Substituting Whole Wheat Flour with Pigeon Pea (Cajanus cajan) Flour in Chapati: Effect on Nutritional Characteristics, Color Profiles, and In Vitro Starch and Protein Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pigeon Pea Flour
2.3. Preparation of Chapati
2.4. Proximate Analysis
2.5. Color Measurement
2.6. Starch Digestion
2.7. Total Starch and Starch Fraction
2.8. Amino-Group-Containing Residue
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.2. Color Profiles
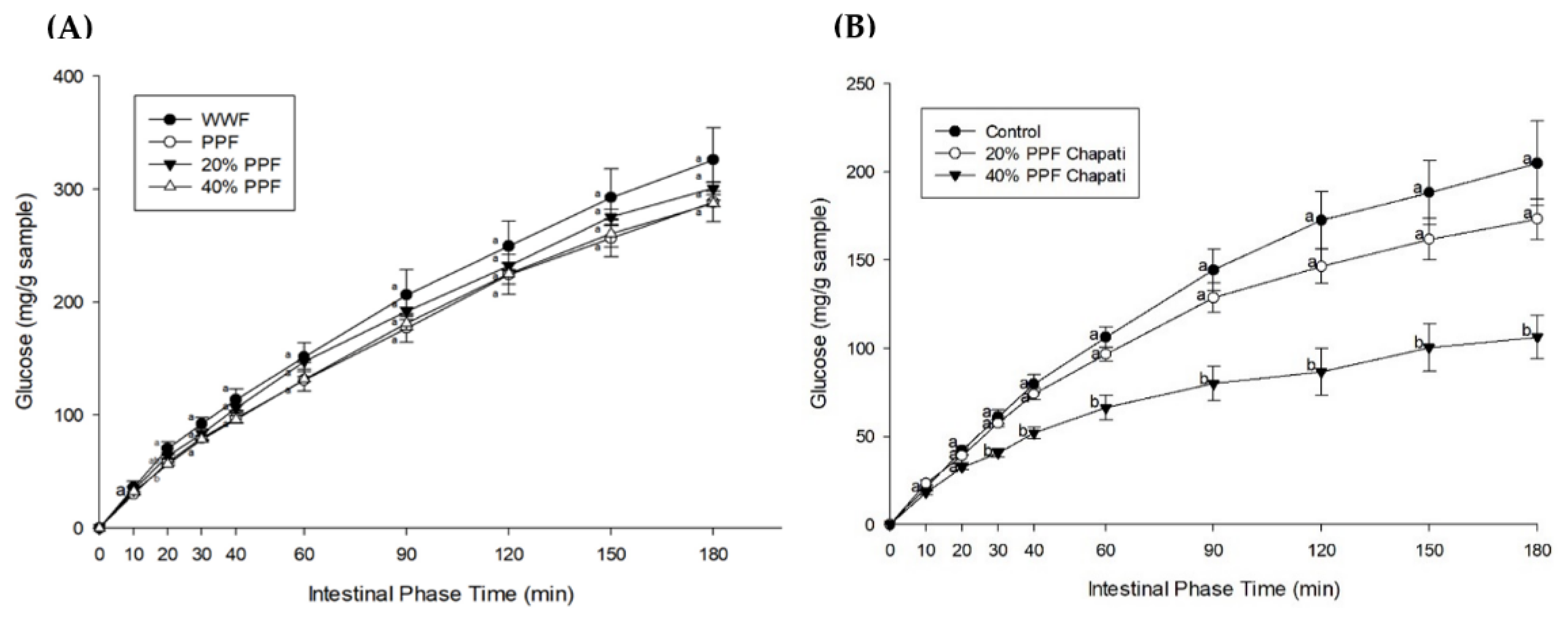
3.3. Starch Digestibility and Fraction
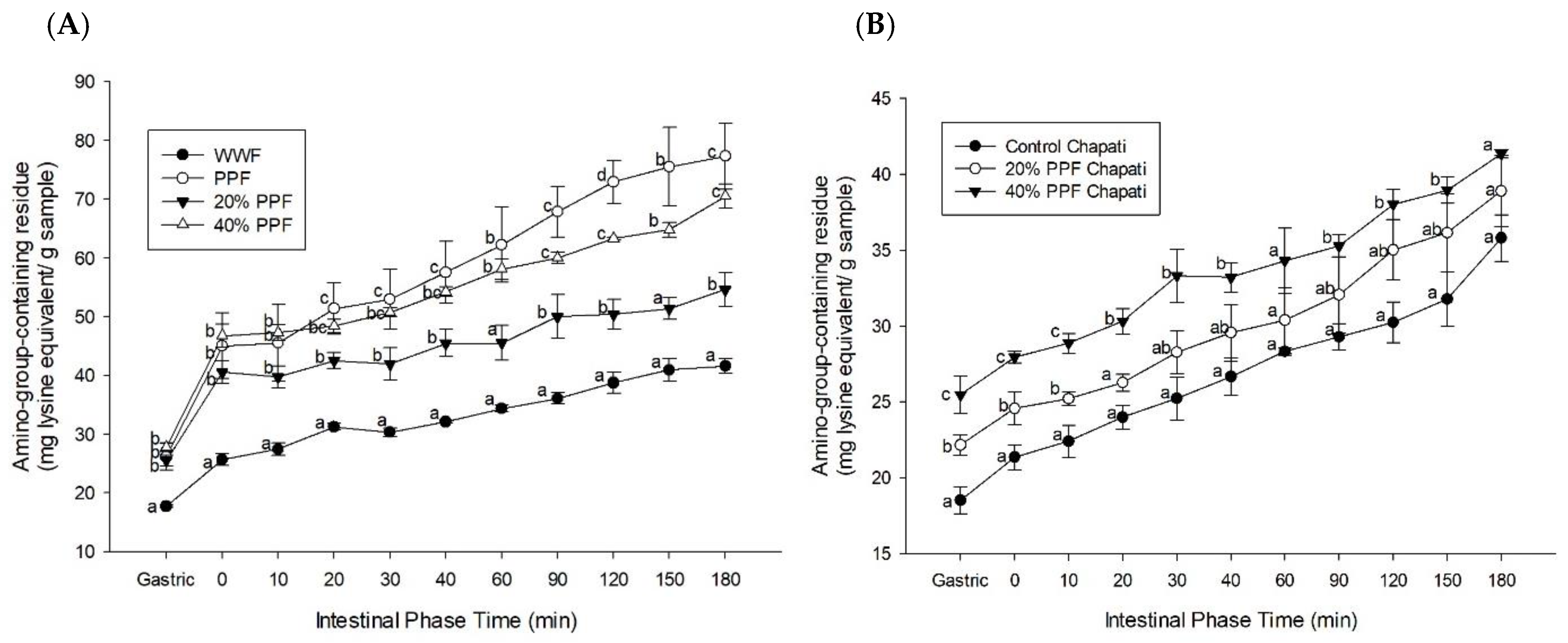
3.4. Amino-Group-Containing Residue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional Update for Physicians: Plant-Based Diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapp, C.; Barnard, N.; Katcher, H. A Plant-Based Diet for Type 2 Diabetes: Scientific Support and Practical Strategies. Diabetes Educ. 2010, 36, 33–48. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, E2671. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated With a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant Proteins in Relation to Human Protein and Amino Acid Nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef] [Green Version]
- Millward, D.J. The Nutritional Value of Plant-Based Diets in Relation to Human Amino Acid and Protein Requirements. Proc. Nutr. Soc. 1999, 58, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Prasada Rao, U.J.S.; Haridas Rao, P. Studies on Protein and Its High-Molecular-Weight Subunit Composition in Relation to Chapati-Making Quality of Indian Wheat Cultivars. J. Sci. Food Agric. 2003, 83, 225–231. [Google Scholar] [CrossRef]
- Yadav, D.N.; Singh, K.K.; Rehal, J. Studies on Fortification of Wheat Flour with Defatted Rice Bran for Chapati Making. J. Food Sci. Technol. 2012, 49, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Gujral, H.S.; Solah, V. Effect of Incorporating Finger Millet in Wheat Flour on Mixolab Behavior, Chapatti Quality and Starch Digestibility. Food Chem. 2017, 231, 156–164. [Google Scholar] [CrossRef]
- Akhtar, S.; Layla, A.; Sestili, P.; Ismail, T.; Afzal, K.; Rizvanov, A.A.; Asad, M.H.H.B. Glycemic and Insulinemic Responses of Vegetables and Beans Powders Supplemented Chapattis in Healthy Humans: A Randomized, Crossover Trial. BioMed Res. Int. 2019, 2019, 7425367. [Google Scholar] [CrossRef]
- Sarkar, S.; Panda, S.; Yadav, K.K.; Kandasamy, P. Pigeon Pea (Cajanus cajan) an Important Food Legume in Indian Scenario—A Review. Legume Res.-Int. J. 2018, 43, 601–610. [Google Scholar] [CrossRef]
- Ajani, A.O.; Adegoke, G.O. Nutritional Quality and Sensory Acceptability of Fermented Breadfruit—Pigeon Pea Based Custard. Ann. Food Sci. Technol. 2018, 19, 265–274. [Google Scholar]
- Li, L. The Effect of Soy Protein Isolate, Starch and Salt on Quality of Ready-to-Eat Restructured Beef Products. Int. J. Food Eng. Technol. 2019, 3, 31–44. [Google Scholar] [CrossRef]
- Mora, E.; Andrés, C. Sensorial Descriptors for Vegetarian Pigeon Pea Hamburger Patties According to a Focus Group. Cuad. Investig. UNED 2015, 7, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Eneche, E.H. Biscuit-Making Potential of Millet/Pigeon Pea Flour Blends. Plant Foods Hum. Nutr. 1999, 54, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Aluko, R.E. Development of Value-Added Nutritious Crackers with High Antidiabetic Properties from Blends of Acha (Digitaria exilis) and Blanched Pigeon Pea (Cajanus cajan). Food Sci. Nutr. 2018, 6, 1791–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gbenga-Fabusiwa, F.J.; Oladele, E.P.; Oboh, G.; Adefegha, S.A.; Fabusiwa, O.F.; Osho, P.O.; Enikuomehin, A.; Oshodi, A.A. Glycemic Response in Diabetic Subjects to Biscuits Produced from Blends of Pigeon Pea and Wheat Flour. Plant Foods Hum. Nutr. 2019, 74, 553–559. [Google Scholar] [CrossRef]
- Pande, S.; Sakhare, S.D.; Bhosale, M.G.; Haware, D.J.; Inamdar, A.A. Atta (Whole Wheat Flour) with Multi-Wholegrains: Flour Characterization, Nutritional Profiling and Evaluation of Chapati Making Quality. J. Food Sci. Technol. 2017, 54, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W. (Ed.) Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019; ISBN 978-0-935584-89-9. [Google Scholar]
- Sullivan, D.M.; Carpenter, D.E. (Eds.) Methods of Analysis for Nutrition Labeling; AOAC International: Arlington, VA, USA, 1993; ISBN 978-0-935584-52-3. [Google Scholar]
- Mokrzycki, W.; Tatol, M. Color Difference Delta E-A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Sopade, P.A.; Gidley, M.J. A Rapid In-Vitro Digestibility Assay Based on Glucometry for Investigating Kinetics of Starch Digestion. Starch-Stärke 2009, 61, 245–255. [Google Scholar] [CrossRef]
- Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Thiranusornkij, L.; Thamnarathip, P.; Chandrachai, A.; Kuakpetoon, D.; Adisakwattana, S. Comparative Studies on Physicochemical Properties, Starch Hydrolysis, Predicted Glycemic Index of Hom Mali Rice and Riceberry Rice Flour and Their Applications in Bread. Food Chem. 2019, 283, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A Starch Hydrolysis Procedure to Estimate Glycemic Index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Englyst, K.N.; Liu, S.; Englyst, H.N. Nutritional Characterization and Measurement of Dietary Carbohydrates. Eur. J. Clin. Nutr. 2007, 61 (Suppl. 1), S19–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Haven, M.O.; Jørgensen, H. The Challenging Measurement of Protein in Complex Biomass-Derived Samples. Appl. Biochem. Biotechnol. 2014, 172, 87–101. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Development of an in Vitro Protein Digestibility Assay Mimicking the Chicken Digestive Tract. Anim. Nutr. 2018, 4, 401–409. [Google Scholar] [CrossRef]
- Kunyanga, C.N.; Imungi, J.K.; Vellingiri, V. Nutritional Evaluation of Indigenous Foods with Potential Food-Based Solution to Alleviate Hunger and Malnutrition in Kenya. J. Appl. Biosci. 2013, 67, 5277–5288. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Chavan, J.K.; Kadam, S.S. Pigeon Pea as an Important Food Source. Crit. Rev. Food Sci. Nutr. 1986, 23, 103–145. [Google Scholar] [CrossRef]
- Saxena, K.B.; Kumar, R.V.; Sultana, R. Quality Nutrition through Pigeon pea—A Review. Health 2010, 2, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.; Voraputhaporn, W.; Rao, P.V.; Jambunathan, R. Physicochemical Characteristics of Pigeonpea and Mung Bean Starches and Their Noodle Quality. J. Food Sci. 1989, 54, 1293–1297. [Google Scholar] [CrossRef]
- Adeola, A.A.; Ohizua, E.R. Physical, Chemical, and Sensory Properties of Biscuits Prepared from Flour Blends of Unripe Cooking Banana, Pigeon Pea, and Sweet Potato. Food Sci. Nutr. 2018, 6, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.R.; Otten, L. A Relationship Between Colour Development and Moisture Content During Roasting of Peanuts. Can. Inst. Food Sci. Technol. J. 1989, 22, 34–39. [Google Scholar] [CrossRef]
- Bajka, B.H.; Pinto, A.M.; Ahn-Jarvis, J.; Ryden, P.; Perez-Moral, N.; van der Schoot, A.; Stocchi, C.; Bland, C.; Berry, S.E.; Ellis, P.R.; et al. The Impact of Replacing Wheat Flour with Cellular Legume Powder on Starch Bioaccessibility, Glycaemic Response and Bread Roll Quality: A Double-Blind Randomised Controlled Trial in Healthy Participants. Food Hydrocoll. 2021, 114, 106565. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Functional Study of Raw and Cooked Blue Maize Flour: Starch Digestibility, Total Phenolic Content and Antioxidant Activity. J. Cereal Sci. 2017, 76, 179–185. [Google Scholar] [CrossRef]
- Englyst, K.N.; Hudson, G.J.; Englyst, H.N. Starch Analysis in Food. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2006; ISBN 978-0-470-02731-8. [Google Scholar]
- Roman, L.; Martinez, M.M. Structural Basis of Resistant Starch (RS) in Bread: Natural and Commercial Alternatives. Foods 2019, 8, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gbenga-Fabusiwa, F.J.; Oladele, E.P.; Oboh, G.; Adefegha, S.A.; Oshodi, A.A. Nutritional Properties, Sensory Qualities and Glycemic Response of Biscuits Produced from Pigeon Pea-Wheat Composite Flour. J. Food Biochem. 2018, 42, e12505. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Torres, A.; Frias, J.; Vidal-Valverde, C. Semolina Supplementation with Processed Lupin and Pigeon Pea Flours Improve Protein Quality of Pasta. Food Sci. Technol. 2010, 43, 617–622. [Google Scholar] [CrossRef]
- Khatun, A.; Waters, D.L.E.; Liu, L. The Impact of Rice Protein on in Vitro Rice Starch Digestibility. Food Hydrocoll. 2020, 109, 106072. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L. Understanding the Mechanism of Starch Digestion Mitigation by Rice Protein and Its Enzymatic Hydrolysates. Food Hydrocoll. 2018, 84, 473–480. [Google Scholar] [CrossRef]
- López-Barón, N.; Gu, Y.; Vasanthan, T.; Hoover, R. Plant Proteins Mitigate in Vitro Wheat Starch Digestibility. Food Hydrocoll. 2017, 69, 19–27. [Google Scholar] [CrossRef]
- Joymak, W.; Ngamukote, S.; Chantarasinlapin, P.; Adisakwattana, S. Unripe Papaya By-Product: From Food Wastes to Functional Ingredients in Pancakes. Foods 2021, 10, 615. [Google Scholar] [CrossRef]
- Chusak, C.; Ying, J.A.Y.; Zhien, J.L.; Pasukamonset, P.; Henry, C.J.; Ngamukote, S.; Adisakwattana, S. Impact of Clitoria ternatea (Butterfly Pea) Flower on in Vitro Starch Digestibility, Texture and Sensory Attributes of Cooked Rice Using Domestic Cooking Methods. Food Chem. 2019, 295, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Chusak, C.; Chanbunyawat, P.; Chumnumduang, P.; Chantarasinlapin, P.; Suantawee, T.; Adisakwattana, S. Effect of Gac Fruit (Momordica Cochinchinensis) Powder on in Vitro Starch Digestibility, Nutritional Quality, Textural and Sensory Characteristics of Pasta. LWT 2020, 118, 108856. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Tagle Freire, D.; Boom, R.M.; Janssen, A.E.M. The Influence of Starch and Fibre on In Vitro Protein Digestibility of Dry Fractionated Quinoa Seed (Riobamba Variety). Food Biophys. 2019, 14, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The Digestion Rate of Protein Is an Independent Regulating Factor of Postprandial Protein Retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.; Tavan, M. A Review of the Alternative Protein Industry. Curr. Opin. Food Sci. 2022, 47, 100869. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Oshodi, A.A.; Olaofe, O.; Hall, G.M. Amino Acid, Fatty Acid and Mineral Composition of Pigeon Pea (Cajanus cajan). Int. J. Food Sci. Nutr. 1993, 43, 187–191. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
| Experiments | WWF | PPF | WWF Chapati | 20% PPF Chapati | 40% PPF Chapati |
|---|---|---|---|---|---|
| Total calories (kcal) | 363.32 ± 0.33 b | 374.06 ± 0.98 a | 274.08 ± 0.48 c | 266.25 ± 0.19 d | 265.41 ± 1.00 d |
| Total carbohydrate (g) | 71.82 ± 0.02 a | 60.53 ± 1.55 b | 55.73 ± 0.3 c | 52.15 ± 0.40 d | 50.24 ± 0.03 e |
| Ash (g) | 1.24 ± 0.03 b | 1.51 ± 0.10 a | 0.97 ± 0.02 e | 1.14 ± 0.01 d | 1.35 ± 0.01 c |
| Total fat (g) | 2.44 ± 0.17 b | 3.06 ± 0.06 a | 1.60 ± 0.04 c | 1.58 ± 0.03 c | 1.69 ± 0.02 c |
| Protein (N × 6.25, g) | 13.52 ± 0.28 b | 26.10 ± 1.16 a | 9.19 ± 0.52 d | 10.88 ± 0.37 c | 12.31 ± 0.32 c |
| Total dietary fiber (g) | 10.08 ± 0.19 a | 10.41 ± 0.02 a | 10.34 ± 0.17 a | 11.02 ± 0.04 b | 11.08 ± 0.11 b |
| Moisture (g) | 10.98 ± 0.15 a | 8.80 ± 0.23 b | 32.51 ± 0.16 d | 34.26 ± 0.01 c | 34.41 ± 0.26 c |
| Samples | L* | a* | b* |
|---|---|---|---|
| WWF | 36.96 ± 0.32 a | 1.31 ± 0.02 c | 6.26 ± 0.08 d |
| PPF | 35.98 ± 0.35 a | 0.78 ± 0.02 d | 6.84 ± 0.12 c |
| WWF Chapati | 21.79 ± 0.65 c | 3.10 ± 0.09 a | 8.63 ± 0.06 b |
| 20% PPF Chapati | 22.55 ± 0.47 c | 2.77 ± 0.05 ab | 8.77 ± 0.10 ab |
| 40% PPF Chapati | 23.49 ± 0.32 b | 2.47 ± 0.14 b | 9.17 ± 0.15 a |
| Samples | pGI | %HI | AUC (mg·min/g Sample) | TS (g/50 g Sample) | %RDS | %SDS | %RS |
|---|---|---|---|---|---|---|---|
| WWF Chapati | 51.55 ± 0.20 a | 21.57 ± 0.58 a | 30,644.34 ± 818.86 a | 21.55 ± 0.27 a | 17.87 ± 1.30 a | 47.64 ± 2.32 a | 34.49 ± 3.25 a |
| 20% PPF Chapati | 49.55 ± 0.68 b | 17.93 ± 1.24 b | 25,475.88 ± 1767.09 b | 20.54 ± 0.04 b | 15.38 ± 1.07 a | 40.59 ± 3.37 a | 44.02 ± 4.43 a |
| 40% PPF Chapati | 47.19 ± 0.31 c | 13.62 ± 0.56 c | 19,356.81 ± 795.26 c | 18.55 ± 0.09 c | 14.54 ± 0.98 a | 27.67 ± 3.32 b | 57.99 ± 2.67 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachanarula, S.; Chantarasinlapin, P.; Adisakwattana, S. Substituting Whole Wheat Flour with Pigeon Pea (Cajanus cajan) Flour in Chapati: Effect on Nutritional Characteristics, Color Profiles, and In Vitro Starch and Protein Digestion. Foods 2022, 11, 3157. https://doi.org/10.3390/foods11203157
Sachanarula S, Chantarasinlapin P, Adisakwattana S. Substituting Whole Wheat Flour with Pigeon Pea (Cajanus cajan) Flour in Chapati: Effect on Nutritional Characteristics, Color Profiles, and In Vitro Starch and Protein Digestion. Foods. 2022; 11(20):3157. https://doi.org/10.3390/foods11203157
Chicago/Turabian StyleSachanarula, Sirin, Praew Chantarasinlapin, and Sirichai Adisakwattana. 2022. "Substituting Whole Wheat Flour with Pigeon Pea (Cajanus cajan) Flour in Chapati: Effect on Nutritional Characteristics, Color Profiles, and In Vitro Starch and Protein Digestion" Foods 11, no. 20: 3157. https://doi.org/10.3390/foods11203157






