Integrated Metabolomics and Network Pharmacology to Decipher the Latent Mechanisms of Protopanaxatriol against Acetic Acid-Induced Gastric Ulcer
Abstract
1. Introduction
2. Results
2.1. Anti-GU Effect
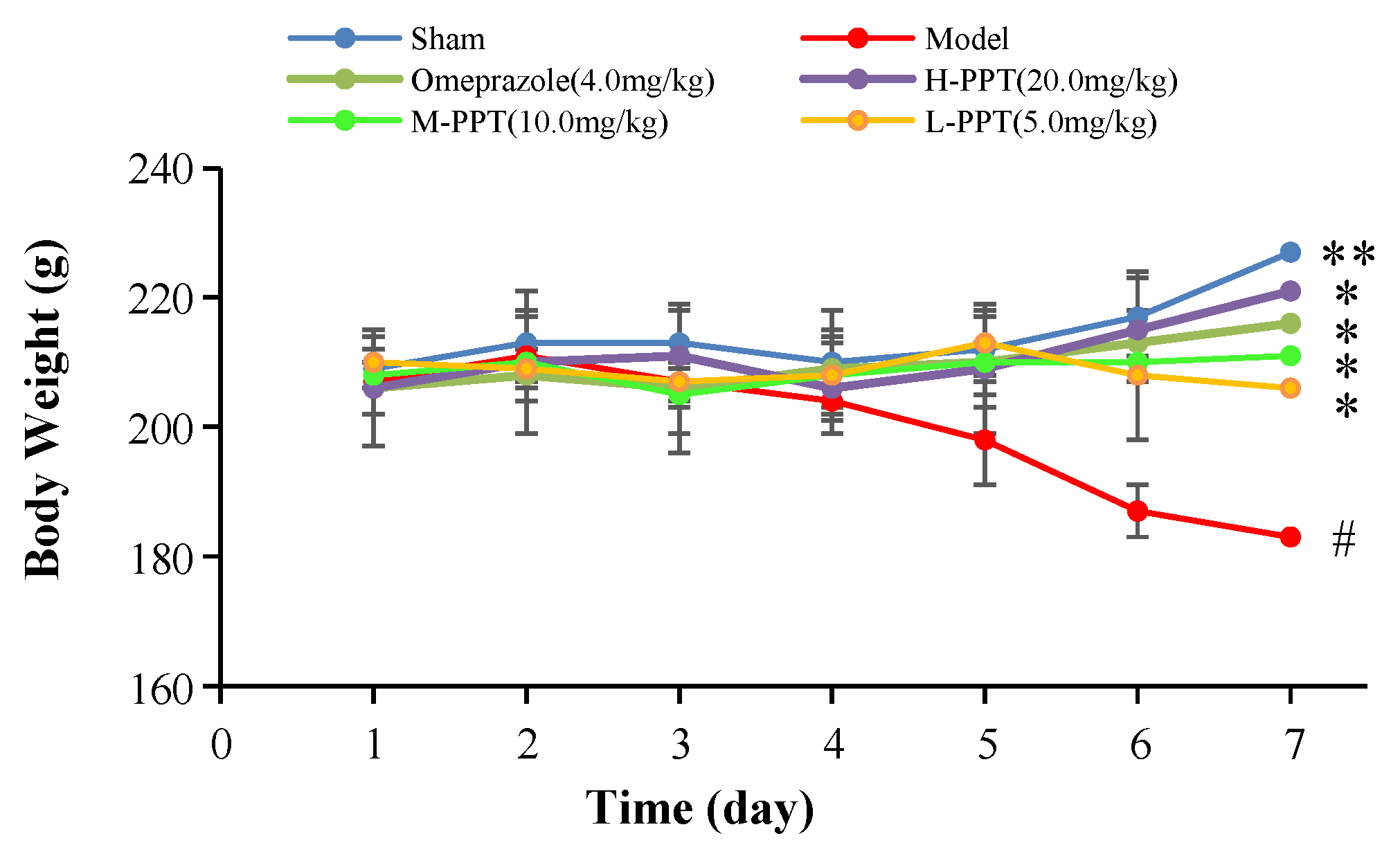
2.1.1. Body Weights of the Rats
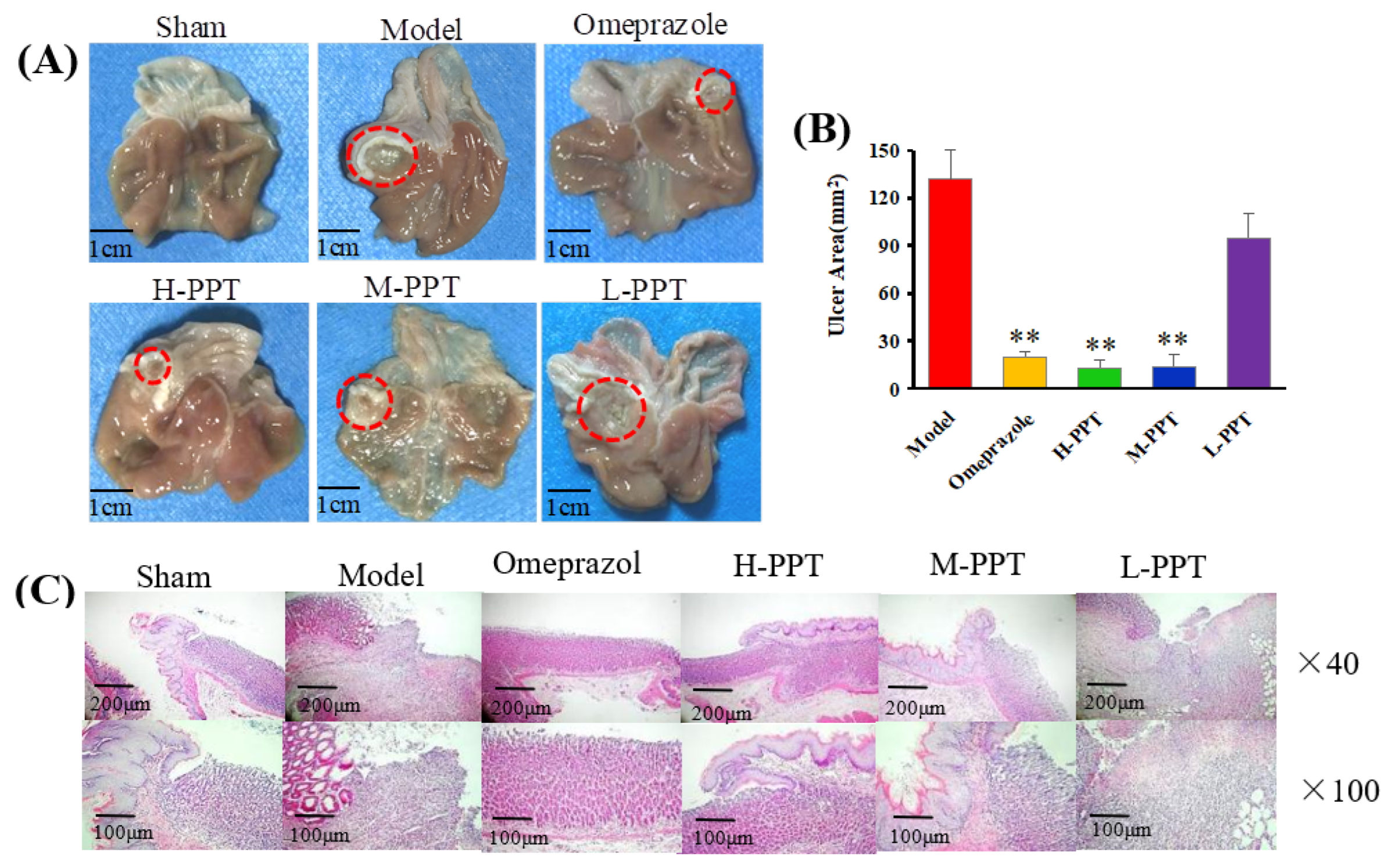
2.1.2. Macroscopic Results
2.1.3. Histological Evaluation of Gastric Ulcers
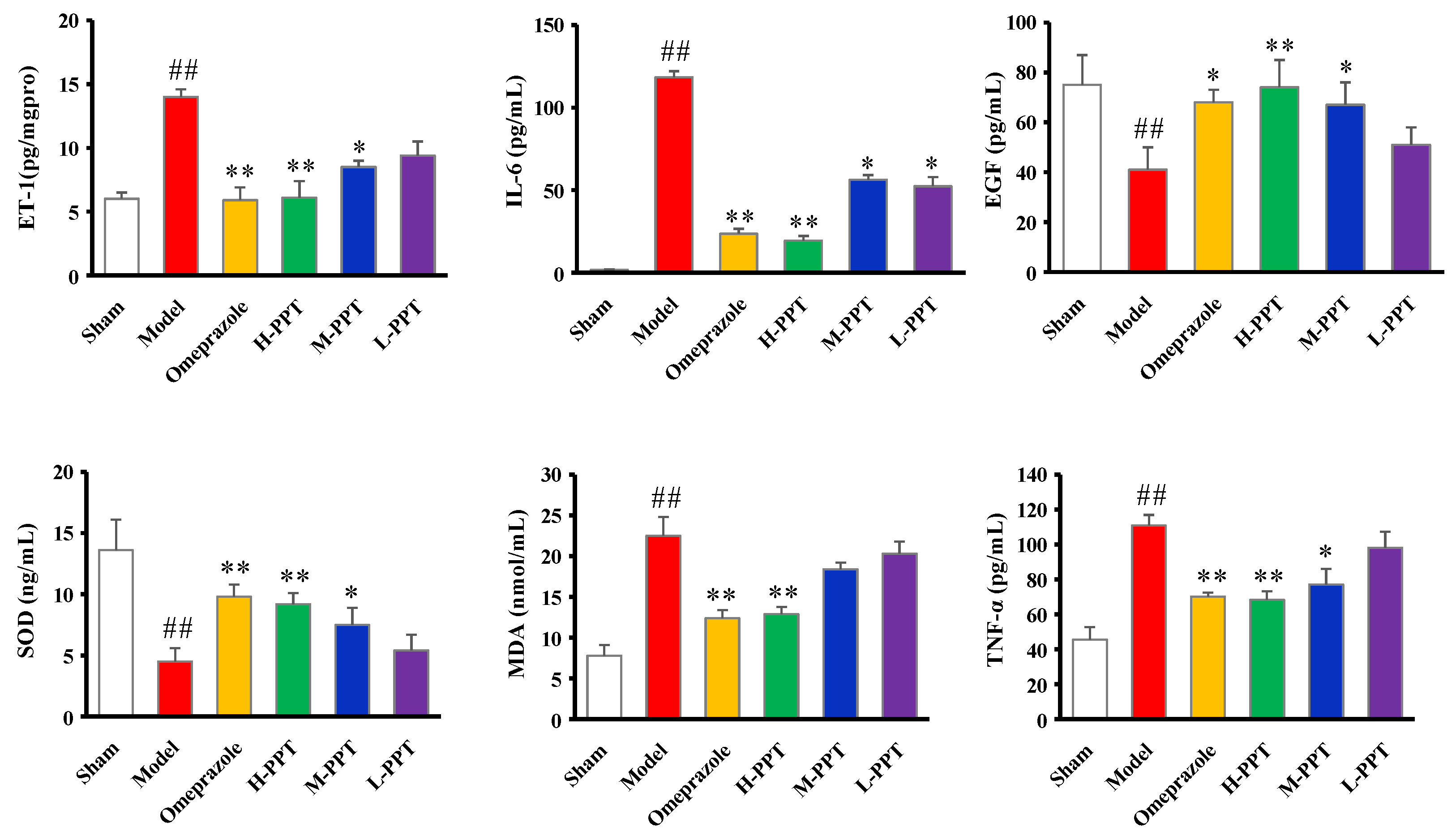
2.1.4. ET-1, IL-6, EGF, SOD, MDA and TNF-α Levels in Serum
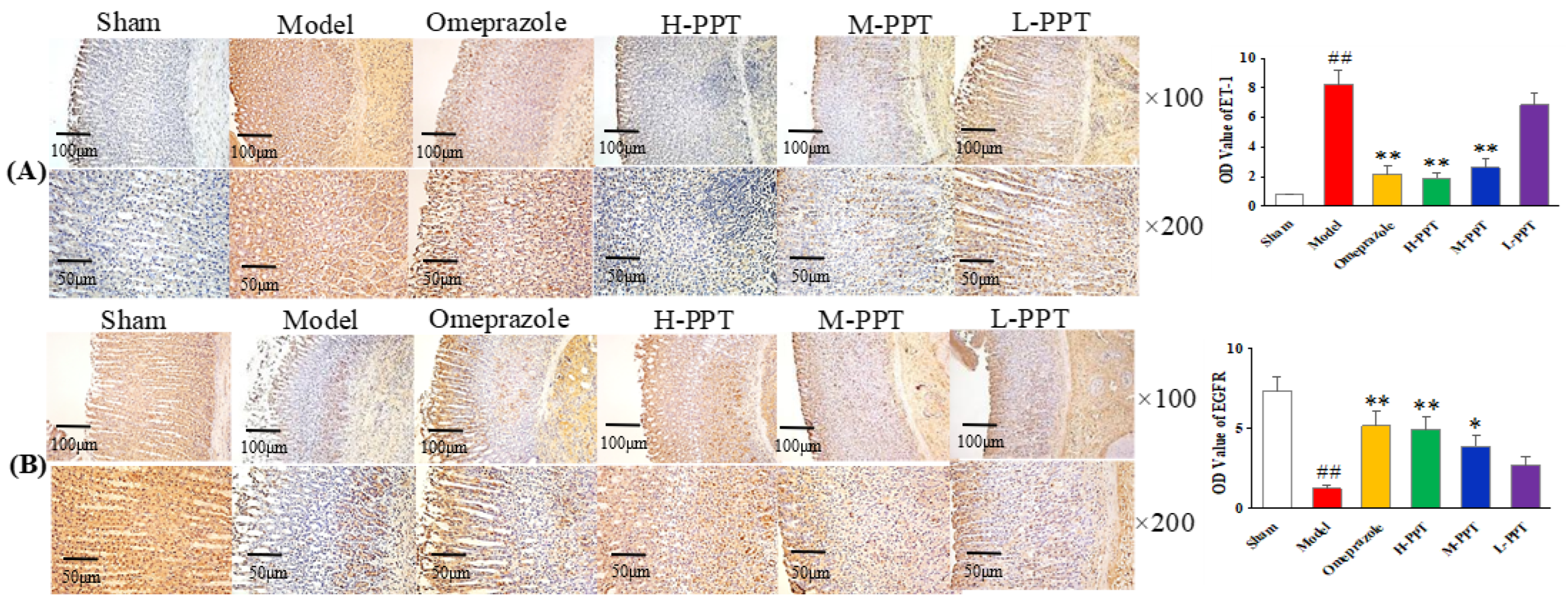
2.1.5. Immunohistochemical Analysis
2.2. Metabolomics Study
2.2.1. Method Validation
2.2.2. Identification of the Differential Metabolites and Pathway Analysis
2.3. Network Pharmacology
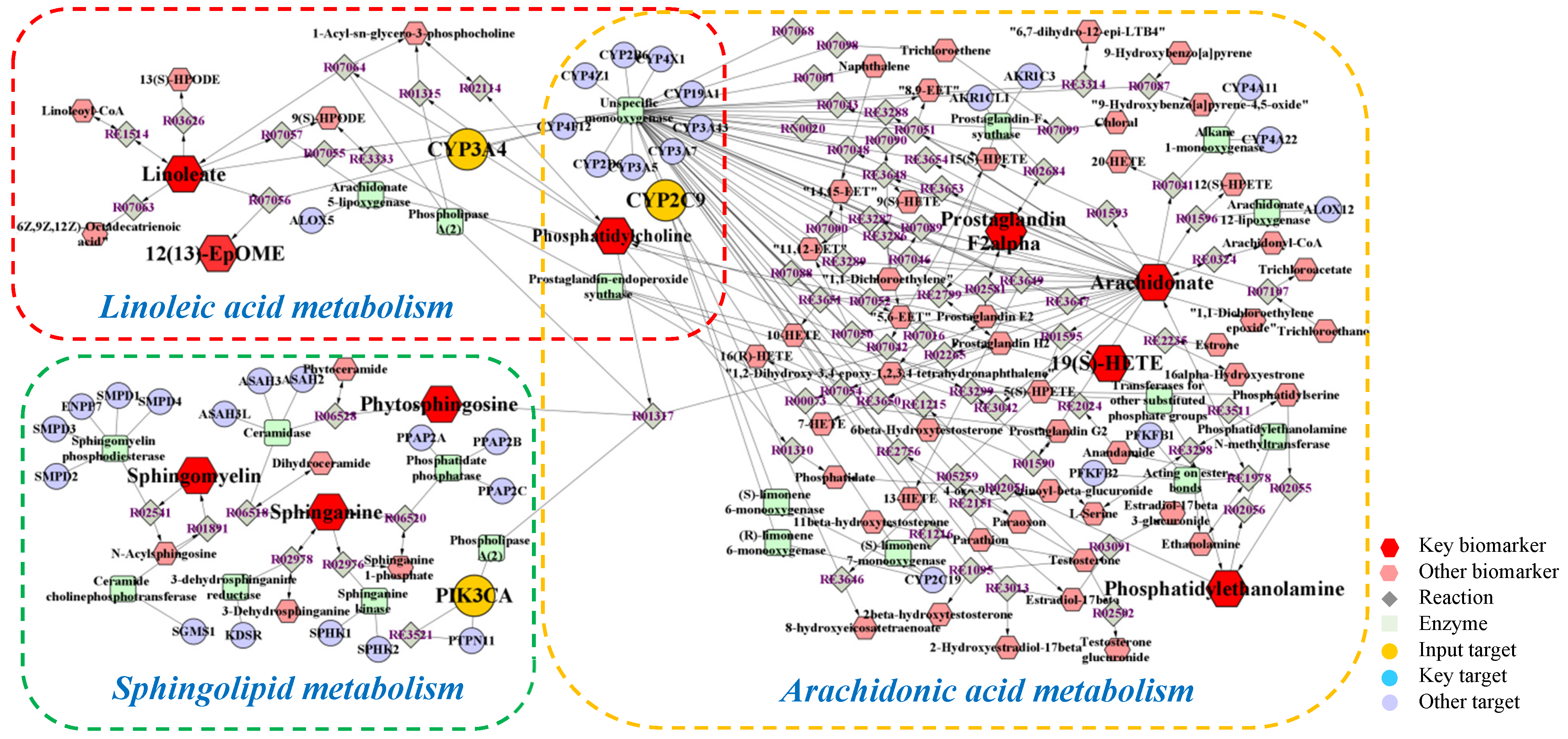
2.4. Integrated Analysis of Metabolomics and Network Pharmacology
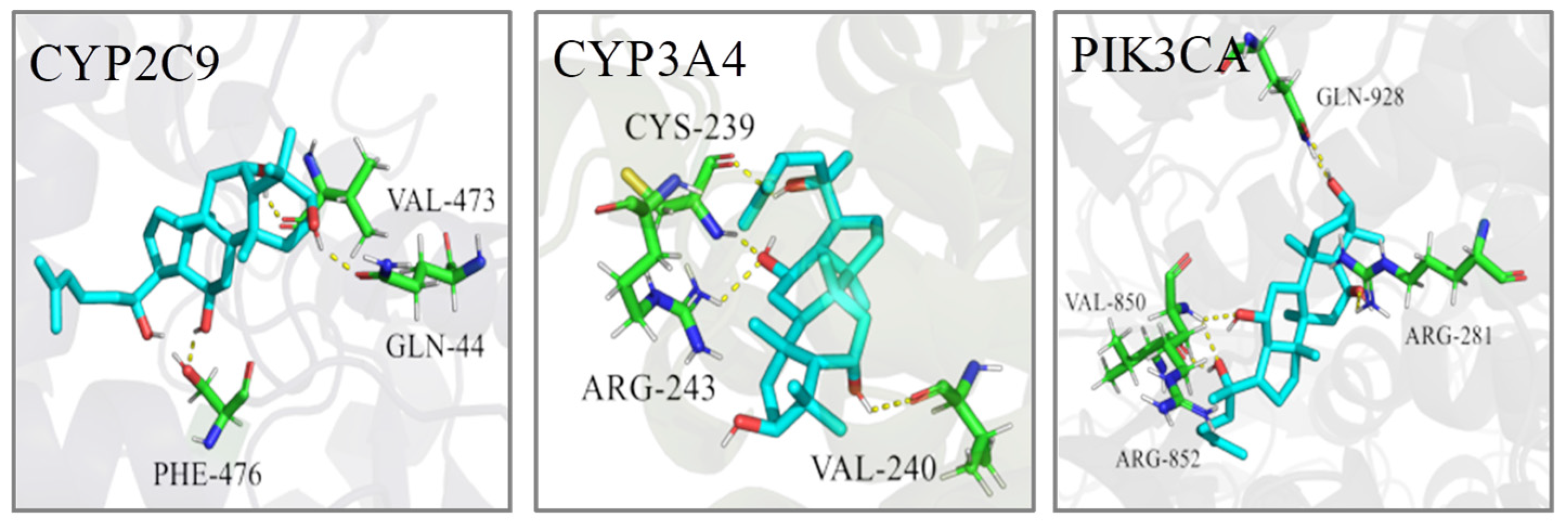
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. UPLC-QTOF-MS Conditions
4.2.2. Experimental Design
4.2.3. Preparation of Samples
4.2.4. Therapeutic Effects
Body Weights
Macroscopic Evaluation of Stomach
Histological Analysis
Biochemical Estimations in Serum
Immunohistochemical Analysis
4.2.5. Metabolomics Study
Method Validation of Metabolomics Study
Data Analysis of Metabolomics Strategies
4.2.6. Network Pharmacology Analysis
4.2.7. Integrated Analysis Involving Metabolomics and Network Pharmacology
4.2.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AUC | Area under curve |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| ESI | Electrospray ionization |
| ET-1 | Endothelin-1 |
| GU | Gastric ulcer |
| H&E | Hematoxylin and eosin |
| HETE | Hydroxyeicosatetraenoic acid |
| H-PPT | High-dose PPT |
| L-PPT | Low-dose PPT |
| LysoPC | Lysophosphatidylcholine |
| M-PPT | Moderate-dose PPT |
| MDA | Malondialdehyde |
| OPLS-DA | Orthogonal Projections to Latent Structures Discriminant Analysis |
| PC | Phosphatidylcholine |
| PCA | Principal Component Analysis |
| PDB | Protein Data Bank |
| QC | Quality Control |
| QTOF-MS | Quadrupole Time of Flight-Mass Spectrometry |
| ROC | Receiver operating characteristic |
| RSD | Relative Standard Deviation |
| RT | Retention Time |
| SD | Standard deviation |
| SM | Sphingomyelin |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor-α |
| UPLC | Ultra-Performance Liquid Chromatography |
| VIP | Variable importance in the projection |
References
- Wu, Y.; Murray, G.K.; Byrne, E.M.; Sidorenko, J.; Visscher, P.M.; Wray, N.R. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun. 2021, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Halim, P.A.; Georgey, H.H.; George, M.Y.; El Kerdawy, A.M.; Said, M.F. Design and Synthesis of Novel 4-Fluorobenzamide-based Derivatives as Promising Anti-inflammatory and Analgesic Agents with an Enhanced Gastric Tolerability and COX-inhibitory Activity. Bioorg. Chem. 2021, 115, 105253. [Google Scholar]
- Kavitt, R.T.; Lipowska, A.M.; Anyane-Yeboa, A.; Gralnek, I. Diagnosis and treatment of peptic ulcer disease. Am. J. Med. 2019, 132, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; More, S.K.; Vomhof-DeKrey, E.E.; Golovko, M.Y.; Basson, M.D. Small molecule fAK activator promotes human intestinal epithelial monolayer wound closure and mouse ulcer healing. Sci. Rep. 2019, 9, 14669. [Google Scholar] [CrossRef] [PubMed]
- Matthis, A.L.; Kaji, I.; Engevik, K.A.; Akiba, Y.; Kaunitz, J.D.; Montrose, M.H.; Aihara, E. Deficient Active Transport Activity in Healing Mucosa After Mild Gastric Epithelial Damage. Dig. Dis. Sci. 2020, 65, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N.; Park, J.M.; Kim, E.H.; Hahm, K.B. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J. Gastrointest. Pathophysiol. 2014, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic ulcer disease: A brief review of conventional therapy and herbal treatment options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, D.K.; Cho, S.W.; Hong, H.D. Ginseng, the root of Panax ginseng C.A. Meyer, protects ethanol-induced gastric damages in rat through the induction of cytoprotective heat-shock protein 27. Dig. Dis. Sci. 2008, 53, 606–613. [Google Scholar] [CrossRef]
- Jeong, C.S. Effect of butanol fraction of Panax ginseng head on gastric lesion and ulcer. Arch. Pharmacal Res. 2002, 25, 61–66. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sugimoto, S.; Nakamura, S.; Sakumae, H.; Matsuda, H. Medicinal flowers. XVI. New dammarane-type triterpene tetraglycosides and gastroprotective principles from flower buds of Panax ginseng. Chem. Pharm. Bull. 2007, 55, 1034–1038. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Choi, G.E.; Song, G.Y.; Bae, J.S. Pulmonary Protective Functions of Rare Ginsenoside Rg4 on Particulate Matter-induced Inflammatory Responses. Biotechnol. Bioprocess Eng. 2019, 24, 445–453. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J. Toxicol. Environ. Health Part A 2019, 82, 338–350. [Google Scholar] [CrossRef]
- Shin, D.; Moon, H.W.; Oh, Y.; Kim, K.; Kim, D.D.; Lim, C.J. Defensive Properties of Ginsenoside Re against UV-B-Induced Oxidative Stress through Up-Regulating Glutathione and Superoxide Dismutase in HaCaT Keratinocytes. Iran. J. Pharm. Res. 2018, 17, 249–260. [Google Scholar]
- Christensen, L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar]
- Chen, X.L.; Si, H.R.; Li, L.; Fu, D.X.; Liu, J.T.; Liu, J.P.; Li, P.Y. The optimization of semi-synthetic protopanaxatriol process based on Box-Behnken design-response surface methodology. J. Chin. Med. Mat. 2021, 44, 2162–2167. [Google Scholar]
- Liu, Y.; Li, W.; Li, P.; Deng, M.C.; Yang, S.L.; Yang, L. The Inhibitory Effect of Intestinal Bacterial Metabolite of Ginsenosides on CYP3A Activity. Biol. Pharm. Bull. 2004, 27, 1555–1560. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, C.C.; Pu, F.F.; Lin, P.Y.; Qian, T.X. Metabolite profiling of ginsenoside Rg1 after oral administration in rat. Biomed. Chromatogr. 2015, 28, 1320–1324. [Google Scholar] [CrossRef]
- Gong, Z.G.; Hu, J.; Wu, X.; Xu, Y.J. The Recent Developments in Sample Preparation for Mass Spectrometry-Based Metabolomics. Crit. Rev. Anal. Chem. 2017, 47, 325–331. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Khalid, H.M.; Saleem, F. Metabolomics: A Way Forward for Crop Improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Choi, B.M.; Seo, E.A.; Kim, D.H.; Shin, M.K.; Kim, J.D.; Kim, J.B.; Chung, H.T. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004, 205, 23–29. [Google Scholar] [CrossRef]
- Li, S.; Xue, X.; Yang, X.; Zhou, S.; Wang, S.; Meng, J. A Network Pharmacology Approach Used to Estimate the Active Ingredients of Moutan Cortex Charcoal and the Potential Targets in Hemorrhagic Diseases. Biol. Pharm. Bull. 2019, 42, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Luo, Z.; Zhou, Y.; Zhang, L.; Wu, Y.; Ding, L.; Shi, Y. Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach. Biomed. Pharmacother. 2019, 117, 109094. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Kim, K.; Lim, C.J. Photoprotective properties of 20(S)-protopanaxatriol, an aglycone of ginseng saponins: Protection from ultraviolet-B radiation-induced oxidative stress in human epidermal keratinocytes. Mol. Med. Rep. 2016, 14, 2839–2845. [Google Scholar] [CrossRef]
- Yang, S.; Cao, C.; Chen, S.; Hu, L.Y.; Bao, W.; Shi, H.D. Serum Metabolomics Analysis of Quercetin against Acrylamide-Induced Toxicity in Rats. J. Agric. Food Chem. 2016, 64, 9237–9245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, W.T.; Lu, Y.; Hu, S.N.; Gao, R.; Sun, Z.X.; Chen, X.N.; Ma, J.M.; Guo, S.; Du, S.Y. Network pharmacology-based identification of protective mechanism of Panax notoginseng saponins on aspirin induced gastrointestinal injury. Biomed. Pharmacother. 2018, 105, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to beside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.Y.; Zhao, X.; Qia, Y.; Sun, P.; Zhu, K.; Li, J.; Sun, B.Z. Lactobacillus fermentum Suo Attenuates HCl/Ethanol Induced Gastric Injury in Mice through Its Antioxidant Effects. Nutrients 2016, 8, 155. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, W.C.; Lu, L.L.; Zheng, J.Y.; Hu, X.S.; Yu, Z.H.; Zhu, L.X. Study on the antiulcer effects of Veronicastrum axillare on gastric ulcer in rats induced by ethanol based on tumor necrosis factor-alpha (TNF-alpha) and endothelin-1 (ET-1). Asian Pac. J. Trop. Biomed. 2013, 3, 925–930. [Google Scholar] [CrossRef]
- Lee, K.P.; Choi, N.H.; Sudjarwo, G.W.; Ahn, S.H.; Park, I.S.; Lee, S.R.; Hong, H. Protective Effect of Areca catechu Leaf Ethanol Extract Against Ethanol-Induced Gastric Ulcers in ICR Mice. J. Med. Food 2016, 19, 127–132. [Google Scholar] [CrossRef]
- Karolin Kamel, A.A. Comparative evaluation of the anti-ulcer activity of curcumin and omeprazole during the acute phase of gastric ulcer-efficacy of curcumin in gastric ulcer prevention against omeprazole. Food Nutr. Sci. 2011, 6, 628–640. [Google Scholar]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdeldayem, U.A.; Ali, S.S.; Al Jaouni, S.K. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxidative Med. Cell. Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kawi, S.H.; Hashem, K.S.; Saad, M.K.; Fekry, G.; Abdel-Hameed, E.M. The ameliorative effects of cinnamon oil against ethanol-induced gastric ulcer in rats by regulating oxidative stress and promoting angiogenesis. J. Mol. Histol. 2022, 53, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Xu, H.Y.; He, H.B.; Li, X.M.; Feng, M.L.; He, Y.M.; Jiang, W.J.; Wang, J.Z.; Xu, D.X.; Zou, K. Total triterpenes from the fruits of Chaenomeles speciosa (Sweet) Nakai protects against indomethacin-induced gastric mucosal injury: Involvement of TFF1-mediated EGF/EGFR and apoptotic pathways. J. Pharm. Pharmacol. 2020, 72, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Park, H.S.; Kim, K.R.; Choi, H.N.; Jang, K.Y.; Chung, M.J.; Kang, M.J.; Lee, D.G.; Moon, W.S. Increased expression of epidermal growth factor receptor and betacellulin during the early stage of gastric ulcer healing. Mol. Med. Rep. 2008, 1, 505–510. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, X.Q.; Dai, Y.J.; Wang, Q.H.; Wang, G.Y.; Liu, D.P.; Gu, X.Z.; Yu, D.R.; Ma, Y.L.; et al. Treatment Mechanism of Gardeniae Fructus and Its Carbonized Product Against Ethanol-Induced Gastric Lesions in Rats. Front. Pharmacol. 2019, 10, 750. [Google Scholar] [CrossRef]
- Schepp, W.; Steffen, B.; Ruoff, H.J.; Schusdziarra, V.; Classen, M. Modulation of rat gastric mucosal prostaglandin E2 release by dietary linoleic acid: Effects on gastric acid secretion and stress-induced mucosal damage. Gastroenterology 1988, 95, 18–25. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Shen, X.T.; Xuan, S.X.; Yang, B.; Ruan, Q.F.; Cui, H.; Zhao, Z.X.; Jin, J. Serum and colon metabolomics study reveals the anti-ulcerative colitis effect of Croton crassifolius Geisel. Phytomedicine 2021, 87, 153570. [Google Scholar] [CrossRef]
- Baek, S.W.; Kim, N.K.; Jin, H.J.; Koh, C.W.; Kim, C.K.; Kwon, O.H.; Kim, J.S.; Cho, M.H.; Park, C.K. Anti-ulcer Actions of Phytosphingosine Hydrochloride in Different Experimental Rat Ulcer Models. Arzneimittelforschung 2005, 55, 461–465. [Google Scholar] [CrossRef]
- Kanfer, J.N.; Young, O.M.; Shapiro, D.; Brady, R.O. The metabolism of sphingomyelin. I. Purification and properties of a sphingomyelin-cleaving enzyme from rat liver tissue. J. Biol. Chem. 1966, 241, 1081–1084. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, S.Q. Effects of sodium ferulate on the oxidative damage and arachidonic acid metabolism in patients with ulcerative colitis. Lishizhen Med. Mater. Med. Res. 2008, 19, 1168–1169. [Google Scholar]
- Ishibashi, S.; Iwakiri, R.; Shimoda, R.; Ootani, H.; Kawasaki, S.; Tadano, J.; Kikkawa, A.; Ootani, A.; Oda, K.; Fujise, T.; et al. Normalization of Phospholipids Concentration of the Gastric Mucosa was Observed in Patients with Peptic Ulcer after Eradication of Helicobacter pylori. Helicobacter 2010, 7, 245–249. [Google Scholar] [CrossRef]
- Lemahieu, W.; Maes, B.D.; Verbeke, K.; Vanrenterghem, Y. Impact of gastric acid suppressants on cytochrome P450 3A4 and P-glycoprotein: Consequences for FK506 assimilation. Kidney Int. 2005, 67, 1152–1160. [Google Scholar] [CrossRef]
- Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar]
- Pilotto, A.; Seripa, D.; Franceschi, M.; Scarcelli, C.; Colaizzo, D.; Grandone, E.; Niro, V.; Andriulli, A.; Leandro, G.; Mario, F.D.; et al. Genetic susceptibility to nonsteroidal anti-inflammatory drug-related gastroduodenal bleeding: Role of cytochrome P450 2C9 polymorphisms. Gastroenterology 2007, 133, 465–471. [Google Scholar] [CrossRef]
- Martinez, C.; Blanco, G.; Ladero, J.M.; García-Martín, E.; Taxonera, C.; Gamito, F.G.; Diaz-Rubio, M.; Agúndez, J.A.G. Genetic predisposition to acute gastrointestinal bleeding after NSAIDs use. Br. J. Pharmacol. 2004, 141, 205–208. [Google Scholar] [CrossRef]
- Hiromasa, I.; Shinji, T.; Ryosuke, N.; Risa, K.; Tomohide, U.; Mai, O.; Yuichi, U.; Kengo, K. Metabolism of steroids by cytochrome P450 2C9 variants. Biopharm. Drug Dispos. 2018, 39, 371–377. [Google Scholar]
- Obach, R.S.; Zhang, Q.Y.; Dunbar, D.; Kaminsky, L.S. Metabolic characterization of the major human small intestinal cytochrome p450s. Drug Metab. Dispos. 2001, 29, 347–352. [Google Scholar]
- Kanazu, T.; Yamaguchi, Y.; Okamura, N.; Baba, T.; Koike, M. Model for the drug-drug interaction responsible for Cyp3a enzyme inhibition. II: Establishment and evaluation of dexamethasone-pretreated female rats. Xenobiotica 2004, 34, 403–413. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Liu, Q. Glycyrrhizin affects the pharmacokinetics of omeprazole in rats through inducing the activity of CYP3A4, CYP2C19, and P-gp. Lat. Am. J. Pharm. 2018, 37, 2177–2184. [Google Scholar]
- Pu, J.; Wang, F.; Tang, W.; Zhu, M.S. Biotransformation of Ilaprazole in Human Liver Microsomes and Human: Role of CYP3A4 in Ilaprazole Clearance and Drug-Drug Interaction. Drug Metab. Dispos. 2018, 46, 1453–1461. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Newcomb, P.; Hollowood, A.; Moganaden; Gupta, J.; Feakins, R.; Storey, A.; Farthing, M.J.G.; Alderson, D.; Holly, J. A comparison study of gastric cancer risk in patients with duodenal and gastric ulcer: Roles of gastric mucosal histology and p53 codon 72 polymorphism. Dig. Dis. Sci. 2004, 49, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.K.; He, L.; Liang, M.; Liu, J.F. Chitosan/PIK3CA siRNA nanoparticle-mediated PIK3CA gene interference decreases the invasive capacity of gastric cancer cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2014, 34, 1503–1506. [Google Scholar] [PubMed]
- Shi, J.; Yao, D.M.; Liu, W.; Wang, N.; Lv, H.J.; Zhang, G.J.; Ji, M.J.; Xu, L.; He, N.Y.; Shi, B.Y.; et al. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC Cancer 2012, 12, 1471–2407. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Yang, J.L.; Cheng, C.; Huang, Y.H.; Du, F.F.; Wang, F.Q.; Niu, W.; Xu, F.; Jiang, R.R.; Gao, X.M.; et al. Combinatorial metabolism notably affects human systemic exposure to ginsenosides from orally administered extract of Panax notoginseng roots (Sanqi). Drug Metab. Dispos. 2013, 41, 1457–1469. [Google Scholar] [CrossRef]
- Hao, H.P.; Lai, L.; Zheng, C.N.; Wang, Q.O.; Yu, G.; Zhou, X.Y.; Wu, L.A.; Gong, P.; Wang, G.J. Microsomal Cytochrome P450-Mediated Metabolism of Protopanaxatriol Ginsenosides: Metabolite Profile, Reaction Phenotyping, and Structure-Metabolism Relationship. Drug Metab. Dispos. 2010, 38, 1731. [Google Scholar] [CrossRef]
- Wang, W.Y.; Ni, Y.Y.; Wang, L.; Che, X.; Liu, W.H.; Meng, Q.G. Stereoselective oxidation metabolism of 20(S)-protopanaxatriol in human liver microsomes and in rats. Xenobiotica 2015, 45, 385–395. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.W.; Li, W.; Ma, H.; Sun, J.; Deng, M.C.; Yang, L. Ginsenoside Metabolites, Rather Than Naturally Occurring Ginsenosides, Lead to Inhibition of Human Cytochrome P450 Enzymes. Toxicol. Sci. 2006, 91, 356–364. [Google Scholar] [CrossRef]
- Divya, S.; Cho, W.C.; Ghanshyam, U. Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview. Front. Physiol. 2015, 6, 363. [Google Scholar]
- Peng, Z.; Wu, W.W.; Yi, P. The Efficacy of Ginsenoside Rg3 Combined with First-line Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 11, 2519. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, T.; Ma, C.Y.; Song, W.W.; Zhang, J.; Yu, Z.X. Ginsenoside metabolite compound K enhances the efficacy of cisplatin in lung cancer cells. J. Thorac. Dis. 2015, 7, 400–406. [Google Scholar]
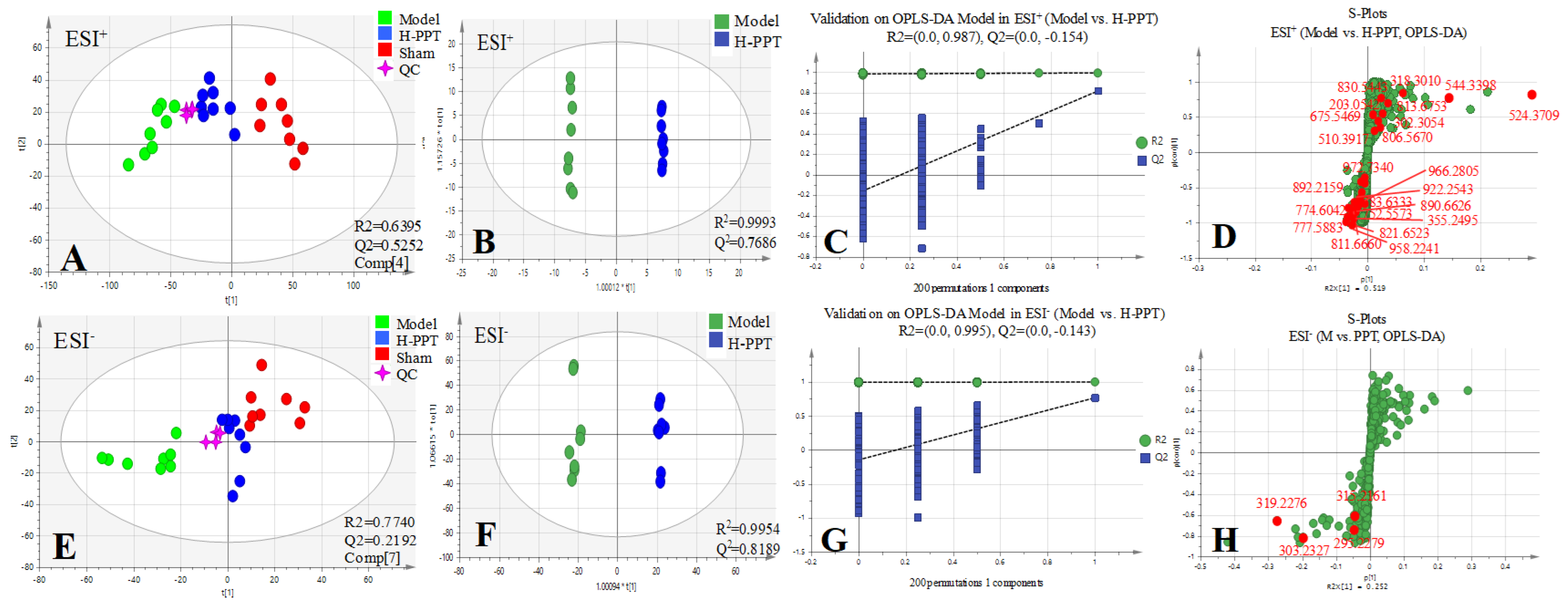
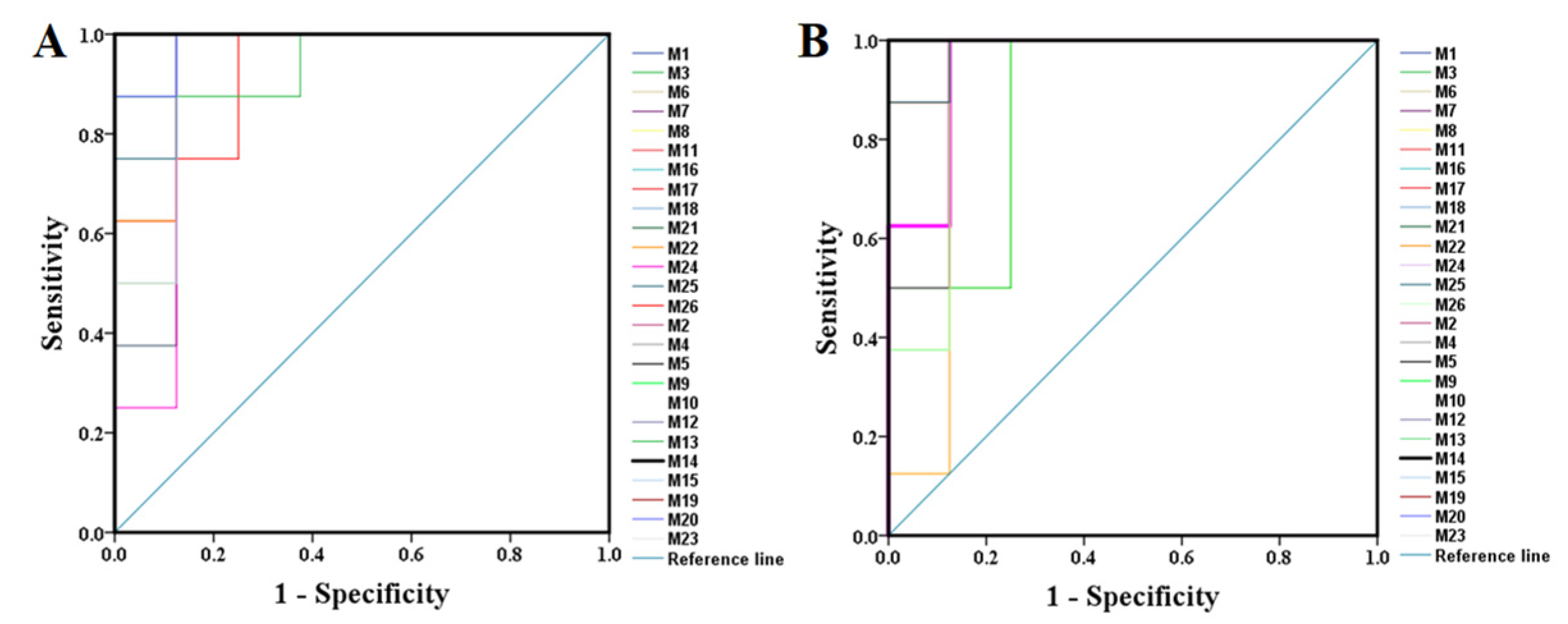
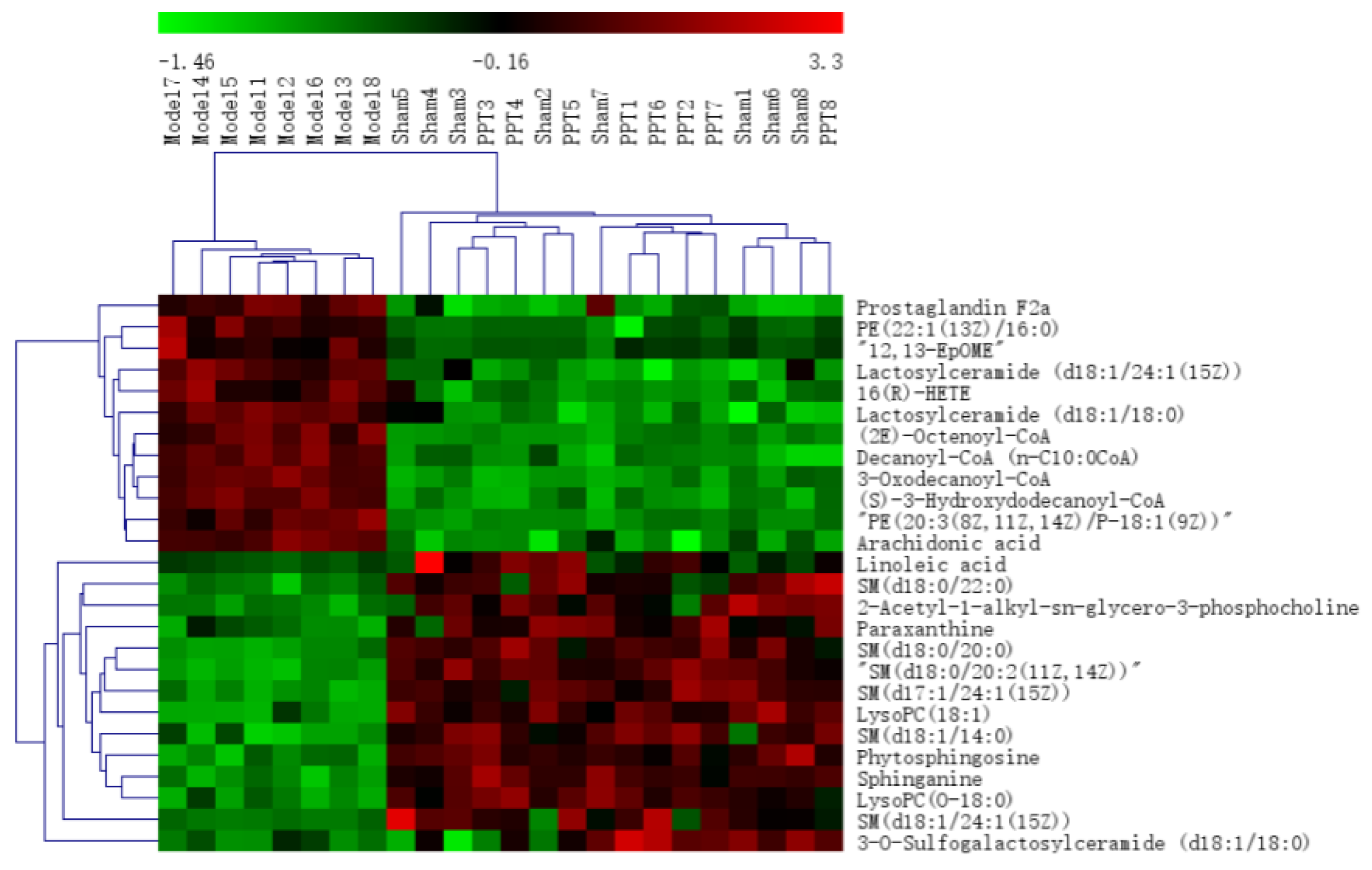
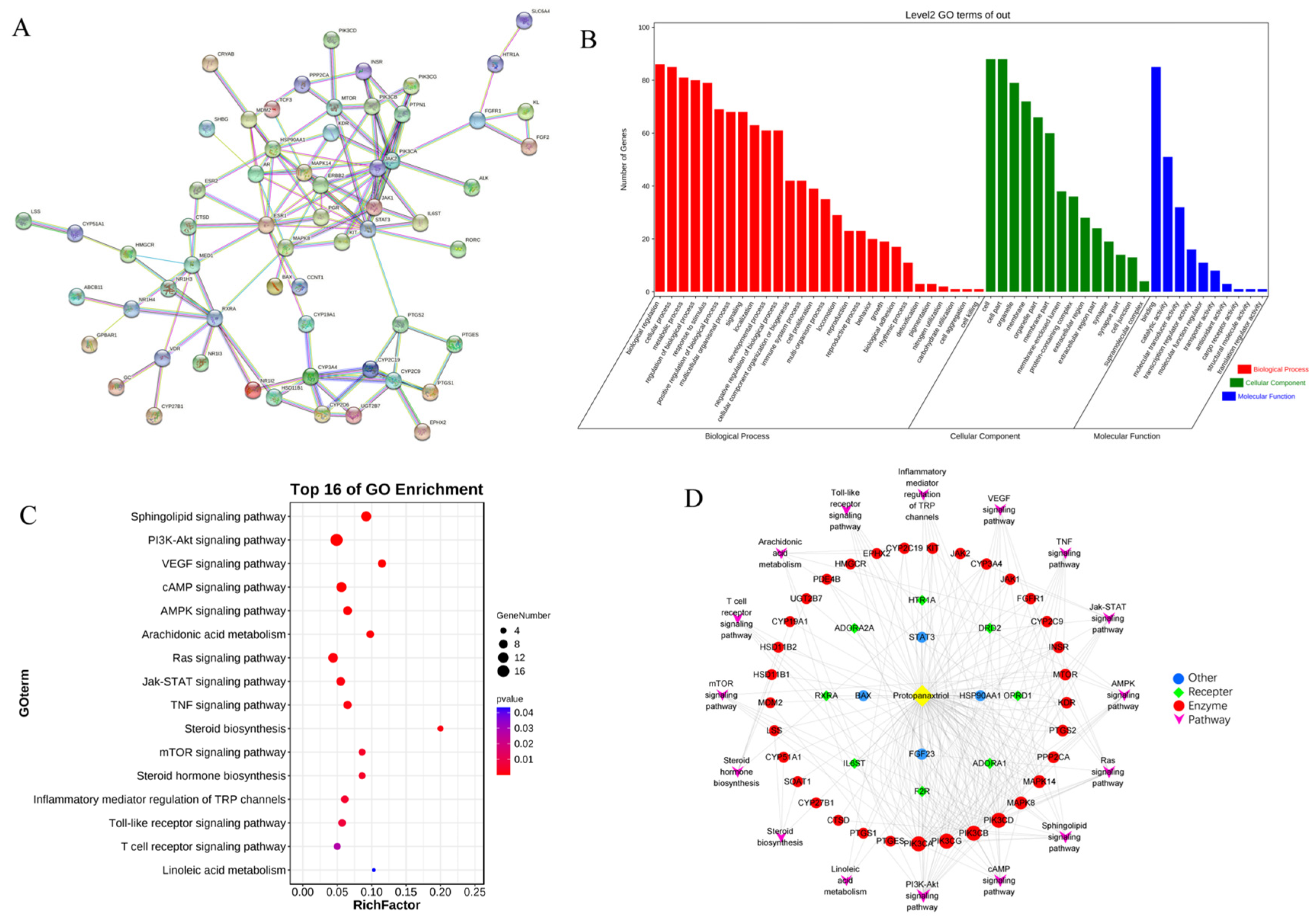

| Tests | ESI+ Mode | ESI- Mode | ||
|---|---|---|---|---|
| Peak Intensity | RT | Peak Intensity | RT | |
| System stability | 0.38–4.19 | 0.24–1.75 | 0.56–1.94 | 0.16–1.13 |
| Precision | 0.35–3.41 | 0.10–0.41 | 0.19–4.65 | 0.07–0.68 |
| Reproducibility | 0.06–0.39 | 0.94–1.17 | 0.14–0.67 | 1.07–1.93 |
| Sample stability | 1.07–4.47 | 0.07–0.98 | 0.97–2.96 | 0.10–1.75 |
| Compound No. | Sham & Model | Model & H-PPT | Compound No. | Sham & Model | Model & H-PPT | ||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | p | AUC | p | AUC | p | AUC | p | ||
| 1 | 0.922 | 0.005 | 0.922 | 0.005 | 14 | 1.000 | 0.001 | 1.000 | 0.001 |
| 2 | 1.000 | 0.001 | 1.000 | 0.001 | 15 | 0.938 | 0.001 | 1.000 | 0.001 |
| 3 | 1.000 | 0.001 | 1.000 | 0.001 | 16 | 1.000 | 0.001 | 1.000 | 0.001 |
| 4 | 0.984 | 0.001 | 0.984 | 0.001 | 17 | 1.000 | 0.001 | 1.000 | 0.001 |
| 5 | 1.000 | 0.001 | 1.000 | 0.001 | 18 | 0.984 | 0.001 | 0.984 | 0.001 |
| 6 | 0.984 | 0.001 | 0.906 | 0.006 | 19 | 1.000 | 0.001 | 1.000 | 0.001 |
| 7 | 0.922 | 0.005 | 0.938 | 0.003 | 20 | 0.984 | 0.001 | 1.000 | 0.001 |
| 8 | 0.938 | 0.003 | 0.922 | 0.005 | 21 | 1.000 | 0.001 | 1.000 | 0.001 |
| 9 | 1.000 | 0.001 | 1.000 | 0.001 | 22 | 0.953 | 0.002 | 0.984 | 0.001 |
| 10 | 1.000 | 0.001 | 0.922 | 0.005 | 23 | 1.000 | 0.001 | 0.953 | 0.002 |
| 11 | 1.000 | 0.001 | 1.000 | 0.001 | 24 | 0.906 | 0.006 | 0.953 | 0.002 |
| 12 | 1.000 | 0.001 | 1.000 | 0.001 | 25 | 0.969 | 0.002 | 0.875 | 0.002 |
| 13 | 0.953 | 0.002 | 0.891 | 0.009 | 26 | 0.922 | 0.005 | 0.984 | 0.001 |
| No. | RT | Mass | Compound Name | VIP | Formula | Adducts | Δm | HMDB ID | Pathways | Content Level |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 0.60 | 203.0542 | Paraxanthine | 2.42 | C7H8N4O2 | M+Na | −1.48 | 0001860 | CM | M < H-PPT |
| 2 a | 10.99 | 774.6042 | PE(22:1(13Z)/16:0) | 1.68 | C43H84NO8P | M+H | 3.74 | 0009517 | GM | M > H-PPT |
| 3 a | 11.07 | 811.6660 | SM(d18:0/22:0) | 2.37 | C45H93N2O6P | M+Na | −1.11 | 0012091 | SM | M < H-PPT |
| 4 a | 11.23 | 752.5573 | PE(20:3(8Z,11Z,14Z)/P-18:1(9Z)) | 2.00 | C43H78NO7P | M+H | −2.79 | 0009381 | GM | M > H-PPT |
| 5 a | 11.24 | 892.2159 | (2E)-Octenoyl-CoA | 2.48 | C29H48N7O17P3S | M+H | 4.60 | 0003949 | FAE | M > H-PPT |
| 6 a | 11.25 | 783.6333 | SM(d18:0/20:0) | 2.31 | C43H89N2O6P | M+Na | −2.94 | 0012090 | SM | M < H-PPT |
| 7 a | 11.27 | 777.5883 | SM(d18:0/20:2(11Z,14Z)) | 2.70 | C43H83N2O6P | M+Na | −0.39 | 0013465 | SM | M < H-PPT |
| 8 a | 11.36 | 821.6523 | SM(d17:1/24:1(15Z)) | 3.09 | C46H91N2O6P | M+Na | 1.34 | 0011696 | SM | M < H-PPT |
| 9 a | 11.37 | 958.2241 | 3-Oxodecanoyl-CoA | 2.11 | C31H52N7O18P3S | M+Na | 4.28 | 0003939 | FAE | M > H-PPT |
| 10 a | 11.39 | 972.7340 | Lactosylceramide(d18:1/24:1(15Z)) | 1.46 | C54H101NO13 | M+H | −1.13 | 0004872 | SM | M > H-PPT |
| 11 a | 11.46 | 675.5469 | SM(d18:1/14:0) | 1.51 | C37H75N2O6P | M+H | 4.14 | 0012097 | SM | M < H-PPT |
| 12 a | 11.49 | 890.6626 | Lactosylceramide (d18:1/18:0) | 2.00 | C48H91NO13 | M+H | 4.49 | 0011591 | SM | M > H-PPT |
| 13 a | 11.54 | 922.2543 | Decanoyl-CoA (n-C10:0CoA) | 2.28 | C31H54N7O17P3S | M+H | −4.88 | 0006404 | FAE | M > H-PPT |
| 14 a | 11.61 | 966.2805 | (S)-3-Hydroxydodecanoyl-CoA | 1.66 | C33H58N7O18P3S | M+H | −4.66 | 0003936 | FAE | M > H-PPT |
| 15 a | 12.39 | 355.2495 | Prostaglandin F2a | 2.48 | C20H34O5 | M+H | 3.09 | 0001139 | AM | M > H-PPT |
| 16 * | 12.72 | 318.3010 | Phytosphingosine | 5.59 | C18H39NO3 | M+H | 0.63 | 0004610 | SM | M < H-PPT |
| 17 * | 14.94 | 302.3054 | Sphinganine | 2.46 | C18H39NO2 | M+H | −1.65 | 0000269 | SM | M < H-PPT |
| 18 a | 16.09 | 544.3398 | LysoPC(18:1(9Z)) | 13.28 | C26H52NO7P | M+Na | 3.49 | 0002815 | GM | M > H-PPT |
| 19 * | 17.08 | 295.2279 | 12,13-EpOME | 1.23 | C18H32O3 | M-H | 2.03 | 0004702 | LM | M > H-PPT |
| 20 * | 18.11 | 319.2276 | 19(S)-HETE | 6.00 | C20H32O3 | M-H | 0.94 | 0004680 | AM | M > H-PPT |
| 21 a | 20.54 | 524.3709 | PC(O-16:0/2:0) | 26.76 | C26H54NO7P | M+H | −1.33 | 0062195 | EM | M > H-PPT |
| 22 a | 21.15 | 510.3917 | LysoPC(O-18:0) | 1.46 | C26H56NO6P | M+H | −1.37 | 0011149 | EM | M > H-PPT |
| 23 * | 22.81 | 303.2327 | Arachidonic acid | 4.54 | C20H32O2 | M-H | 0.99 | 0001043 | AM | M > H-PPT |
| 24 * | 23.14 | 279.2325 | Linoleic acid | 1.71 | C18H32O2 | M-H | 3.96 | 0000673 | LM | M < H-PPT |
| 25 a | 26.33 | 813.6753 | SM(d18:1/24:1(15Z)) | 1.95 | C47H93N2O6P | M+H | 0.37 | 0012107 | SM | M < H-PPT |
| 26 a | 26.39 | 830.5443 | 3-O-Sulfogalactosylceramide (d18:1/18:0) | 1.63 | C42H81NO11S | M+Na | 1.81 | 0012314 | SM | M < H-PPT |
| Pathway Name | Match Status | p | −log (p) | Holm p | FDR | Impact |
|---|---|---|---|---|---|---|
| Sphingolipid metabolism | 5/21 | 2.397 × 10−6 | 5.6203 | 2.0135 × 10−4 | 2.0135 × 10−4 | 0.1582 |
| Linoleic acid metabolism | 3/5 | 0.0013 | 2.8806 | 0.1066 | 0.0276 | 1.000 |
| Fatty acid elongation | 4/27 | 4.2911 × 10−4 | 7.7538 | 0.0339 | 0.0116 | 0.1075 |
| Arachidonic acid metabolism | 3/36 | 0.0080 | 2.0991 | 0.6367 | 0.1337 | 0.3329 |
| Ether lipid metabolism | 2/13 | 0.2146 | 0.6684 | 1.0000 | 1.0000 | 0.1446 |
| Glycerophospholipid metabolism | 2/30 | 0.0667 | 1.1762 | 1.0000 | 0.7998 | 0.1219 |
| Caffeine metabolism | 1/12 | 0.1348 | 0.8710 | 1.0000 | 1.0000 | 0.6923 |
| No. | Target | PDB ID | Bind Energy (Kcal/mol) | Related Pathway |
|---|---|---|---|---|
| 1 | CYP2C9 | 1R9O | −6.78 | AM, LM |
| 2 | CYP3A4 | 2V0M | −9.40 | LM |
| 3 | PIK3CA | 3ZIM | −9.27 | SM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Tan, L.; Liu, J.; Fu, D.; Wang, C.; Li, P.; Li, Z.; Liu, J. Integrated Metabolomics and Network Pharmacology to Decipher the Latent Mechanisms of Protopanaxatriol against Acetic Acid-Induced Gastric Ulcer. Int. J. Mol. Sci. 2022, 23, 12097. https://doi.org/10.3390/ijms232012097
Wang C, Tan L, Liu J, Fu D, Wang C, Li P, Li Z, Liu J. Integrated Metabolomics and Network Pharmacology to Decipher the Latent Mechanisms of Protopanaxatriol against Acetic Acid-Induced Gastric Ulcer. International Journal of Molecular Sciences. 2022; 23(20):12097. https://doi.org/10.3390/ijms232012097
Chicago/Turabian StyleWang, Cuizhu, Luying Tan, Juntong Liu, Dongxing Fu, Caixia Wang, Pingya Li, Zhuo Li, and Jinping Liu. 2022. "Integrated Metabolomics and Network Pharmacology to Decipher the Latent Mechanisms of Protopanaxatriol against Acetic Acid-Induced Gastric Ulcer" International Journal of Molecular Sciences 23, no. 20: 12097. https://doi.org/10.3390/ijms232012097
APA StyleWang, C., Tan, L., Liu, J., Fu, D., Wang, C., Li, P., Li, Z., & Liu, J. (2022). Integrated Metabolomics and Network Pharmacology to Decipher the Latent Mechanisms of Protopanaxatriol against Acetic Acid-Induced Gastric Ulcer. International Journal of Molecular Sciences, 23(20), 12097. https://doi.org/10.3390/ijms232012097







