The Paradoxical Effect of Cannabis Use on Cognition in Chronic Psychotic Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Assessment
2.3.1. Psychopathological Measures
2.3.2. Translation of Psychopathological Instruments
2.3.3. Cognitive Assessment
2.3.4. Cannabis Use Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHS | Auditory Hallucinations Subscale |
| BACS | Brief Assessment of Cognition in Schizophrenia |
| BNA | Brief Neurocognitive Assessment |
| BNSS | Brief Negative Symptom Scale |
| CBD | Cannabidiol |
| CB1/CB2 | Cannabinoid Receptor Type 1/Type 2 |
| CHR-P | Clinical High Risk for Psychosis |
| CDSS | Calgary Depression Scale for Schizophrenia |
| CU | Cannabis Users |
| DS | Delusions Subscale |
| DSCF | Dwass–Steel–Critchlow–Fligner (test) |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| EE | Emotional Expressivity |
| FDR | False Discovery Rate |
| FEP | First-Episode Psychosis |
| FTD | Formal Thought Disorder |
| H-CU | High-Frequency Cannabis Users |
| IQ | Intelligence Quotient |
| L-CU | Low-Frequency Cannabis Users |
| MAP | Motivation and Pleasure |
| MCCB | MATRICS Consensus Cognitive Battery |
| MCI | Mild Cognitive Impairment |
| MoCA | Montreal Cognitive Assessment |
| No-CU | Non-Cannabis Users |
| PLE | Psychotic-like Experiences |
| PSYRATS | Psychotic Symptom Rating Scales |
| SCIP | Screen for Cognitive Impairment in Psychiatry |
| SCZ | Schizophrenia |
| SD | Standard Deviation |
| TLC | Thought, Language, and Communication Scale |
| THC | Δ9-Tetrahydrocannabinol |
| WAIS-R | Wechsler Adult Intelligence Scale–Revised |
References
- World Health Organization. Cannabis. The Health and Social Effects of Nonmedical Cannabis Use; WHO Press, World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. Alcohol, E-Cigarettes, Cannabis: Concerning Trends in Adolescent Substance Use, Shows New WHO/Europe Report; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Johnson-Ferguson, L.; Di Forti, M. From heavy cannabis use to psychosis: Is it time to take action? Ir. J. Psychol. Med. 2023, 40, 13–18. [Google Scholar] [CrossRef]
- Punniakotti, M.; Ahmad, R.; Villanueva, J.; Roy, T.; Mohammed, K.; Sani, D.; Mohammad, S.; Sundaram, M.; Haque, M.; Rahman, S. Cannabis and Health: Exploring Risks, Benefits and Research Horizons. Adv. Hum. Biol. 2025, 10-4103. [Google Scholar] [CrossRef]
- Colizzi, M.; Weltens, N.; McGuire, P.; Lythgoe, D.; Williams, S.; Van Oudenhove, L.; Bhattacharyya, S. Delta-9-tetrahydrocannabinol increases striatal glutamate levels in healthy individuals: Implications for psychosis. Mol. Psychiatry 2020, 25, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- European Union Drugs Agency. Cannabis—The Current Situation in Europe (European Drug Report 2025); European Union Drugs Agency: Lisbon, Portugal, 2025. [Google Scholar]
- García Álvarez, L.; Gomar, J.J.; García-Portilla, M.P.; Bobes, J. Cannabis use and cognitive impairment in schizophrenia and first-episode psychosis. Adicciones 2019, 31, 89–94. [Google Scholar] [CrossRef] [PubMed]
- West, M.L.; Sharif, S. Cannabis and Psychosis. Child Adolesc. Psychiatr. Clin. N. Am. 2023, 32, 69–83. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Duczmal, D.; Bazan-Wozniak, A.; Niedzielska, K.; Pietrzak, R. Cannabinoids-Multifunctional Compounds, Applications and Challenges-Mini Review. Molecules 2024, 29, 4923. [Google Scholar] [CrossRef]
- Urits, I.; Charipova, K.; Gress, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Kassem, H.; Ngo, A.L.; Kaye, A.D.; Viswanath, O. Adverse Effects of Recreational and Medical Cannabis. Psychopharmacol. Bull. 2021, 51, 94–109. [Google Scholar]
- Volkow, N.D.; Swanson, J.M.; Evins, A.E.; DeLisi, L.E.; Meier, M.H.; Gonzalez, R.; Bloomfield, M.A.; Curran, H.V.; Baler, R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 2016, 73, 292–297. [Google Scholar] [CrossRef]
- Harvey, P.D.; Koren, D.; Reichenberg, A.; Bowie, C.R. Negative symptoms and cognitive deficits: What is the nature of their relationship? Schizophr. Bull. 2006, 32, 250–258. [Google Scholar] [CrossRef]
- Sevy, S.; Davidson, M. The cost of cognitive impairment in schizophrenia. Schizophr. Res. 1995, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.; Harvey, P.D. Cognitive impairment in schizophrenia. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 11–37. [Google Scholar] [CrossRef]
- Ringen, P.A.; Nesvåg, R.; Helle, S.; Lagerberg, T.V.; Lange, E.H.; Løberg, E.M.; Agartz, I.; Andreassen, O.A.; Melle, I. Premorbid cannabis use is associated with more symptoms and poorer functioning in schizophrenia spectrum disorder. Psychol. Med. 2016, 46, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C. Cannabis, cannabinoids and psychosis: A balanced view. World Psychiatry 2023, 22, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Bozikas, V.P.; Kosmidis, M.H.; Karavatos, A. Disproportionate impairment in semantic verbal fluency in schizophrenia: Differential deficit in clustering. Schizophr. Res. 2005, 74, 51–59. [Google Scholar] [CrossRef]
- Collins, A.G.; Brown, J.K.; Gold, J.M.; Waltz, J.A.; Frank, M.J. Working memory contributions to reinforcement learning impairments in schizophrenia. J. Neurosci. 2014, 34, 13747–13756. [Google Scholar] [CrossRef]
- Orellana, G.; Slachevsky, A.; Peña, M. Executive attention impairment in first-episode schizophrenia. BMC Psychiatry 2012, 12, 154. [Google Scholar] [CrossRef]
- Helldin, L.; Kane, J.M.; Karilampi, U.; Norlander, T.; Archer, T. Remission and cognitive ability in a cohort of patients with schizophrenia. J. Psychiatr. Res. 2006, 40, 738–745. [Google Scholar] [CrossRef]
- Hurford, I.M.; Marder, S.R.; Keefe, R.S.; Reise, S.P.; Bilder, R.M. A brief cognitive assessment tool for schizophrenia: Construction of a tool for clinicians. Schizophr. Bull. 2011, 37, 538–545. [Google Scholar] [CrossRef]
- Kroon, E.; Kuhns, L.; Hoch, E.; Cousijn, J. Heavy cannabis use, dependence and the brain: A clinical perspective. Addiction 2020, 115, 559–572. [Google Scholar] [CrossRef]
- Cunha, P.J.; Rosa, P.G.; Ayres Ade, M.; Duran, F.L.; Santos, L.C.; Scazufca, M.; Menezes, P.R.; dos Santos, B.; Murray, R.M.; Crippa, J.A.; et al. Cannabis use, cognition and brain structure in first-episode psychosis. Schizophr. Res. 2013, 147, 209–215. [Google Scholar] [CrossRef]
- de la Serna, E.; Mayoral, M.; Baeza, I.; Arango, C.; Andrés, P.; Bombin, I.; González, C.; Rapado, M.; Robles, O.; Rodríguez-Sánchez, J.M.; et al. Cognitive functioning in children and adolescents in their first episode of psychosis: Differences between previous cannabis users and nonusers. J. Nerv. Ment. Dis. 2010, 198, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Setién-Suero, E.; Ayesa-Arriola, R.; Peña, J.; Crespo-Facorro, B.; Ojeda, N. Longitudinal effects of cannabis use on attentional processes in patients with first episode of psychosis. Schizophr. Res. 2022, 244, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.; Gonzalez, R.; Carey, C.L.; Natarajan, L.; Wolfson, T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J. Int. Neuropsychol. Soc. 2003, 9, 679–689. [Google Scholar] [CrossRef]
- Schreiner, A.M.; Dunn, M.E. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Exp. Clin. Psychopharmacol. 2012, 20, 420–429. [Google Scholar] [CrossRef]
- Di Forti, M.; Quattrone, D.; Freeman, T.P.; Tripoli, G.; Gayer-Anderson, C.; Quigley, H.; Rodriguez, V.; Jongsma, H.E.; Ferraro, L.; La Cascia, C.; et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019, 6, 427–436. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Green, M.F.; Kern, R.S.; Baade, L.E.; Barch, D.M.; Cohen, J.D.; Essock, S.; Fenton, W.S.; Frese, F.J., 3rd; Gold, J.M.; et al. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef]
- Kern, R.S.; Nuechterlein, K.H.; Green, M.F.; Baade, L.E.; Fenton, W.S.; Gold, J.M.; Keefe, R.S.; Mesholam-Gately, R.; Mintz, J.; Seidman, L.J.; et al. The MATRICS Consensus Cognitive Battery, part 2: Co-norming and standardization. Am. J. Psychiatry 2008, 165, 214–220. [Google Scholar] [CrossRef]
- Keefe, R.S.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef]
- Purdon, S. Purdon (2005) SCIP Manual; Purdon Neuropsychological Labs Inc.: Palo Alto, CA, USA, 2005. [Google Scholar]
- Fervaha, G.; Hill, C.; Agid, O.; Takeuchi, H.; Foussias, G.; Siddiqui, I.; Kern, R.S.; Remington, G. Examination of the validity of the Brief Neurocognitive Assessment (BNA) for schizophrenia. Schizophr. Res. 2015, 166, 304–309. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Rosca, E.C.; Cornea, A.; Simu, M. Montreal Cognitive Assessment for evaluating the cognitive impairment in patients with schizophrenia: A systematic review. Gen. Hosp. Psychiatry 2020, 65, 64–73. [Google Scholar] [CrossRef]
- Daderwal, M.C.; Sreeraj, V.S.; Suhas, S.; Rao, N.P.; Venkatasubramanian, G. Montreal Cognitive Assessment (MoCA) and Digit Symbol Substitution Test (DSST) as a screening tool for evaluation of cognitive deficits in schizophrenia. Psychiatry Res. 2022, 316, 114731. [Google Scholar] [CrossRef]
- Corral, S.; Gaspar, P.A.; Castillo-Passi, R.I.; Mayol Troncoso, R.; Mundt, A.P.; Ignatyev, Y.; Nieto, R.R.; Figueroa-Muñoz, A. Montreal Cognitive Assessment (MoCA) as a screening tool for cognitive impairment in early stages of psychosis. Schizophr. Res. Cogn. 2024, 36, 100302. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013. [Google Scholar]
- Franzen, M. The Wechsler Adult Intelligence Scale-Revised and Wechsler Adult Intelligence Scale-III. In Reliability and Validity in Neuropsychological Assessment; Springer Nature: Boston, MA, USA, 2000; pp. 55–70. [Google Scholar]
- Ang, M.S.; Rekhi, G.; Lee, J. Validation of the Brief Negative Symptom Scale and its association with functioning. Schizophr. Res. 2019, 208, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Dollfus, S.; Nordentoft, M.; Falkai, P.; Kaiser, S.; Giordano, G.M.; Vandevelde, A.; Nielsen, M.O.; Glenthoj, L.B.; et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e23. [Google Scholar] [CrossRef] [PubMed]
- Addington, D.; Addington, J.; Maticka-Tyndale, E. Assessing depression in schizophrenia: The Calgary Depression Scale. Br. J. Psychiatry Suppl. 1993, 163, 39–44. [Google Scholar] [CrossRef]
- Haddock, G.; McCarron, J.; Tarrier, N.; Faragher, E.B. Scales to measure dimensions of hallucinations and delusions: The psychotic symptom rating scales (PSYRATS). Psychol. Med. 1999, 29, 879–889. [Google Scholar] [CrossRef]
- Andreasen, N.C. Scale for the assessment of thought, language, and communication (TLC). Schizophr. Bull. 1986, 12, 473–482. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yücel, M.; Solowij, N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef]
- Solowij, N. Cannabis and Cognitive Functioning; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Schoeler, T.; Monk, A.; Sami, M.B.; Klamerus, E.; Foglia, E.; Brown, R.; Camuri, G.; Altamura, A.C.; Murray, R.; Bhattacharyya, S. Continued versus discontinued cannabis use in patients with psychosis: A systematic review and meta-analysis. Lancet Psychiatry 2016, 3, 215–225. [Google Scholar] [CrossRef]
- Schnell, T.; Koethe, D.; Daumann, J.; Gouzoulis-Mayfrank, E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology 2009, 205, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.; Mendrek, A.; Durand, M.; Lakis, N.; Lipp, O.; Stip, E.; Lalonde, P.; Grignon, S.; Potvin, S. Cannabis abuse is associated with better emotional memory in schizophrenia: A functional magnetic resonance imaging study. Psychiatry Res. 2013, 214, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.; Oldenhof, E.; Ahmed, S.H.; Belin, D.; Billieux, J.; Bowden-Jones, H.; Carter, A.; Chamberlain, S.R.; Clark, L.; Connor, J.; et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: An international Delphi consensus study. Addiction 2019, 114, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Setién-Suero, E.; Suárez-Pinilla, P.; Ferro, A.; Tabarés-Seisdedos, R.; Crespo-Facorro, B.; Ayesa-Arriola, R. Childhood trauma and substance use underlying psychosis: A systematic review. Eur. J. Psychotraumatol. 2020, 11, 1748342. [Google Scholar] [CrossRef]
- Leeson, V.C.; Harrison, I.; Ron, M.A.; Barnes, T.R.E.; Joyce, E.M. The Effect of Cannabis Use and Cognitive Reserve on Age at Onset and Psychosis Outcomes in First-Episode Schizophrenia. Schizophr. Bull. 2011, 38, 873–880. [Google Scholar] [CrossRef]
- Argote, M.; Sescousse, G.; Brunelin, J.; Baudin, G.; Schaub, M.P.; Rabin, R.; Schnell, T.; Ringen, P.A.; Andreassen, O.A.; Addington, J.M.; et al. Association between cannabis use and symptom dimensions in schizophrenia spectrum disorders: An individual participant data meta-analysis on 3053 individuals. EClinicalMedicine 2023, 64, 102199. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Y.; Tang, X.; Cui, H.; Hu, Y.; Xu, L.; Liu, H.; Wang, Z.; Chen, T.; Hu, Q.; et al. Cognitive Impairments in Drug-Naive Patients with First-Episode Negative Symptom-Dominant Psychosis. JAMA Netw. Open 2024, 7, e2415110. [Google Scholar] [CrossRef]
- Roser, P.; Juckel, G.; Rentzsch, J.; Nadulski, T.; Gallinat, J.; Stadelmann, A.M. Effects of acute oral Delta9-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur. Neuropsychopharmacol. 2008, 18, 569–577. [Google Scholar] [CrossRef]
- Bloomfield, M.A.; Ashok, A.H.; Volkow, N.D.; Howes, O.D. The effects of Δ(9)-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [CrossRef]
- Batalla, A.; Bos, J.; Postma, A.; Bossong, M.G. The Impact of Cannabidiol on Human Brain Function: A Systematic Review. Front. Pharmacol. 2020, 11, 618184. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weinstein, A.M. Synthetic and Non-synthetic Cannabinoid Drugs and Their Adverse Effects-A Review From Public Health Prospective. Front. Public Health 2018, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Bruci, Z.; Papoutsis, I.; Athanaselis, S.; Nikolaou, P.; Pazari, E.; Spiliopoulou, C.; Vyshka, G. First systematic evaluation of the potency of Cannabis sativa plants grown in Albania. Forensic. Sci. Int. 2012, 222, 40–46. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022; 60p. [Google Scholar] [CrossRef]
- Freeman, A.M.; Mokrysz, C.; Hindocha, C.; Lawn, W.; Morgan, C.J.; Freeman, T.P.; Saunders, R.; Curran, H.V. Does variation in trait schizotypy and frequency of cannabis use influence the acute subjective, cognitive and psychotomimetic effects of delta-9-tetrahydrocannabinol? A mega-analysis. J. Psychopharmacol. 2021, 35, 804–813. [Google Scholar] [CrossRef]
- Jager, G.; Ramsey, N.F. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr. Drug Abus. Rev. 2008, 1, 114–123. [Google Scholar] [CrossRef]
- Hunt, D.A.; Keefe, J.; Whitehead, T.; Littlefield, A. Understanding Cannabis. J. Nurse Pract. 2020, 16, 645–649. [Google Scholar] [CrossRef]
- Ribolsi, M.; Fiori Nastro, F.; Pelle, M.; Esposto, E.; Jannini, T.B.; Di Lorenzo, G. Targeting Psychotic and Cognitive Dimensions in Clinical High Risk for Psychosis (CHR-P): A Narrative Review. J. Clin. Med. 2025, 14, 5432. [Google Scholar] [CrossRef]
- Ribolsi, M.; Prosperi Porta, D.; Sacco, R.; Di Lorenzo, G.; Fiori Nastro, F.; Albergo, G.; Di Lazzaro, V.; Costa, A. Psychopathological characteristics in ultra-high risk for psychosis with and without comorbid ADHD. Early Interv. Psychiatry 2024, 18, 578–582. [Google Scholar] [CrossRef]
- Fiori Nastro, F.; Pelle, M.; Clemente, A.; Corinto, F.; Prosperi Porta, D.; Sonnino, Y.; Gelormini, C.; Di Lorenzo, G.; Ribolsi, M. Investigating Aberrant Salience in Autism Spectrum Disorder and Psychosis risk: A Cross-Group Analysis. Early Interv. Psychiatry 2025, 19, e70099. [Google Scholar] [CrossRef]
- Riccioni, A.; Siracusano, M.; Vasta, M.; Ribolsi, M.; Fiori Nastro, F.; Gialloreti, L.E.; Di Lorenzo, G.; Mazzone, L. Clinical profile and conversion rate to full psychosis in a prospective cohort study of youth affected by autism spectrum disorder and attenuated psychosis syndrome: A preliminary report. Front. Psychiatry 2022, 13, 950888. [Google Scholar] [CrossRef]
| Variables |
Total Sample
(n = 105; 100%) | CU (n = 56; 53.3%) | No-CU (n = 49; 46.7%) | Statistics a,b | |
|---|---|---|---|---|---|
| Value | p | ||||
| Age | 40.3 ± 11.84 | 34.8 ± 9.34 | 46.6 ± 11.3 | 569 | <0.001 |
| Years of education | 12.8 ± 3.82 | 13.0 ± 3.81 | 12.5 ± 3.85 | 1272 | 0.495 |
| Sex: | 8.89 | 0.004 | |||
| Females | 34 (32.4%) | 11 (10.5%) | 23 (21.9%) | ||
| Males | 71 (67.6%) | 45 (42.9%) | 26 (24.8%) | ||
| Years of illness | 11.8 ± 8.1 | 8.52 ± 5.81 | 15.6 ± 8.66 | 693 | <0.001 |
| Number of hospital admissions | 3.0 ± 1.8 | 3.04 ± 1.80 | 3.04 ± 1.86 | 1365 | 0.963 |
| Use of antipsychotics: | 2.60 | 0.122 | |||
| Clozapine | 27 (25.7%) | 18 (17.1%) | 9 (8.6%) | ||
| Other antipsychotics | 78 (74.3%) | 38 (36.2%) | 40 (38.1%) | ||
| Long-acting injectable antipsychotics: | 0.793 | 0.422 | |||
| Yes | 39 (37.1%) | 23 (21.9%) | 16 (15.2%) | ||
| No | 66 (62.9%) | 33 (31.4%) | 33 (31.4%) | ||
| Diagnosis: | 0.0321 | 0.858 | |||
| non-affective psychosis | 78 (74.3%) | 42 (75%) | 36 (73.5%) | ||
| affective psychosis | 27 (25.7%) | 14 (25%) | 13 (26.5%) | ||
| Motivation-pleasure deficit (BNSS) | 12.9 ± 7.06 | 11 ± 5.86 | 15 ± 7.74 | 975 | 0.011 |
| Emotional expressivity deficit (BNSS) | 4.42 ± 5.01 | 4.45 ± 4.95 | 4.39 ± 5.13 | 1334 | 0.805 |
| CDSS | 3.90 ± 3.14 | 3.29 ± 2.29 | 4.59 ± 3.80 | 1153 | 0.156 |
| PSYRATS Delusion scale | 16.5 ± 3.28 | 16.8 ± 3.35 | 16.3 ± 3.22 | 1237 | 0.384 |
| PSYRATS Hallucination scale | 22.9 ± 10.9 | 22.5 ± 9.91 | 23.3 ± 12.0 | 1228 | 0.354 |
| TLC | 17.8 ± 6.80 | 17 ± 6.14 | 18.7 ± 7.43 | 1185 | 0.230 |
| Variable | Z-Statistic a | Raw p-Value | FDR p-Value |
|---|---|---|---|
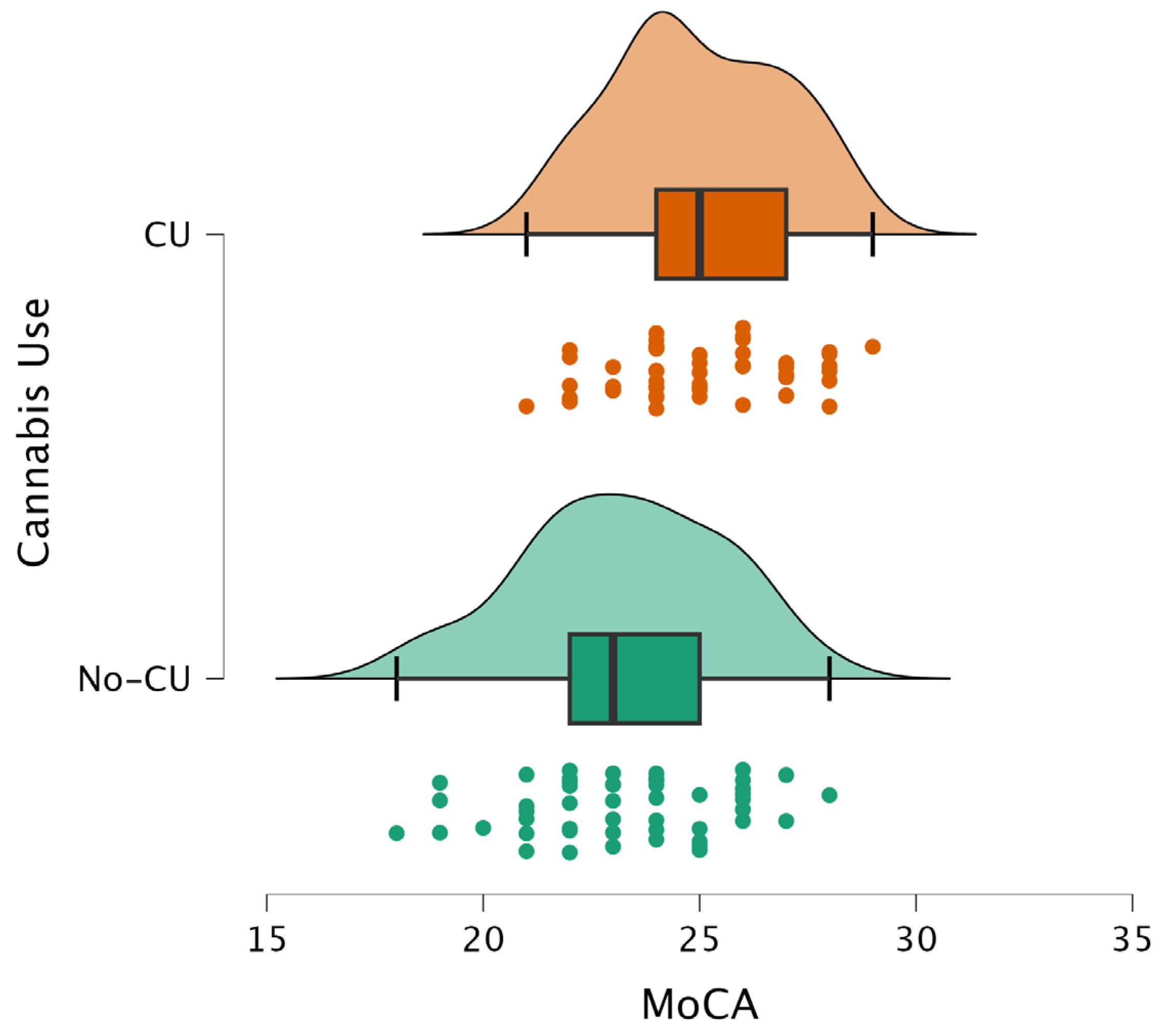
| MoCA general score | −3.696 | 0.0003 | 0.002400 |
| VisuoSpatial/Executive | −2.496 | 0.0142 | 0.022720 |
| Naming | −0.289 | 0.7955 | 0.909143 |
| Attention | −2.853 | 0.0039 | 0.010400 |
| Language | −1.080 | 0.2629 | 0.350533 |
| Abstraction | −3.214 | 0.0006 | 0.002400 |
| Delayed Recall | −0.059 | 0.9723 | 0.972300 |
| Orientation | −2.798 | 0.0063 | 0.012600 |
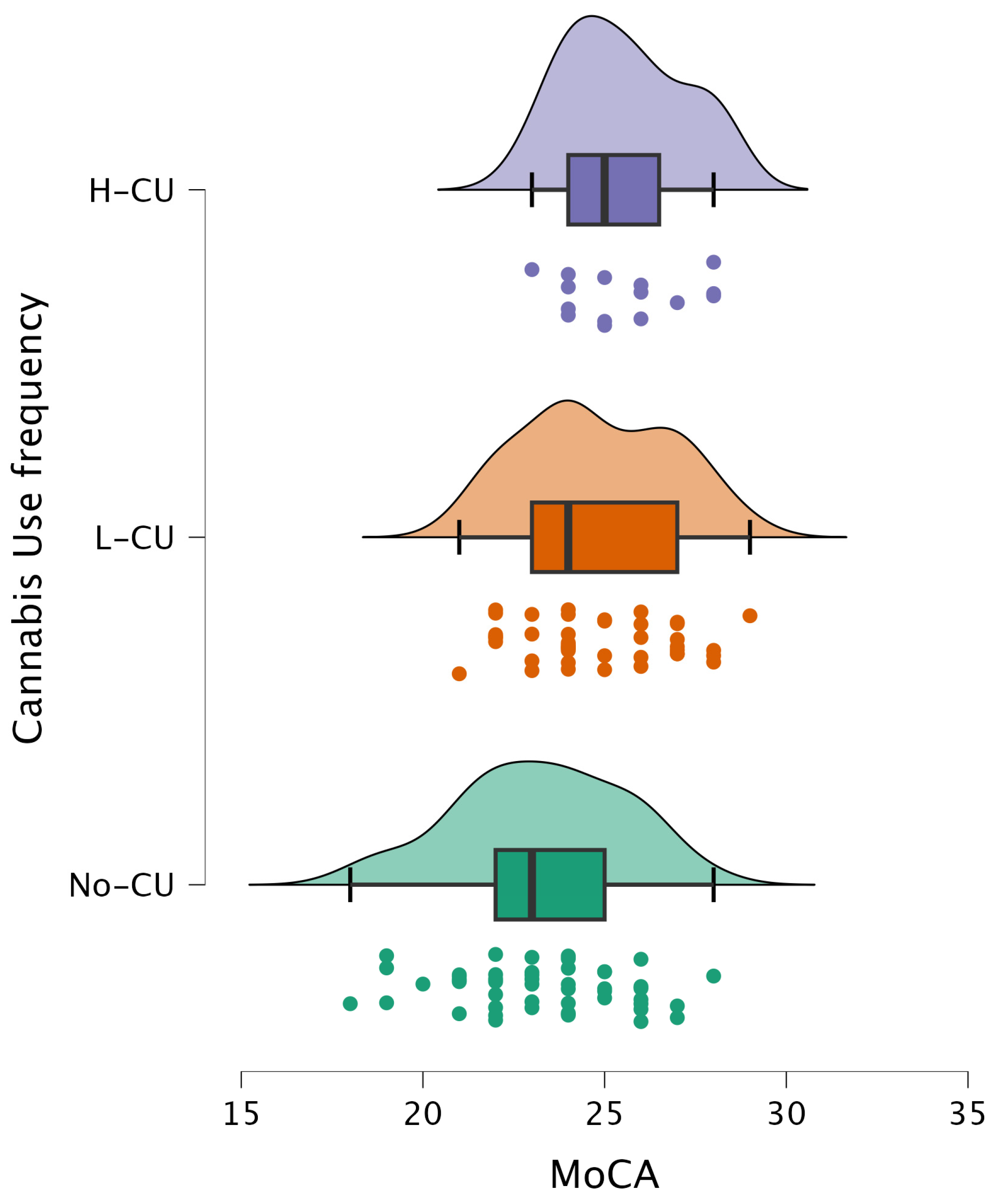
| Variable | H-Statistic a | Raw p-Value | FDR p-Value |
|---|---|---|---|
| MoCA general score | 15.051 | 0.0003 | 0.002400 |
| VisuoSpatial/Executive | 8.746 | 0.0102 | 0.026240 |
| Naming | 0.596 | 0.8061 | 0.806100 |
| Attention | 8.154 | 0.0154 | 0.026240 |
| Language | 1.285 | 0.5495 | 0.628000 |
| Abstraction | 11.419 | 0.0029 | 0.011600 |
| Delayed Recall | 1.600 | 0.4571 | 0.609467 |
| Orientation | 7.978 | 0.0164 | 0.026240 |
| MoCA Variable | Comparison | Z-Statistic a | p-Value b |
|---|---|---|---|
| MoCA general score | No-CU vs. L-CU | 4.23 | 0.0073 |
| No-CU vs. H-CU | 4.62 | 0.0026 | |
| L-CU vs. H-CU | 1.75 | 0.4246 | |
| Visuospatial/Executive | No-CU vs. L-CU | 2.40 | 0.2050 |
| No-CU vs. H-CU | 3.97 | 0.0122 | |
| L-CU vs. H-CU | 2.32 | 0.2330 | |
| Attention | No-CU vs. L-CU | 3.55 | 0.0307 |
| No-CU vs. H-CU | 3.04 | 0.0802 | |
| L-CU vs. H-CU | −0.08 | 0.9989 | |
| Abstraction | No-CU vs. L-CU | 3.70 | 0.0208 |
| No-CU vs. H-CU | 4.02 | 0.0099 | |
| L-CU vs. H-CU | 1.58 | 0.5039 | |
| Orientation | No-CU vs. L-CU | 3.80 | 0.0156 |
| No-CU vs. H-CU | 2.12 | 0.2904 | |
| L-CU vs. H-CU | −0.71 | 0.8729 | |
| Naming | No-CU vs. L-CU | 0.78 | 0.8144 |
| No-CU vs. H-CU | −0.46 | 0.9307 | |
| L-CU vs. H-CU | −1.04 | 0.8144 | |
| Language | No-CU vs. L-CU | 1.58 | 0.5213 |
| No-CU vs. H-CU | 0.66 | 0.8976 | |
| L-CU vs. H-CU | −0.52 | 0.9373 | |
| Delayed Recall | No-CU vs. L-CU | −0.63 | 0.8955 |
| No-CU vs. H-CU | 1.54 | 0.5228 | |
| L-CU vs. H-CU | 1.65 | 0.4767 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gorea, F.; Pelle, M.; Fiori Nastro, F.; Gelormini, C.; Elezi, F.; Ribolsi, M.; Di Lorenzo, G. The Paradoxical Effect of Cannabis Use on Cognition in Chronic Psychotic Disorders. Pathophysiology 2026, 33, 11. https://doi.org/10.3390/pathophysiology33010011
Gorea F, Pelle M, Fiori Nastro F, Gelormini C, Elezi F, Ribolsi M, Di Lorenzo G. The Paradoxical Effect of Cannabis Use on Cognition in Chronic Psychotic Disorders. Pathophysiology. 2026; 33(1):11. https://doi.org/10.3390/pathophysiology33010011
Chicago/Turabian StyleGorea, Fiorela, Martina Pelle, Federico Fiori Nastro, Carmine Gelormini, Fatime Elezi, Michele Ribolsi, and Giorgio Di Lorenzo. 2026. "The Paradoxical Effect of Cannabis Use on Cognition in Chronic Psychotic Disorders" Pathophysiology 33, no. 1: 11. https://doi.org/10.3390/pathophysiology33010011
APA StyleGorea, F., Pelle, M., Fiori Nastro, F., Gelormini, C., Elezi, F., Ribolsi, M., & Di Lorenzo, G. (2026). The Paradoxical Effect of Cannabis Use on Cognition in Chronic Psychotic Disorders. Pathophysiology, 33(1), 11. https://doi.org/10.3390/pathophysiology33010011






