Addressing Psychosocial Factors in Cognitive Impairment Screening from a Holistic Perspective: The DeCo-Booklet Methodology Design and Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bibliographic Review
2.2. Data Collection Booklet Design
2.2.1. Socio-Demographic Factors
2.2.2. Cognitive Impairment
2.2.3. Meaning in Life
2.2.4. Psychosocial Factors
2.2.5. Health Problems
2.2.6. Lifestyle
2.3. Healthy Aging
2.4. Population
2.5. Statistical Analysis
2.6. Ethical Approval
| Variable | Tools or Scale | No. of Items | Study |
|---|---|---|---|
| Meaning in Life | |||
| Whole construct | Meaning in Life Questionnaire (MLQ) | 10 | McGee et al., 2017 [41] Aftab et al., 2019 [42] |
| Sense of coherence | Orientation to Life Questionnaire(OLQ) | 13 | Bartrés-Faz et al., 2018 [26] Macià et al., 2021 [43] Sutin et al., 2020 [23] |
| Purpose in life | Purpose in life subscale of Ryff’s Well-Being Scale (PiL) | 6 | Bartrés-Faz et al., 2018 [26] Macià et al., 2021 [43] |
| 7 | Sutin et al., 2018 [44] Lewis et al., 2021 [45] Kim et al., 2019 [22] | ||
| 6 and 7 | Sutin et al., 2021 [21] | ||
| PiL scale from Ryff and Keyes’ Psychological Wellbeing Scale | 10 | Wingo et al., 2020 [46] Boyle et al., 2021 [47] | |
| Engagement with life | Engaged Living Scale(ELS) | 16 | Bartrés-Faz et al., 2018 [26] Macià et al., 2021 [43] |
| Psychosocial Factors | |||
| Resilience | Brief Resilience Coping Scale (BRCS) | 4 | Meléndez et al., 2018 [48] |
| Connor–Davidson Resilience Scale (CD-RISC) | 25 | Eyre et al., 2017 [49] | |
| Stress | Perceived Stress Scale(PSS) | 4 | Turner et al., 2017 [50] Feeney et al., 2018 [51] |
| 10 | Elkana et al., 2020 [52] | ||
| 14 | Katz et al., 2016 [53] Jiang et al., 2017 [54] | ||
| Loneliness | UCLA Loneliness Scale | 3 | Lara et al., 2019 [55] Jang et al., 2021 [56] |
| 11 | Lee et al., 2021 [57] | ||
| 20 | Kwon et al., 2017 [58] Gené-Badia et al., 2020 [59] | ||
| De Jong Gierveld Loneliness Scale | 6 | Burholt et al., 2017 [60] Fung et al., 2019 [61] Evans et al., 2019 [62] | |
| Social isolation | Lubben Social Network Scale(LSNS) | 6 | Burholt et al., 2017 [60] Evans et al., 2019 [62] Li et al., 2019 [63] Gené-Badia et al., 2020 [59] Jang et al., 2021 [56] Foong et al., 2021 [64] |
| 12 | Siette et al., 2020 [65] | ||
| Shankar Index | 5 | Lara et al., 2019 [55] | |
3. Results
3.1. Selection of the Tools to Assess Meaning in Life and Psychosocial Factors
3.2. Data Collection Booklet and Pilot Study
3.2.1. Sociodemographic Factors
3.2.2. Cognitive Impairment
3.2.3. Meaning in Life
3.2.4. Psychosocial Variables
3.2.5. Health Problems
3.2.6. Lifestyle
3.2.7. Healthy Aging
3.3. Data Analysis
3.3.1. Correlations between Variables
3.3.2. Correlations between Variables and Cognitive Impairment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild Cognitive Impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef]
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Canevelli, M.; Grande, G.; Lacorte, E.; Quarchioni, E.; Cesari, M.; Mariani, C.; Bruno, G.; Vanacore, N. Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Morris, J.C.; Berg-Weger, M.; Borson, S.; Carpenter, B.D.; del Campo, N.; Dubois, B.; Fargo, K.; Fitten, L.J.; Flaherty, J.H.; et al. Brain Health: The Importance of Recognizing Cognitive Impairment: An IAGG Consensus Conference. J. Am. Med. Dir. Assoc. 2015, 16, 731–739. [Google Scholar] [CrossRef]
- WHO. Risk Reduction of Cognitive Decline And Dementia; WHO: Geneva, Switzerland, 2019; ISBN 9789241550543.
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- World Health Organization. 10 Facts on Ageing and Health. Available online: http://www.who.int/features/factfiles/ageing/en/ (accessed on 17 May 2022).
- Spanish National Institute of Statistics (INE). Projections of the Population 2020–2070; INE: Madrid, Spain, 2020.
- WHO. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; ISBN 9241565047.
- Michel, J.P.; Sadana, R. “Healthy Aging” Concepts and Measures. J. Am. Med. Dir. Assoc. 2017, 18, 460–464. [Google Scholar] [CrossRef]
- Rowe, J.W.; Kahn, R.L. Successful Aging 2.0: Conceptual Expansions for the 21st Century. J. Gerontol.–Ser. B Psychol. Sci. Soc. Sci. 2015, 70, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.W.; Kahn, R.L. Human Aging: Usual and Successful. Science 1987, 237, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Pikhart, H.; Sacker, A. Domains and Measurements of Healthy Aging in Epidemiological Studies: A Review. Gerontologist 2019, 59, e294–e310. [Google Scholar] [CrossRef]
- Lara, J.; Godfrey, A.; Evans, E.; Heaven, B.; Brown, L.J.E.; Barron, E.; Rochester, L.; Meyer, T.D.; Mathers, J.C. Towards Measurement of the Healthy Ageing Phenotype in Lifestyle-Based Intervention Studies. Maturitas 2013, 76, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Deaton, A.; Stone, A.A. Psychological Wellbeing, Health and Ageing. Lancet 2015, 385, 640–648. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.F.; Frazier, P.; Kaler, M.; Oishi, S. The Meaning in Life Questionnaire: Assessing the Presence of and Search for Meaning in Life. J. Couns. Psychol. 2006, 53, 80–93. [Google Scholar] [CrossRef]
- Martela, F.; Steger, M.F. The Three Meanings of Meaning in Life: Distinguishing Coherence, Purpose, and Significance. J. Posit. Psychol. 2016, 11, 531–545. [Google Scholar] [CrossRef]
- King, L.A.; Hicks, J.A. The Science of Meaning in Life. Annu. Rev. Psychol. 2021, 72, 561–584. [Google Scholar] [CrossRef]
- Heintzelman, S.J.; King, L.A. Life Is Pretty Meaningful. Am. Psychol. 2014, 69, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Luchetti, M.; Stephan, Y.; Strickhouser, J.E.; Terracciano, A. The Association between Purpose/Meaning in Life and Verbal Fluency and Episodic Memory: A Meta-Analysis of >140,000 Participants from up to 32 Countries. Int. Psychogeriatr. 2021, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Shin, S.H.; Scicolone, M.A.; Parmelee, P. Purpose in Life Protects Against Cognitive Decline Among Older Adults. Am. J. Geriatr. Psychiatry 2019, 27, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Luchetti, M.; Stephan, Y.; Terracciano, A. Meaning in Life and Risk of Cognitive Impairment: A 9-Year Prospective Study in 14 Countries. Arch. Gerontol. Geriatr. 2020, 88, 104033. [Google Scholar] [CrossRef] [PubMed]
- Vos, J. Cardiovascular Disease and Meaning in Life: A Systematic Literature Review and Conceptual Model. Palliat. Support. Care 2021, 19, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Czekierda, K.; Banik, A.; Park, C.L.; Luszczynska, A. Meaning in Life and Physical Health: Systematic Review and Meta-Analysis. Health Psychol. Rev. 2017, 11, 387–418. [Google Scholar] [CrossRef] [PubMed]
- Bartrés-Faz, D.; Cattaneo, G.; Solana, J.; Tormos, J.M.; Pascual-Leone, A. Meaning in Life: Resilience beyond Reserve. Alzheimer’s Res. Ther. 2018, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Pardo, J.; Sánchez, R.; Puchades, E.; Pérez-Tur, J.; Navarro, A.; Moreno, L. Pharmacist-Physician Interprofessional Collaboration to Promote Early Detection of Cognitive Impairment: Increasing Diagnosis Rate. Front. Pharmacol. 2021, 12, 579489. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.T.; Pardo, J.; Muñoz-Almaraz, F.J.; Guerrero, M.D.; Moreno, L. Decision Tree for Early Detection of Cognitive Impairment by Community Pharmacists. Front. Pharmacol. 2018, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Moreno, L.; Gil, M.; García-Lluch, G.; Sendra-Lillo, J.; Alacreu, M. Pharmacists’ Knowledge of Factors Associated with Dementia: The a-to-z Dementia Knowledge List. Int. J. Environ. Res. Public Health 2021, 18, 9934. [Google Scholar] [CrossRef] [PubMed]
- Buschke, H.; Kuslansky, G.; Katz, M.; Stewart, W.F.; Sliwinski, M.J.; Eckholdt, H.M.; Lipton, R.B. Screening for Dementia with the Memory Impairment Screen. Neurology 1999, 52, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Carnero Pardo, C.; Lendínez González, A. Utilidad Del Test de Fluencia Verbal Semántica En El Diagnóstico de Demencia. Rev. Neurol. 1999, 29, 709. [Google Scholar] [CrossRef]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Salazar-Ospina, A.; Amariles, P.; Benjumea, D.M.; Gutierrez, F.; Faus, M.J.; Rodriguez, L.F. Effectiveness of the Dader Method for Pharmaceutical Care in Patients with Bipolar I Disorder: EMDADER-TAB: Study Protocol for a Randomized Controlled Trial. Trials 2014, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- WHOCC. ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 5 September 2022).
- International Classification of Diseases (ICD). Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 6 September 2022).
- Noain, A.; Garcia-Cardenas, V.; Gastelurrutia, M.A.; Malet-Larrea, A.; Martinez-Martinez, F.; Sabater-Hernandez, D.; Benrimoj, S.I. Cost Analysis for the Implementation of a Medication Review with Follow-up Service in Spain. Int. J. Clin. Pharm. 2017, 39, 750–758. [Google Scholar] [CrossRef] [PubMed]
- BOT Plus 2. Base de Datos de Medicamentos. Available online: https://botplusweb.portalfarma.com/botplus.aspx (accessed on 18 May 2022).
- CheckTheMeds. Available online: https://www.checkthemeds.com/ (accessed on 5 September 2022).
- Gabriel, R.; Brotons, C.; Tormo, M.J.; Segura, A.; Rigo, F.; Elosua, R.; Carbayo, J.A.; Gavrila, D.; Moral, I.; Tuomilehto, J.; et al. The ERICE-Score: The New Native Cardiovascular Score for the Low-Risk and Aged Mediterranean Population of Spain. Rev. Española Cardiol. Engl. Ed. 2015, 68, 205–215. [Google Scholar] [CrossRef]
- Rowe, J.W.; Kahn, R.L. Successful Aging. Gerontologist 1997, 37, 433–440. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.S.; Zhao, H.C.; Myers, D.R.; Kim, S.M. Positive Psychological Assessment and Early-Stage Dementia. Clin. Gerontol. 2017, 40, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Aftab, A.; Lee, E.E.; Klaus, F.; Daly, R.; Wu, T.C.; Tu, X.; Huege, S.; Jeste, D.V. Meaning in Life and Its Relationship With Physical, Mental, and Cognitive Functioning: A Study of 1,042 Community-Dwelling Adults Across the Lifespan. J. Clin. Psychiatry 2019, 81, 11357. [Google Scholar] [CrossRef] [PubMed]
- Macià, D.; Cattaneo, G.; Solana, J.; Tormos, J.M.; Pascual-Leone, A.; Bartrés-Faz, D. Meaning in Life: A Major Predictive Factor for Loneliness Comparable to Health Status and Social Connectedness. Front. Psychol. 2021, 12, 627547. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Psychological Well-Being and Risk of Dementia. Int. J. Geriatr. Psychiatry 2018, 33, 743–747. [Google Scholar] [CrossRef]
- Lewis, N.A.; Hill, P.L. Sense of Purpose Promotes Resilience to Cognitive Deficits Attributable to Depressive Symptoms. Front. Psychol. 2021, 12, 2517. [Google Scholar] [CrossRef]
- Wingo, A.P.; Wingo, T.S.; Fan, W.; Bergquist, S.; Alonso, A.; Marcus, M.; Levey, A.I.; Lah, J.J. Purpose in Life Is a Robust Protective Factor of Reported Cognitive Decline among Late Middle-Aged Adults: The Emory Healthy Aging Study. J. Affect. Disord. 2020, 263, 310–317. [Google Scholar] [CrossRef]
- Boyle, P.A.; Wang, T.; Yu, L.; Barnes, L.L.; Wilson, R.S.; Bennett, D.A. Purpose in Life May Delay Adverse Health Outcomes in Old Age. Am. J. Geriatr. Psychiatry 2021, 30, 174–181. [Google Scholar] [CrossRef]
- Meléndez, J.C.; Satorres, E.; Redondo, R.; Escudero, J.; Pitarque, A. Wellbeing, Resilience, and Coping: Are There Differences between Healthy Older Adults, Adults with Mild Cognitive Impairment, and Adults with Alzheimer-Type Dementia? Arch. Gerontol. Geriatr. 2018, 77, 38–43. [Google Scholar] [CrossRef]
- Eyre, H.A.; Siddarth, P.; Acevedo, B.; Van Dyk, K.; Paholpak, P.; Ercoli, L.; St Cyr, N.; Yang, H.; Khalsa, D.S.; Lavretsky, H. A Randomized Controlled Trial of Kundalini Yoga in Mild Cognitive Impairment. Int. Psychogeriatr. 2017, 29, 557–567. [Google Scholar] [CrossRef]
- Turner, A.D.; James, B.D.; Capuano, A.W.; Aggarwal, N.T.; Barnes, L.L. Perceived Stress and Cognitive Decline in Different Cognitive Domains in a Cohort of Older African Americans. Am. J. Geriatr. Psychiatry 2017, 25, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Feeney, J.; O’Sullivan, M.; Kenny, R.A.; Robertson, I.H. Change in Perceived Stress and 2-Year Change in Cognitive Function among Older Adults: The Irish Longitudinal Study on Ageing. Stress Health 2018, 34, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Elkana, O.; Adelson, M.; Sason, A.; Doniger, G.M.; Peles, E. Improvement in Cognitive Performance after One Year of Methadone Maintenance Treatment. Psychiatry Res. 2020, 294, 113526. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Derby, C.A.; Wang, C.; Sliwinski, M.J.; Ezzati, A.; Zimmerman, M.E.; Zwerling, J.L.; Lipton, R.B. Influence of Perceived Stress on Incident Amnestic Mild Cognitive Impairment. Alzheimer Dis. Assoc. Disord. 2016, 30, 93–98. [Google Scholar] [CrossRef]
- Jiang, J.M.; Seng, E.K.; Zimmerman, M.E.; Kim, M.; Lipto, R.B. Positively Worded Subscale Score of the Perceived Stress Scale Is Associated with Cognitive Domain Function. J. Behav. Brain Sci. 2017, 7, 311–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lara, E.; Caballero, F.F.; Rico-Uribe, L.A.; Olaya, B.; Haro, J.M.; Ayuso-Mateos, J.L.; Miret, M. Are Loneliness and Social Isolation Associated with Cognitive Decline? Int. J. Geriatr. Psychiatry 2019, 34, 1613–1622. [Google Scholar] [CrossRef]
- Jang, Y.; Park, N.S.; Chiriboga, D.A. Cognitive Health Risks Posed by Social Isolation and Loneliness in Older Korean Americans. Alzheimers. Dement. 2021, 17, e049804. [Google Scholar] [CrossRef]
- Lee, J.H.; Luchetti, M.; Aschwanden, D.; Sesker, A.A.; Strickhouser, J.E.; Terracciano, A.; Sutin, A.R. Cognitive Impairment and the Trajectory of Loneliness in Older Adulthood: Evidence from the Health and Retirement Study. J. Aging Health 2021, 34, 3–13. [Google Scholar] [CrossRef]
- Kwon, D.-Y.; Jung, J.-M.; Park, M.H. Loneliness in Elderly Patients with Mild Cognitive Impairment: A Pilot Study. Dement. Neurocognitive Disord. 2017, 16, 110–113. [Google Scholar] [CrossRef]
- Gené-Badia, J.; Comice, P.; Belchín, A.; Erdozain, M.Á.; Cáliz, L.; Torres, S.; Rodríguez, R. Profiles of Loneliness and Social Isolation in Urban Population. Aten. Primaria 2020, 52, 224–232. [Google Scholar] [CrossRef]
- Burholt, V.; Windle, G.; Morgan, D.J. A Social Model of Loneliness: The Roles of Disability, Social Resources, and Cognitive Impairment. Gerontologist 2017, 57, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.W.T.; Lee, A.T.C.; Cheng, S.T.; Lam, L.C.W. Loneliness Interacts with Family Relationship in Relation to Cognitive Function in Chinese Older Adults. Int. Psychogeriatr. 2019, 31, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.E.M.; Llewellyn, D.J.; Matthews, F.E.; Woods, R.T.; Brayne, C.; Clare, L. Social Isolation, Cognitive Reserve, and Cognition in Older People with Depression and Anxiety. Aging Ment. Health 2019, 23, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Lian, Z.; Zhu, Z.; Liu, Y. Social Networks, Community Engagement, and Cognitive Impairment among Community-Dwelling Chinese Older Adults. Dement. Geriatr. Cogn. Dis. Extra 2019, 9, 330–337. [Google Scholar] [CrossRef]
- Foong, H.F.; Ibrahim, R.; Hamid, T.A.; Haron, S.A. Social Networks Moderate the Association between Physical Fitness and Cognitive Function among Community-Dwelling Older Adults: A Population-Based Study. BMC Geriatr. 2021, 21, 679. [Google Scholar] [CrossRef]
- Siette, J.; Georgiou, A.; Brayne, C.; Westbrook, J.I. Social Networks and Cognitive Function in Older Adults Receiving Home- and Community-Based Aged Care. Arch. Gerontol. Geriatr. 2020, 89, 104083. [Google Scholar] [CrossRef]
- Antonovsky, A. The Structure and Properties of the Sense of Coherence Scale. Soc. Sci. Med. 1993, 36, 725–733. [Google Scholar] [CrossRef]
- Ryff, C.D.; Keyes, C.L.M. The Structure of Psychological Well-Being Revisited. J. Pers. Soc. Psychol. 1995, 69, 719–727. [Google Scholar] [CrossRef]
- Trompetter, H.R.; Ten Klooster, P.M.; Schreurs, K.M.G.; Fledderus, M.; Westerhof, G.J.; Bohlmeijer, E.T. Measuring Values and Committed Action with the Engaged Living Scale (ELS): Psychometric Evaluation in a Nonclinical Sample and a Chronic Pain Sample. Psychol. Assess. 2013, 25, 1235–1246. [Google Scholar] [CrossRef]
- Sinclair, V.G.; Wallston, K.A. The Development and Psychometric Evaluation of the Brief Resilient Coping Scale. Assessment 2004, 11, 94–101. [Google Scholar] [CrossRef]
- Hoyl, M.T.; Alessi, C.A.; Harker, J.O.; Josephson, K.R.; Pietruszka, F.M.; Koelfgen, M.; Mervis, J.R.; Fitten, L.J.; Rubenstein, L.Z. Development and Testing of a Five-Item Version of the Geriatric Depression Scale. J. Am. Geriatr. Soc. 1999, 47, 873–878. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. An Ultra-Brief Screening Scale for Anxiety and Depression: The PHQ–4. Psychosomatics 2009, 50, 613–621. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Cacioppo, J.T. A Short Scale for Measuring Loneliness in Large Surveys: Results from Two Population-Based Studies. Res. Aging 2004, 26, 655–672. [Google Scholar] [CrossRef]
- Lubben, J.E. Assessing Social Networks among Elderly Populations. Fam. Community Health 1988, 11, 42–52. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for Themanagement Ofarterial Hypertension. Kardiol. Pol. 2018, 77, 71–159. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- WHO. Manual de Vigilancia STEPS de La OMS; WHO: Geneva, Switzerland, 2009; Volume 463.
- González, L.R.; Pedret, C.V.; Faz, D.B.; Elola-Olaso, C.C.; Padullés, C.S.; Sampol, M.C.; Cladera, J.O.; Capdevila, B.B.; Guix, J.L.M. Cuestionario de Reserva Cognitiva. Valores Obtenidos En Población Anciana Sana y Con Enfermedad de Alzheimer. Rev. Neurol. 2011, 52, 195. [Google Scholar] [CrossRef]
- Ferreira-Pêgo, C.; Nissensohn, M.; Kavouras, S.A.; Babio, N.; Serra-Majem, L.; Águila, A.M.; Mauromoustakos, A.; Pérez, J.Á.; Salas-Salvadó, J. Beverage Intake Assessment Questionnaire: Relative Validity and Repeatability in a Spanish Population with Metabolic Syndrome from the PREDIMED-PLUS Study. Nutrients 2016, 8, 475. [Google Scholar] [CrossRef]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, L.Z.; et al. Overview of the MNA®—Its History and Challenges. J. Nutr. Health Aging 2006, 10, 456–463. [Google Scholar] [PubMed]
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International Physical Activity Questionnaire: Reliability and Validity in a Spanish Population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Jenkins, C.D.; Stanton, B.A.; Niemcryk, S.J.; Rose, R.M. A Scale for the Estimation of Sleep Problems in Clinical Research. J. Clin. Epidemiol. 1988, 41, 313–321. [Google Scholar] [CrossRef]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire a Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef]
- Davies, N.M.; Kehoe, P.G.; Ben-Shlomo, Y.; Martin, R.M. Associations of Anti-Hypertensive Treatments with Alzheimer’s Disease, Vascular Dementia, and Other Dementias. J. Alzheimer’s Dis. 2011, 26, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Kim, H.; Bae, J.; Lee, H.; Oh, I.; Kim, W.J.; Shin, J. Benzodiazepine-Related Cognitive Impairment or Dementia: A Signal Detection Study Using a Case/Non-Case Approach. Psychiatry Investig. 2020, 17, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.J.; Bondi, M.W.; Thomas, K.R.; Campbell, N.L.; Galasko, D.R.; Salmon, D.P.; Sewell, D.; Brewer, J.B.; Feldman, H.H.; Delano-Wood, L.; et al. Association of Anticholinergic Medication and AD Biomarkers with Incidence of MCI among Cognitively Normal Older Adults. Neurology 2020, 95, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Alonso, T.V.; Espí, M.M.; Reina, J.M.; Pérez, D.C.; Pérez, A.R.; Gil Costa, M.; Maside, A.L.; Antón, E.A.; Alonso, J.L.; Gil, M.F. Prevalencia de Deterioro Cognitivo En España. Estudio Gómez de Caso En Redes Centinelas Sanitarias. Neurología 2018, 33, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sommerlad, A.; Ruegger, J.; Singh-Manoux, A.; Lewis, G.; Livingston, G. Marriage and Risk of Dementia: Systematic Review and Meta-Analysis of Observational Studies. J. Neurol. Neurosurg. Psychiatry 2017, 89, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Burgard, S.A.; Needham, B.L. Marital Status and Cognitive Impairment in the United States: Evidence from the National Health and Aging Trends Study. Ann. Epidemiol. 2019, 38, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Yu, L.; Schneider, J.A.; Bennett, D.A. Effect of Purpose in Life on the Relation Between Alzheimer Disease Pathologic Changes on Cognitive Function in Advanced Age. Arch. Gen. Psychiatry 2012, 69, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Barnes, L.L.; Buchman, A.S.; Bennett, D.A. Purpose in Life Is Associated with Mortality among Community-Dwelling Older Persons. Psychosom. Med. 2009, 71, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Schippers, M.C.; Ziegler, N. Life Crafting as a Way to Find Purpose and Meaning in Life. Front. Psychol. 2019, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Van Agteren, J.; Bartholomaeus, J.; Steains, E.; Lo, L.; Gerace, A. Using a Technology-Based Meaning and Purpose Intervention to Improve Well-being: A Randomised Controlled Study. J. Happiness Stud. 2021, 22, 3571–3591. [Google Scholar] [CrossRef]
- Dyer, J.G.; McGuinness, T.M. Resilience: Analysis of the Concept. Arch. Psychiatr. Nurs. 1996, 10, 276–282. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Przybelski, S.A.; MacHulda, M.M.; Knopman, D.S.; Lowe, V.J.; Mielke, M.M.; Reddy, A.L.; Geda, Y.E.; Jack, C.R.; Petersen, R.C.; et al. Better Stress Coping Associated with Lower Tau in Amyloid-Positive Cognitively Unimpaired Older Adults. Neurology 2020, 94, E1571–E1579. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Sidney, S.; Selby, J.; Claiborne Johnston, S.; Yaffe, K. Midlife Cardiovascular Risk Factors and Risk of Dementia in Late Life. Neurology 2005, 64, 277–281. [Google Scholar] [CrossRef]
- Qiu, C.; Fratiglioni, L. A Major Role for Cardiovascular Burden in Age-Related Cognitive Decline. Nat. Rev. Cardiol. 2015, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Bahorik, A.L.; Hoang, T.D.; Forrester, S.; Jacobs, D.R.; Lewis, C.E.; Lloyd-Jones, D.M.; Sidney, S.; Reis, J.P. Cardiovascular Risk Factors and Accelerated Cognitive Decline in Midlife: The CARDIA Study. Neurology 2020, 95, e839–e846. [Google Scholar] [CrossRef]
- Huang, C.C.; Lee, L.H.; Lin, W.S.; Hsiao, T.H.; Chen, I.C.; Lin, C.H. The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults. J. Pers. Med. 2022, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Fisher, D.W.; Yu, T.; Dong, H. The Link between Chronic Pain and Alzheimer’s Disease. J. Neuroinflamm. 2019, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Shafto, M.; Kievit, R.; Matthews, F.; Spink, M.; Valenzuela, M.; Henson, R.N. Lifestyle Activities in Mid-Life Contribute to Cognitive Reserve in Late-Life, Independent of Education, Occupation, and Late-Life Activities. Neurobiol. Aging 2018, 70, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 255–270. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.J.; Connell, C.M.; Heeringa, S.G.; Li, L.W.; Roberts, J.S. Successful Aging in the United States: Prevalence Estimates from a National Sample of Older Adults. J. Gerontol.–Ser. B Psychol. Sci. Soc. Sci. 2010, 65, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Laso, A.; McLaughlin, S.J.; Urdaneta, E.; Yanguas, J. Defining and Estimating Healthy Aging in Spain: A Cross-Sectional Study. Gerontologist 2018, 58, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Hank, K. How “Successful” Do Older Europeans Age? Findings From SHARE. J. Gerontol.–Ser. B Psychol. Sci. Soc. Sci. 2011, 66, 230–236. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Validated scales | Non-validated or ad hoc tools |
| Scales translated into Spanish or tested in the Spanish population | Non-community dwelling population Population aged under 50 years |
| Suitable for a face-to-face interview | Cognitive impairment or Alzheimer’s disease at the baseline |
| Administration time and/or length ensures a suitable interview length (maximum of 10 min) | Literature related to COVID-19 disease |
| Structure of the Data Collection Booklet | Pilot Study Results | |||
|---|---|---|---|---|
| Ref. | No. of Items | Variable, Validated Test/Criteria, and Categories | N (%) or Mean ± SD | Time (min) |
| 1. Sociodemographic Factors | ||||
| 4 | Sex | 1 | ||
| Women | 131 (61.2) | |||
| Men | 81 (37.9) | |||
| Age | 72.8 ± 11.2 | |||
| [50, 65) | 57 (27.1) | |||
| [65, 80) | 85 (40.5) | |||
| [80, 95] | 68 (32.4) | |||
| Marital status | ||||
| Married | 139 (65.6) | |||
| Widow | 57 (26.6) | |||
| Separated/divorced | 6 (2.8) | |||
| Single | 10 (4.7) | |||
| Family history of dementia | ||||
| Yes | 34 (16.0) | |||
| No | 179 (84.0) | |||
| 2. Cognitive Impairment | ||||
| 1 | Subjective memory complaint | 79 (37.1) | <1 | |
| [30] | 8 | Memory Impairment Screen (MIS) [0, 8] | 6.8 ± 2.0 | 2 |
| CI risk [0, 4] | 21 (9.9) | |||
| No CI risk (4, 8] | 191 (90.1) | |||
| [32] | 10 | Short Portable Mental State Questionnaire (SPMSQ) [0–10] | 1.1 ± 1.3 | 3 |
| CI risk [3–4, 10] | 28 (13.2) | |||
| No CI risk [0, 3–4) | 185 (87.0) | |||
| [31] | 1 | Semantic Verbal Fluency (SVF) [0, +∞) | 17.5 ± 7.1 | 1 |
| CI risk [0, 10] | 16 (7.5) | |||
| No CI risk [10, +∞) | 196 (92.5) | |||
| 3. Meaning in Life | ||||
| [66] | 13 | Sense of coherence | 8 | |
| Orientation to Life Questionnaire (OLQ-13) [13, 91] | 69.1 ± 11.9 | |||
| [67] | 6 | Purpose in life | 4 | |
| PiL subscale of Ryff’s Well-Being Scale (PiL) [6, 36] | 29.6 ± 5.6 | |||
| [68] | 16 | Engagement with life | 8 | |
| Engaged Living Scale (ELS) [16, 80] | 70.1 ± 10.8 | |||
| 4. Psychosocial Factors | ||||
| [69] | 4 | Resilience | 2 | |
| Brief Resilient Coping Scale (BRCS) [4, 20] | 16.7 ± 3.0 | |||
| Low [4, 13) | 24 (11.3) | |||
| Intermediate [13, 17] | 104 (48.8) | |||
| High (17, 20] | 85 (39.9) | |||
| [70] | 5 | Depression | 1 | |
| Geriatric Depression Scale (GDS-5) [0, 5] | 0.8 ± 1.3 | |||
| Risk of depression [2, 5] | 46 (21.6) | |||
| No risk of depression [0, 2) | 167 (78.4) | |||
| [71] | 4 | Psychological distress | 2 | |
| Patient Health Questionnaire-4 (PHQ-4) [0–12] | 2.6 ± 2.6 | |||
| None [0, 2] | 126 (59.2) | |||
| Mild [3, 5] | 57 (26.8) | |||
| Moderate [6, 8] | 22 (10.3) | |||
| Severe [9, 12] | 8 (3.8) | |||
| [72] | 4 | Stress | 2 | |
| Perceived Stress Scale (PSS-4) [0, 16] | 3.8 ± 3.1 | |||
| Perceived stress (5.4, 16] | 59 (27.7) | |||
| No perceived stress [0, 5.4) | 154 (72.3) | |||
| [73] | 3 | Loneliness | 1 | |
| UCLA-3 [3, 9] | 3.9 ± 1.5 | |||
| Loneliness [6, 9] | 27 (12.7) | |||
| No loneliness [3, 6) | 186 (87.3) | |||
| [74] | 6 | Social isolation | 5 | |
| Lubben Social Network Scale (LSNS-6) [0, 30] | 18.8 ± 5.0 | |||
| Social isolation [0, 12] | 24 (11.3) | |||
| No social isolation (12, 30] | 189 (88.7) | |||
| 5. Health Problems | ||||
| [75] | 1 | Hypertension | 5 | |
| Yes [systolic BP > 140 mmHg–diastolic BP > 90 mmHg] | 126 (60.0) | |||
| No [systolic BP ≤ 140 mmHg–diastolic BP ≤ 90 mmHg] | 84 (40.0) | |||
| [76] | 1 | Hypercholesterolemia | ||
| Yes [CT > 200 mg/dL–CLDL > 100 mg/dL–CHDL ≤ 35–40 mg/dL] | 94 (44.8) | |||
| No [CT ≤ 200 mg/dL–CLDL ≤ 100 mg/dL–CHDL > 35–40 mg/dL] | 116 (55.2) | |||
| [77] | 1 | Diabetes | ||
| Yes [blood glucose > 126 mg/dL–HbA1c > 6.5%] | 50 (23.5) | |||
| No [blood glucose ≤ 126 mg/dL–HbA1c < 6.5%] | 163 (76.5) | |||
| 1 | Smoking habit | <1 | ||
| Non-smoker | 117 (54.9) | |||
| Former smoker | 53 (24.9) | |||
| Passive smoker | 12 (5.6) | |||
| Smoker | 31 (14.6) | |||
| [39] | 7 | Risk of cardiovascular disease | 1 | |
| ERICE Scale [1, 84] | 28.5 ± 17.4 | |||
| Low [1, 5) | 1 (0.5) | |||
| Mild [5, 9] | 37 (17.4) | |||
| Moderate [10, 14] | 18 (8.5) | |||
| Moderate–high [15, 19] | 17 (8.0) | |||
| High [20, 29] | 42 (19.7) | |||
| Very high [30, 84] | 92 (42.2) | |||
| [40] | 11 | Dependency | 1 | |
| Independent for P-ADL and I-ADL [0, 11] | 0.8 ± 2.0 | |||
| Dependent [1, 11] | 48 (22.5) | |||
| 1 | Hearing loss | <1 | ||
| Yes [self-perceived] | 95 (44.6) | |||
| No | 118 (55.4) | |||
| 1 | Chronic pain | <1 | ||
| Visual analogue scale (VAS) [0–10] | 3.4 ± 2.8 | |||
| No pain [0] | 54 (25.4) | |||
| Mild [1, 3] | 57 (26.8) | |||
| Moderate [4, 6] | 65 (30.5) | |||
| Severe [7, 8] | 23 (10.8) | |||
| Excruciating [9, 10] | 9 (4.2) | |||
| 6. Lifestyle | ||||
| [78] | 2 | Anthropometry | <1 | |
| WHO stepwise method | ||||
| BMI (kg/m2) | 27.2 ± 4.1 | |||
| Normal weight [18.5, 25) | 61 (28.6) | |||
| Overweight [25, 30) | 96 (46.6) | |||
| Obese [30, +∞) | 49 (23.8) | |||
| 1 | Retired | <1 | ||
| Yes | 145 (68.7) | |||
| No | 66 (31.3) | |||
| [79] | 8 | Cognitive reserve | 2 | |
| Cognitive Reserve Questionnaire (CRC) [0, 25] | 10.3 ± 5.1 | |||
| Low [0, 6] | 55 (25.9) | |||
| Intermediate–low [7, 9] | 46 (21.7) | |||
| Intermediate–high [10, 14] | 65 (30.7) | |||
| High [15, 25] | 46 (21.7) | |||
| Nutrition | ||||
| [80] | 14 | Mediterranean Diet Adherence Score (MeDAS) [0, 14] | 9.2 ± 2.1 | 3 |
| Low [0, 6] | 20 (9.4) | |||
| Intermediate [7, 9] | 99 (46.7) | |||
| High [10, 14] | 93 (43.9) | |||
| [81] | 6 | Mini Nutritional Assessment (MNA) [0–14] | 12.2 ± 1.9 | 2 |
| Risk of malnutrition [0, 12) | 74 (34.7) | |||
| Normal nutrition [12, 14] | 139 (65.0) | |||
| [82] | 7 | Physical activity | 5 | |
| International Physical Activity Questionnaire (IPAQ) | ||||
| Low | 50 (23.5) | |||
| Moderate | 95 (44.6) | |||
| High | 68 (31.9) | |||
| Sleep | ||||
| [83] | 4 | Jenkins Sleep Scale (JSS) [0, 20] | 7.38 ± 5.0 | 2 |
| Sleep disorder [12, 20] | 41 (19.3) | |||
| No sleep disorder [0, 12) | 172 (80.8) | |||
| [84] | 8 | STOP-Bang Questionnaire [0, 8] | 3.1 ± 1.4 | 2 |
| Low [0, 2] | 81 (38.0) | |||
| Intermediate [3–4] | 97 (45.5) | |||
| High [5, 8] | 35 (16.4) | |||
| Total | 159 | No. of variables = 31; No. of tests = 23 | 69 | |
| Variable | Totals n (%) 213 (100) | Risk CI n (%) 52 (24.4) | No Risk CI n (%) 161 (75.6) | p-Value |
|---|---|---|---|---|
| 1. Sociodemographic Factors | ||||
| Age | ||||
| ( ± s) | 72.8 ± 11.2 | 78.9 ± 7.7 | 71.0 ± 11.5 | <0.001 a *** |
| [50, 65) | 57 (27.1) | 1 (2.0) | 56 (35.0) | <0.001 b *** |
| [65, 80) | 85 (40.5) | 25 (51.0) | 60 (37.0) | |
| [80, 95] | 68 (32.4) | 23 (47.0) | 45 (28.0) | |
| Marital status | ||||
| Married | 139 (65.6) | 26 (51.0) | 113 (70.2) | 0.013 b * |
| Widow | 57 (26.9) | 22 (43.1) | 35 (21.7) | |
| Separated/Divorced | 6 (2.8) | 0 (0.0) | 6 (3.7) | |
| Single | 10 (4.7) | 3 (5.9) | 7 (4.3) | |
| 2. Cognitive Impairment | ||||
| Subjective memory complaint | ||||
| Yes | 79 (37.1) | 25 (48.1) 27 (51.9) | 54 (33.5) 107 (66.5) | 0.070 c |
| No | 134 (62.9) | |||
| Memory Impairment Screen (MIS) [0, 8] | ||||
| ( ± s) | 6.8 ± 1.9 | 4.9 ± 2.7 | 7.4 ± 1.1 | <0.001 d *** |
| CI risk [0, 4] | 21 (9.9) | 21 (40.4) | 0 (0.0) | <0.001 b *** |
| No CI risk (4, 8] | 191 (90.1) | 31 (59.6) | 160 (100.0) | |
| Short Portable Mental State Questionnaire (SPMSQ) [0, 10] | ||||
| ( ± s) | 1.1 ± 1.3 | 2.4 ± 1.6 | 0.7 ± 0.9 | <0.001 d *** |
| CI risk [3–4, 10) No CI risk [0, 3–4) | 28 (13.1) | 28 (53.8) | 0 (0.0) | <0.001 b *** |
| 185 (86.9) | 24 (46.2) | 161 (100.0) | ||
| Semantic Verbal Fluency (SVF) [0, +∞) ( ± s) | 17.4 ± 7.1 | 13.5 ± 5.1 | 18.7 ± 7.2 | <0.001 d *** |
| CI risk [0, 10] No CI risk [10, +∞) | 16 (7.5) | 16 (30.8) | 0 (0.0) | <0.001 b *** |
| 196 (92.5) | 36 (69.2) | 160 (100.0) | ||
| 3. Meaning in Life | ||||
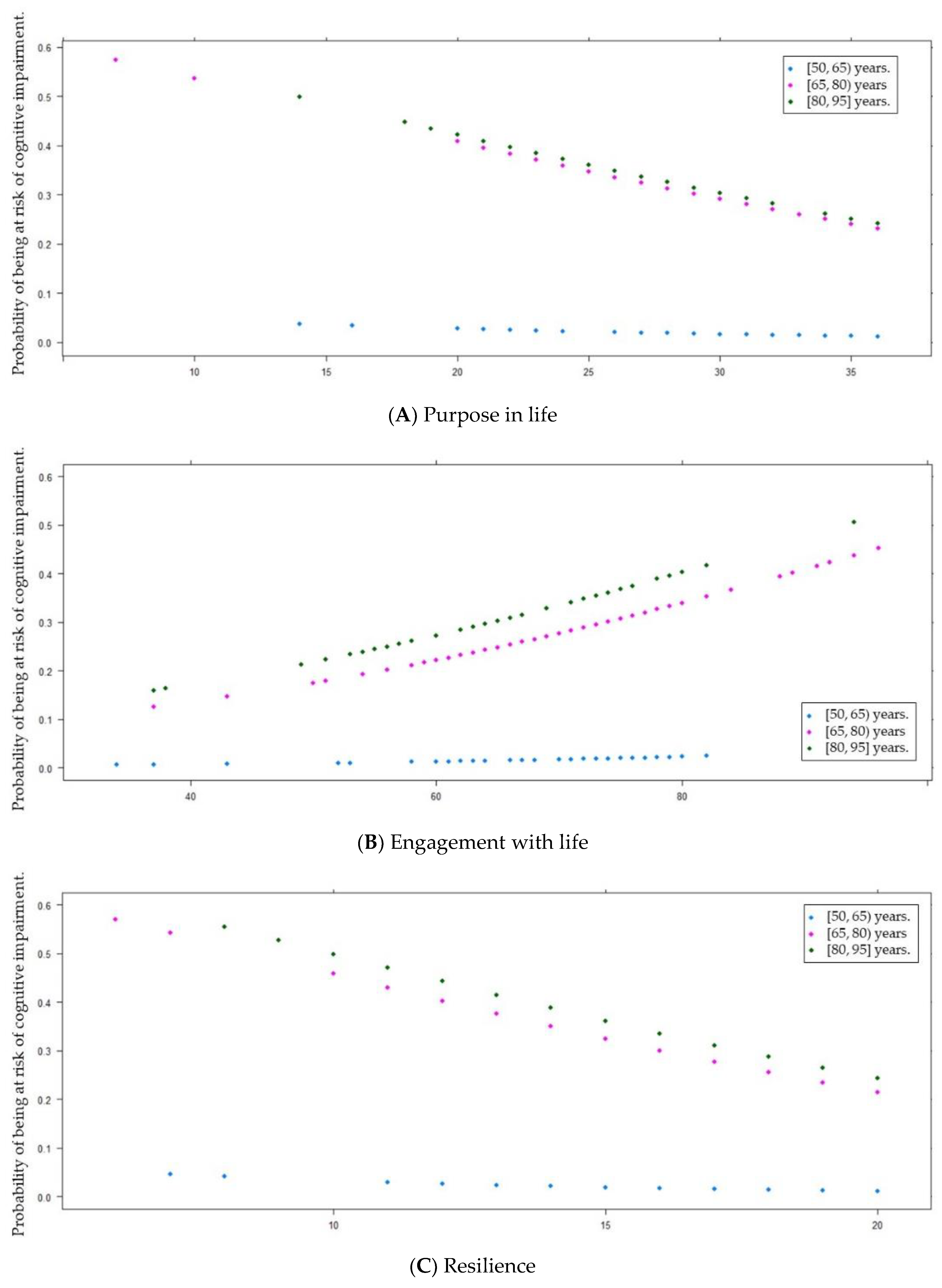
| Purpose in life (PiL) [6, 36] ( ± s) | 29.6 ± 5.6 | 28.2 ± 5.9 | 30.0 ± 5.4 | 0.020 d * |
| Engagement with life (EwL) [16, 80] ( ± s) | 70.1 ± 10.8 | 72.3 ± 13.1 | 69.5 ± 9.9 | 0.016 d * |
| 4. Psychosocial Factors | ||||
| Brief Resilient Coping Scale (BRCS) [4, 20] ( ± s) | 16.3 ± 3.0 | 15.5 ± 3.1 | 16.5 ± 3.0 | 0.018 d * |
| Low [4, 13) Intermediate [13, 17] High (17, 20] | 24 (11.3) | 10 (19.2) | 14 (8.7) | 0.075 c † |
| 104 (48.8) | 26 (50.0) | 78 (48.4) | ||
| 85 (39.9) | 16 (30.8) | 69 (42.9) | ||
| 5. Health Problems | ||||
| Hypertension | ||||
| Yes [systolic BP > 140 mmHg or diastolic BP > 90 mmHg] No [systolic BP ≤ 140 mmHg and diastolic BP ≤ 90 mmHg] | 126 (60.0) | 38 (76.0) | 88 (55.0) | 0.008 c ** |
| 84 (40.0) | 12 (24.0) | 72 (45.0) | ||
| Hypercholesterolemia | ||||
| Yes [Total > 200 mg/dL or LDL >100 mg/dL or HDL ≤ 35–40 mg/dL] No [Total ≤ 200 mg/dL or LDL ≤ 100 mg/dL or HDL > 35–40 mg/dL] | 94 (44.8) | 29 (55.8) | 65 (41.1) | 0.078 c † |
| 116 (55.2) | 23 (44.2) | 93 (58.9) | ||
| Diabetes | ||||
| Yes [Blood glucose > 126 mg/dL or HbA1c > 6.5%] No [Blood glucose ≤ 126 mg/dL and HbA1c ≤ 6.5%] | 50 (23.5) | 18 (34.6) | 32 (19.9) | 0.038 c * |
| 163 (76.5) | 34 (65.4) | 129 (80.1) | ||
| ERICE Scale [1, 84] ( ± s) | 28.4 ± 17.4 | 36.9 ± 15.9 | 26.0 ± 17.1 | <0.001 d *** |
| Low [1, 5) | 1 (0.5) | 0 (0.0) | 1 (0.6) | 0.002 b ** |
| Mild [5, 9] | 37 (17.9) | 3 (6.4) | 34 (21.2) | |
| Moderate [10, 14] | 18 (8.7) | 1 (2.1) | 17 (10.6) | |
| Moderate–high [15, 19] | 17 (8.2) | 2 (4.3) | 15 (9.4) | |
| High [20, 29] | 42 (20.3) | 8 (17.0) | 34 (21.2) | |
| Very high [30, 80] | 92 (44.4) | 33 (70.2) | 59 (36.9) | |
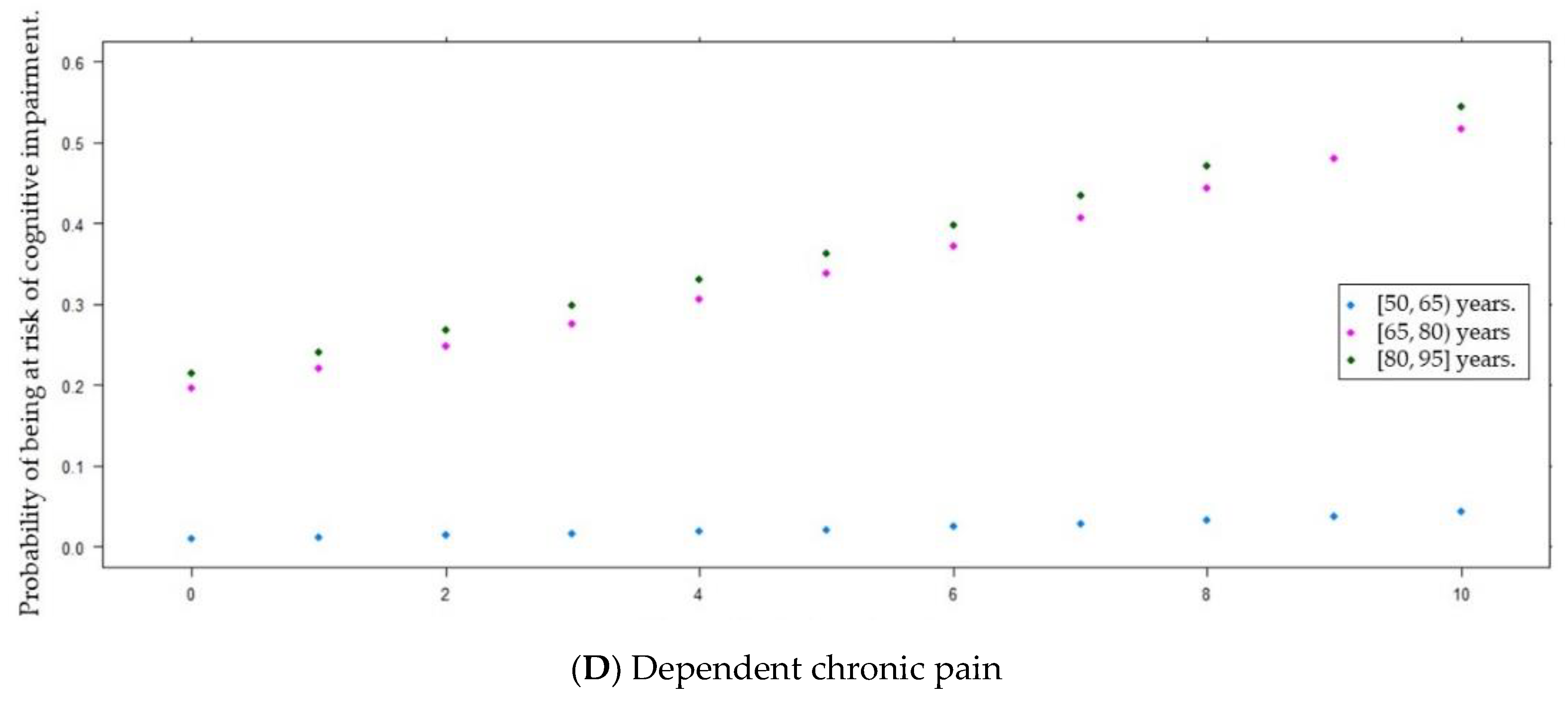
| Dependent chronic pain [0, 10] ( ± s) | 3.4 ± 2.8 | 4.2 ± 2.7 | 3.2 ± 2.8 | 0.013 d * |
| No pain [0] | 54 (26.0) | 5 (10.0) | 49 (31.0) | 0.018 b * |
| Mild [1, 3] | 57 (27.4) | 17 (34.0) | 40 (25.3) | |
| Moderate [4, 6] | 65 (31.2) | 19 (38.0) | 46 (29.1) | |
| Severe [7, 8] | 23 (11.1) | 5 (10.0) | 18 (11.4) | |
| Unbearable [9, 10] | 9 (4.3) | 4 (8.0) | 5 (3.2) | |
| 6. Lifestyle | ||||
| BMI ( ± s) | 27.2 ± 4.1 | 28.3 ± 3.8 | 26.8 ± 4.1 | 0.017 a * |
| Normal weight [18.5, 25) | 61 (29.6) | 8 (16.3) | 53 (33.9) | 0.046 c * |
| Overweight [25, 30) | 96 (46.6) | 26 (51.3) | 70 (44.6) | |
| Obese [30, +∞) | 49 (23.8) | 15 (30.6) | 34 (21.7) | |
| Retired | ||||
| Yes No | 145 (68.7) | 48 (94.1) | 97 (60.6) | <0.001 b *** |
| 66 (31.3) | 3 (5.9) | 63 (39.4) | ||
| Cognitive Reserve Questionnaire (CRC) [0, 25] ( ± s) | 10.3 ± 5.1 | 8.2 ± 5.1 | 11.0 ± 5.0 | <0.001 d *** |
| Low [0, 6] | 55 (25.9) | 22 (43.1) | 33 (20.5) | 0.010 b * |
| Intermediate–low [7, 9] | 46 (21.7) | 11 (21.6) | 35 (21.7) | |
| Intermediate–high [10, 14] | 65 (30.7) | 12 (23.5) | 53 (32.9) | |
| High [15, 25] | 46 (21.7) | 6 (11.8) | 40 (24.8) | |
| STOP–Bang Questionnaire [0, 8] ( ± s) | 3.1 ± 1.4 | 3.5 ± 1.3 | 3.0 ± 1.5 | 0.005 d ** |
| Low [0, 2] | 81 (38.0) | 13 (25.0) | 68 (42.2) | 0.075 b † |
| Intermediate [3, 4] | 97 (45.5) | 28 (53.8) | 69 (42.9) | |
| High [5, 8] | 35 (16.4) | 11 (21.2) | 24 (14.9) | |
| Variable | βi | SD | Wald | d.f. | p-Value | Exp(βi) | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| UL | LL | |||||||
| Model A | ||||||||
| Intercept | −2.51 | 1.34 | −1.87 | 1 | <0.1 † | 0.08 | 0.00 | 0.86 |
| Age (50, 65) (65, 80) (80, 95) | - 3.17 3.23 | - 1.04 1.04 | - 3.05 3.09 | - 1 | - <0.01 ** <0.01 ** | - 23.81 25.20 | - 4.77 4.94 | - 433.32 461.20 |
| Purpose in life | −0.05 | 0.03 | −1.66 | 1 | <0.1 † | 0.95 | 0.89 | 1.01 |
| Model B | ||||||||
| Intercept | −6.10 | 1.60 | −3.80 | 1 | <0.001 *** | 0.00 | 0.00 | 0.04 |
| Age (50, 65) (65, 80) (80, 95) | - 3.08 3.35 | - 1.04 1.04 | - 2.97 3.20 | - 1 | - <0.01 ** <0.01 ** | - 21.71 28.52 | - 4.35 5.59 | - 394.88 522.08 |
| Engagement with life | 0.03 | 0.02 | 1.69 | 1 | <0.1 † | 1.03 | 1.00 | 1.07 |
| Model C | ||||||||
| Intercept | −2.24 | 1.32 | −1.69 | 1 | <0.1 † | 0.11 | 0.00 | 1.09 |
| Age (50, 65) (65, 80) (80, 95) | - 3.20 3.37 | - 1.04 1.04 | - 3.08 3.22 | - 1 | - <0.01 ** <0.01 ** | - 24.59 28.97 | - 4.91 5.72 | - 447.94 529.72 |
| Resilience | −0.11 | 0.07 | −2.01 | 1 | <0.05 * | 0.89 | 0.80 | 1.00 |
| Model D | ||||||||
| Intercept | −4.57 | 1.05 | −4.33 | 1 | <0.001 *** | 0.01 | 0.00 | 0.95 |
| Age (50, 65) (65, 80) (80, 95) | - 3.16 3.27 | - 1.04 1.05 | - 3.03 3.13 | - 1 | - <0.01 ** <0.01 ** | - 23.61 26.33 | - 4.68 5.16 | - 431.38 482.29 |
| Dependent chronic pain | 0.15 | 0.07 | 2.22 | 1 | <0.05 * | 1.16 | 1.02 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, C.; Moreno, L.; Alacreu, M.; Muñoz, F.J.; Martínez, L.A. Addressing Psychosocial Factors in Cognitive Impairment Screening from a Holistic Perspective: The DeCo-Booklet Methodology Design and Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 12911. https://doi.org/10.3390/ijerph191912911
García C, Moreno L, Alacreu M, Muñoz FJ, Martínez LA. Addressing Psychosocial Factors in Cognitive Impairment Screening from a Holistic Perspective: The DeCo-Booklet Methodology Design and Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12911. https://doi.org/10.3390/ijerph191912911
Chicago/Turabian StyleGarcía, Cristina, Lucrecia Moreno, Mónica Alacreu, Francisco J. Muñoz, and Luis A. Martínez. 2022. "Addressing Psychosocial Factors in Cognitive Impairment Screening from a Holistic Perspective: The DeCo-Booklet Methodology Design and Pilot Study" International Journal of Environmental Research and Public Health 19, no. 19: 12911. https://doi.org/10.3390/ijerph191912911
APA StyleGarcía, C., Moreno, L., Alacreu, M., Muñoz, F. J., & Martínez, L. A. (2022). Addressing Psychosocial Factors in Cognitive Impairment Screening from a Holistic Perspective: The DeCo-Booklet Methodology Design and Pilot Study. International Journal of Environmental Research and Public Health, 19(19), 12911. https://doi.org/10.3390/ijerph191912911







