Construction of CagA Expression Vector
2.4. Cellular Transfection and Expression
2.5. Statistical Analyses
3. Results
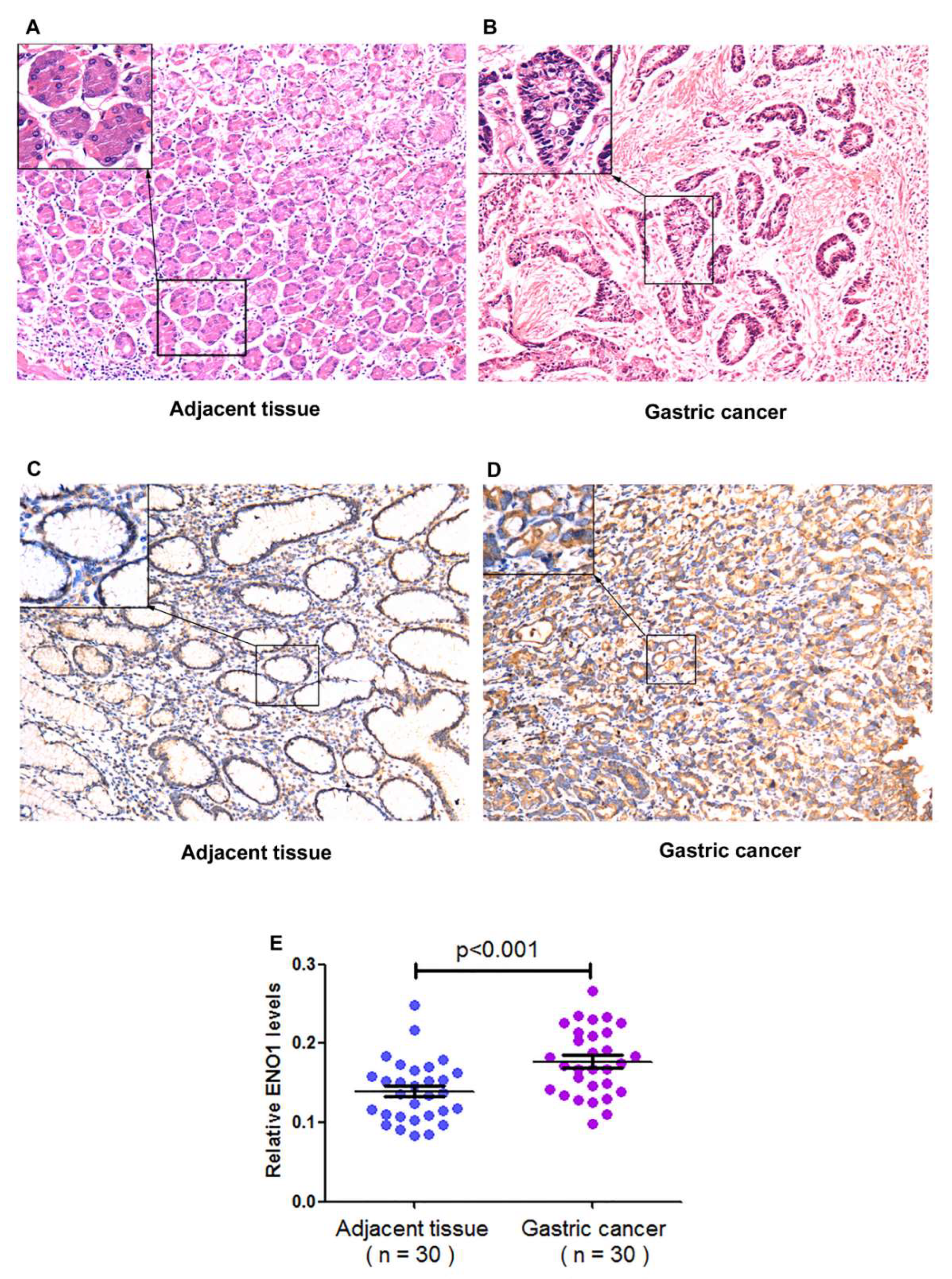
3.1. Collection of Clinical Specimens
3.2. Testing Results of the Gastric Specimens
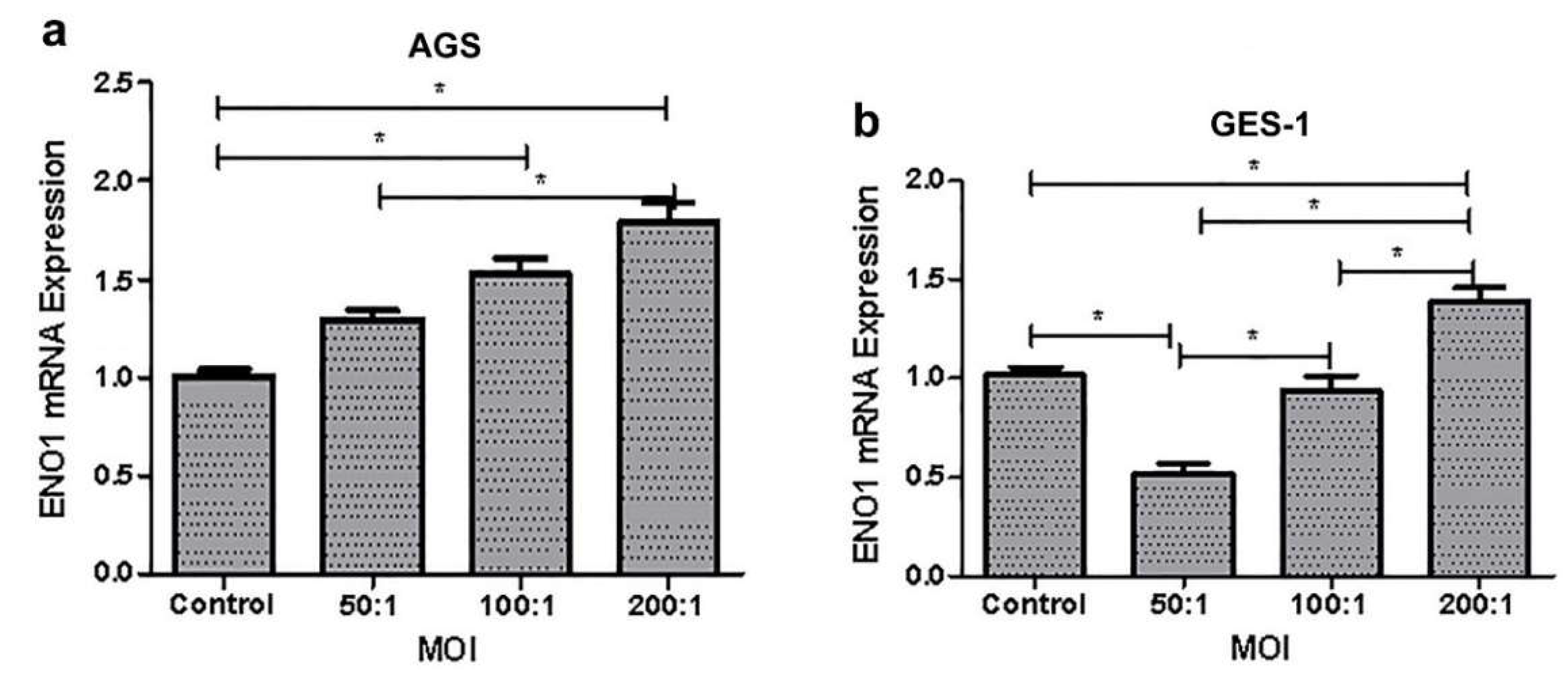
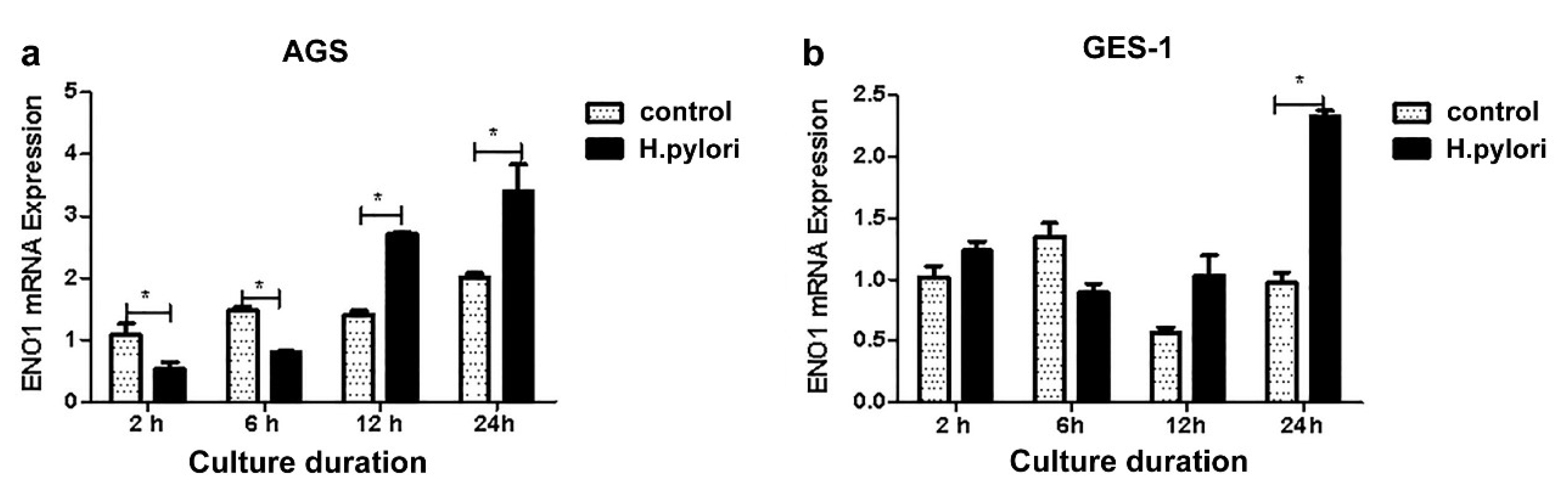
3.3. Co-Culture of Gastric Cells with H. pylori
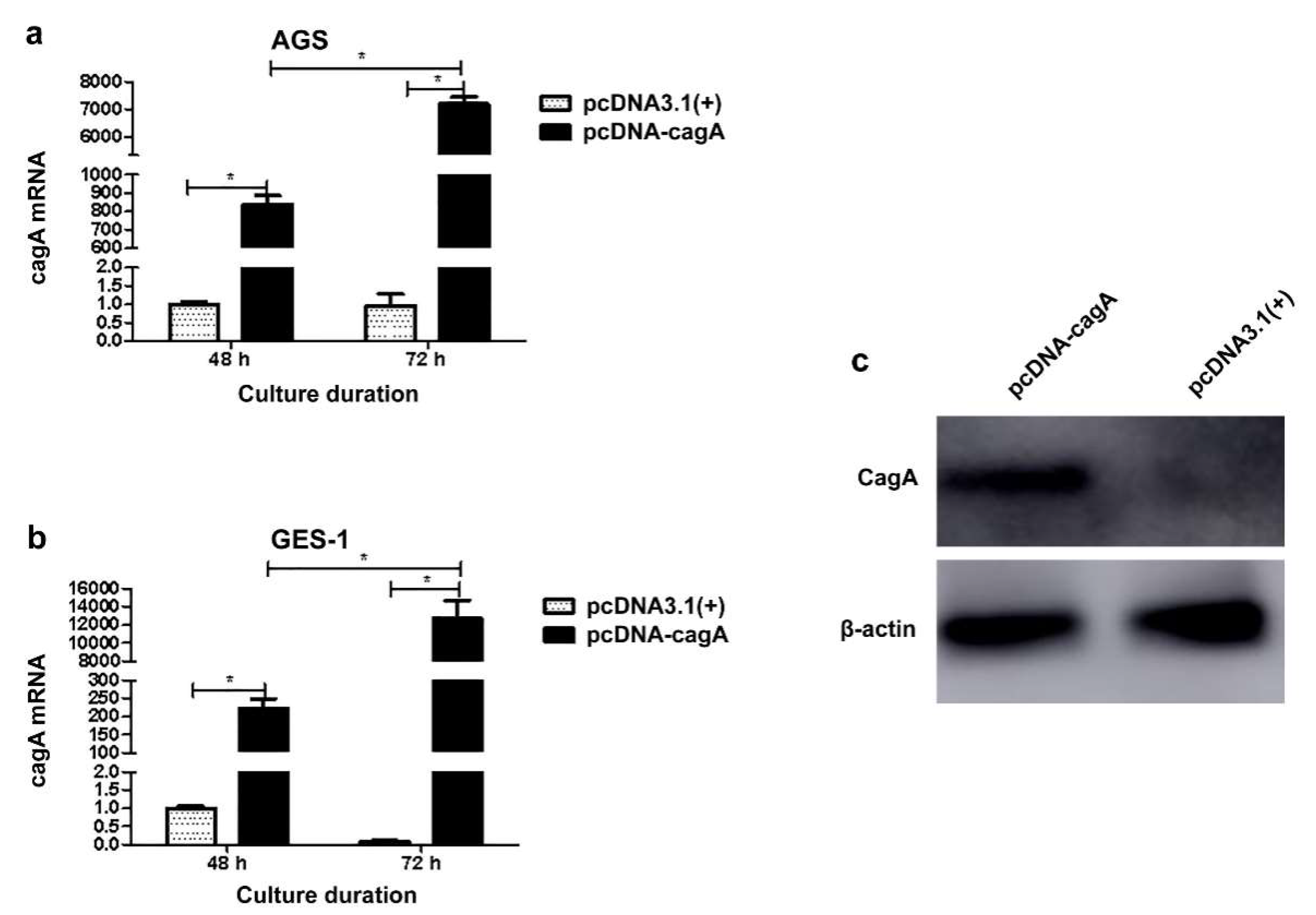
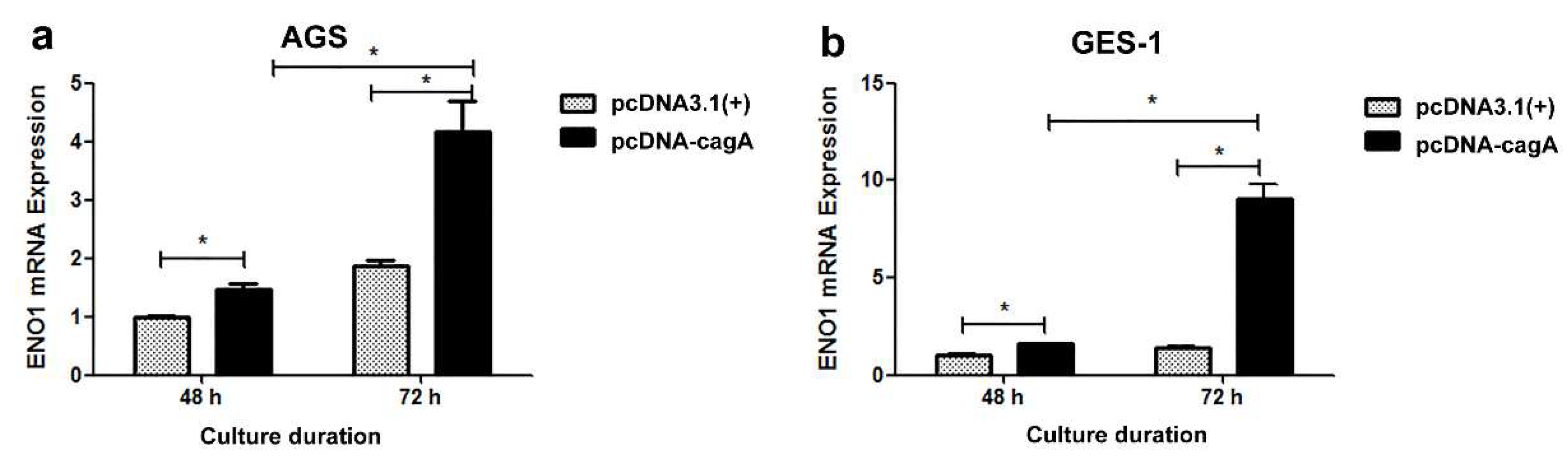
3.4. Cellular Transfection and Impact on ENO1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 2020, 21, E4012. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, B.; Wang, C.; Huo, H.; Aziziaram, Z. Risk assessment of gastric cancer in the presence of Helicobacter pylori cagA and hopQII genes. Cell Mol. Biol. 2022, 67, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; Barbaglia, Y.; Tedeschi, F.; Zalazar, F. Helicobacter pylori infection: A balance between bacteria and host. Rev. Argent Microbiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Palrasu, M.; Zaika, E.; Paulrasu, K.; Caspa Gokulan, R.; Suarez, G.; Que, J.; El-Rifai, W.; Peek, R.M., Jr.; Garcia-Buitrago, M.; Zaika, A.I. Helicobacter pylori pathogen inhibits cellular responses to oncogenic stress and apoptosis. PLoS Pathog. 2022, 18, e1010628. [Google Scholar] [CrossRef]
- Zahmatkesh, M.E.; Jahanbakhsh, M.; Hoseini, N.; Shegefti, S.; Peymani, A.; Dabin, H.; Samimi, R.; Bolori, S. Effects of exosomes derived from Helicobacter pylori outer membrane vesicle-infected hepatocytes on hepatic stellate cell activation and liver fibrosis induction. Front. Cell. Infect. Microbiol. 2022, 12, 857570. [Google Scholar] [CrossRef]
- Sukri, A.; Hanafiah, A.; Mohamad Zin, N.; Kosai, N.R. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS 2020, 128, 150–161. [Google Scholar] [CrossRef]
- Brasil-Costa, I.; Souza, C.O.; Monteiro, L.C.R.; Santos, M.E.S.; Oliveira, E.H.C.; Burbano, R.M.R. H. pylori infection and virulence factors cagA and vacA (s and m regions) in gastric adenocarcinoma from Pará State, Brazil. Pathogens 2022, 11, 414. [Google Scholar] [CrossRef]
- Fock, K.M. Review article: The epidemiology and prevention of gastric cancer. Aliment. Pharmacol. Ther. 2014, 40, 250–260. [Google Scholar] [CrossRef]
- Yakoob, J.; Fatima, S.S.; Abbas, Z.; Mustafa, S.F.; Khan, H.A.; Raghib, M.F.; Awan, S.; Ahmad, Z. Distribution of gastric carcinoma in an area with a high prevalence of Helicobacter pylori. Turk. J. Gastroenterol. 2017, 28, 98–103. [Google Scholar] [CrossRef]
- Chen, S.; Duan, G.; Zhang, R.; Fan, Q. Helicobacter pylori cytotoxin-associated gene A protein upregulates alpha-enolase expression via Src/MEK/ERK pathway: Implication for progression of gastric cancer. Int. J. Oncol. 2014, 45, 764–770. [Google Scholar] [CrossRef]
- Sukri, A.; Hanafiah, A.; Kosai, N.R.; Mohammed Taher, M.; Mohamed, R. New insight on the role of Helicobacter pylori cagA in the expression of cell surface antigens with important biological functions in gastric carcinogenesis. Helicobacter 2022, 27, e12913. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Song, D.; Liu, Y.; Yan, H.; Chen, X. Helicobacter pylori CagA protein attenuates 5-fu sensitivity of gastric cancer cells through upregulating cellular glucose metabolism. Onco. Targets Ther. 2020, 13, 6339–6349. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Ko, C.C.; Chang, J.G.; Chen, S.F.; Wu, M.S.; Lin, J.T.; Chow, L.P. Subcellular and functional proteomic analysis of the cellular responses induced by Helicobacter pylori. Mol. Cell. Proteomics 2006, 5, 702–713. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Sun, M. Correlation of HpSlyD+ Infection with Wnt Pathway Related Protein Expression In Gastric Mucosal Tissues of Patients with Different Gastric Diseases. Master’s Thesis, China Medical University, Taibei, China, 2018. [Google Scholar]
- Roussel, Y.; Wilks, M.; Harris, A.; Mein, C.; Tabaqchali, S. Evaluation of DNA extraction methods from mouse stomachs for the quantification of H. pylori by real-time PCR. J. Microbiol. Methods 2005, 62, 71–81. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, R.; Wang, C.; Yu, F.; Yu, M.; Chen, S.; Fan, Q.; Xi, Y.; Duan, G.E. coli enterotoxin LtB enhances vaccine- induced anti-H. pylori protection by promoting leukocyte migration into gastric mucus via inflammatory lesions. Cells 2019, 8, 982. [Google Scholar] [CrossRef] [Green Version]
- Khalilpour, A.; Kazemzadeh-Narbat, M.; Tamayol, A.; Oklu, R.; Khademhosseini, A. Biomarkers and diagnostic tools for detection of Helicobacter pylori. Appl. Microbiol. Biotechnol. 2016, 100, 4723–4734. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.M.; Zheng, Z.X.; Zhao, X.; Shi, J.; Bi, J.J.; Pei, W.; Feng, Q. Optimization of reference genes for normalization of the quantitative polymerase chain reaction in tissue samples of gastric cancer. Asian Pac. J. Cancer Prev. 2014, 15, 5815–5818. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Shen, K.; Sun, H.; Li, R.; Wang, Z.; Xie, Z. Clinical significance of serum neuron-specific enolase in gastric adenocarcinoma. Medicine 2020, 99, e19829. [Google Scholar] [CrossRef]
- Boroughs, L.K.; Deberardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Capello, M.; Ferri-Borgogno, S.; Cappello, P.; Novelli, F. Alpha-Enolase: A promising therapeutic and diagnostic tumor target. FEBS J. 2011, 278, 1064–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perconti, G.; Ferro, A.; Amato, F.; Rubino, P.; Randazzo, D.; Wolff, T.; Feo, S.; Giallongo, A. The kelch protein NS1-BP interacts with alpha-enolase/MBP-1 and is involved in c-Myc gene transcriptional control. Biochim. Biophys. Acta 2007, 1773, 1774–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Luo, Q.; Long, H.; Hu, Z.; Que, T.; Zhang, X.; Li, Z.; Wang, G.; Yi, L.; Liu, Z.; et al. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol. Cancer 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, F.; Kong, M.; Wang, S.; Nan, L.; Hu, B.; Olszewski, M.A.; Miao, Y.; Ji, D.; Jiang, W.; et al. Validation of a high-throughput multiplex genetic detection system for Helicobacter pylori identification, quantification, virulence, and resistance analysis. Front. Microbiol. 2016, 7, 1401. [Google Scholar] [CrossRef] [Green Version]
- Gonciarz, W.; Krupa, A.; Hinc, K.; Obuchowski, M.; Moran, A.P.; Gajewski, A.; Chmiela, M. The Effect of Helicobacter pylori infection and different H. pylori components on the proliferation and apoptosis of gastric epithelial cells and fibroblasts. PLoS ONE 2019, 14, e0220636. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liu, D.; Zhou, L.; Xie, Y.; Li, Y. FAM60A, increased by Helicobacter pylori, promotes proliferation and suppresses apoptosis of gastric cancer cells by targeting the PI3K/AKT pathway. Biochem. Biophys. Res. Commun. 2020, 521, 1003–1009. [Google Scholar] [CrossRef]
| Primer | Length | Sequence | Length of Products |
|---|---|---|---|
| ENO1 sense | 19 bp | 5′-TGTACCGCCACATCGCTGA-3′ | 147 bp |
| ENO1 anti-sense | 21 bp | 5′-TGAAGTTTGCTGCACCGACTG-3′ | |
| 18S rRNA sense | 20 bp | 5′-CAGCCACCCGAGATTGAGCA-3′ | 244 bp |
| 18S rRNA anti-sense | 20 bp | 5′-TAGTAGCGACGGGCGGGTGT-3′ |
| Characters | CSG (n = 73) | PCD (n = 39) | GC (n = 30) | p |
|---|---|---|---|---|
| Age (years) | 50.25 ± 13.21 | 56.69 ± 11.48 | 60.50 ± 10.03 | <0.001 |
| Male | 42 | 21 | 25 | 0.023 |
| Female | 31 | 18 | 5 | |
| H. pylori + | 42 | 22 | 21 | 0.433 |
| H. pylori − | 31 | 17 | 9 |
| Characters | Groups | n | ENO1 Protein | p |
|---|---|---|---|---|
| H. pylori Infection | H. pylori + | 21 | 0.171 ± 0.043 | 0.244 |
| H. pylori − | 9 | 0.191 ± 0.039 | ||
| Age | <55 | 8 | 0.188 ± 0.040 | 0.425 |
| ≥55 | 22 | 0.173 ± 0.043 | ||
| Location | Cancerous tissues | 30 | 0.177 ± 0.042 | <0.001 |
| Paracancerous tissues | 30 | 0.139 ± 0.039 | ||
| Lymphatic metastasis | + | 19 | 0.183 ± 0.044 | 0.305 |
| − | 11 | 0.167 ± 0.039 | ||
| Differentiation grade | Low | 16 | 0.162 ± 0.040 | 0.038 |
| Moderate/High | 14 | 0.194 ± 0.039 |
| Disease | H. pylori | n | ENO1 mRNA | p |
|---|---|---|---|---|
| CSG§ | + | 42 | 5.93 ± 7.28 | 0.037 |
| − | 31 | 3.89 ± 5.01 | ||
| PCD§ | + | 22 | 2.44 ± 2.52 | 0.630 |
| − | 17 | 1.66 ± 1.24 | ||
| CSG§+PCD§ | + | 64 | 4.37 ± 5.19 | 0.047 |
| − | 48 | 2.89 ± 3.37 | ||
| GCΔ | + | 21 | 0.17 ± 0.04 | 0.244 |
| − | 9 | 0.19 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; He, M.; Li, J.; Wang, H.; Chen, S.; Zhang, X.; Zhang, H.; Duan, G.; Zhang, R. Differential Expression of α-Enolase in Clinical Gastric Tissues and Cultured Normal/Cancer Cells in Response to Helicobacter pylori Infection and cagA Transfection. Medicina 2022, 58, 1453. https://doi.org/10.3390/medicina58101453
Yu F, He M, Li J, Wang H, Chen S, Zhang X, Zhang H, Duan G, Zhang R. Differential Expression of α-Enolase in Clinical Gastric Tissues and Cultured Normal/Cancer Cells in Response to Helicobacter pylori Infection and cagA Transfection. Medicina. 2022; 58(10):1453. https://doi.org/10.3390/medicina58101453
Chicago/Turabian StyleYu, Feiyan, Mengya He, Jian Li, Haiyan Wang, Shuaiyin Chen, Xiaojuan Zhang, Huijuan Zhang, Guangcai Duan, and Rongguang Zhang. 2022. "Differential Expression of α-Enolase in Clinical Gastric Tissues and Cultured Normal/Cancer Cells in Response to Helicobacter pylori Infection and cagA Transfection" Medicina 58, no. 10: 1453. https://doi.org/10.3390/medicina58101453





