Therapeutic Efficacy of Mesenchymal Stem Cells in Modulating Oxidative Stress in Puromycin-Induced Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MSCs from the Rat Bone Marrow
2.2. Animal Model
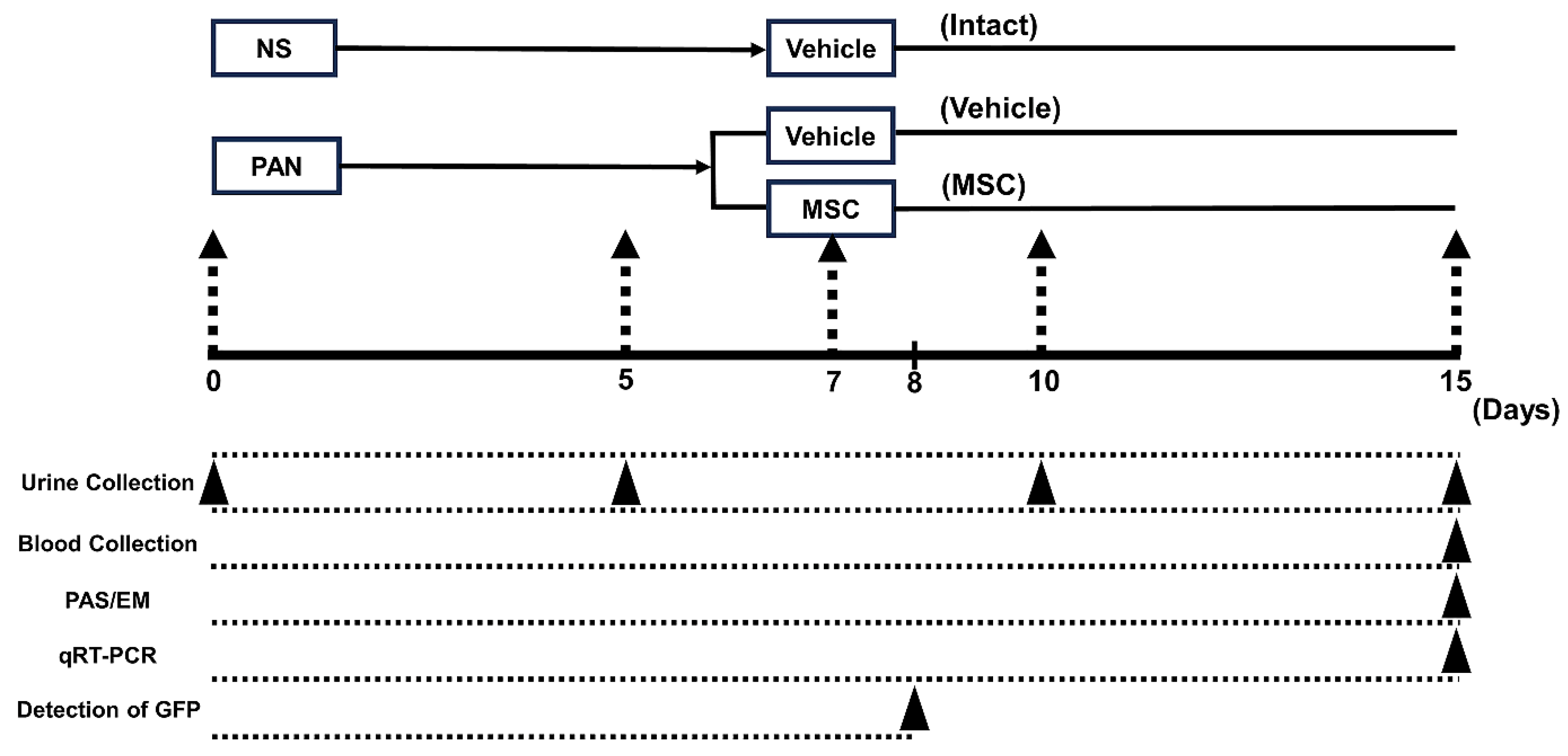
2.3. Experimental Protocol
2.4. Urine Protein
2.5. Blood and Tissue Sample Collection
2.6. Blood Analysis
2.7. PAS Staining
2.8. Electron Microscopic Analysis
2.9. RT-PCR
2.10. Detection of GFP-Expressing MSCs In Vivo
2.11. Statistical Analysis
3. Results
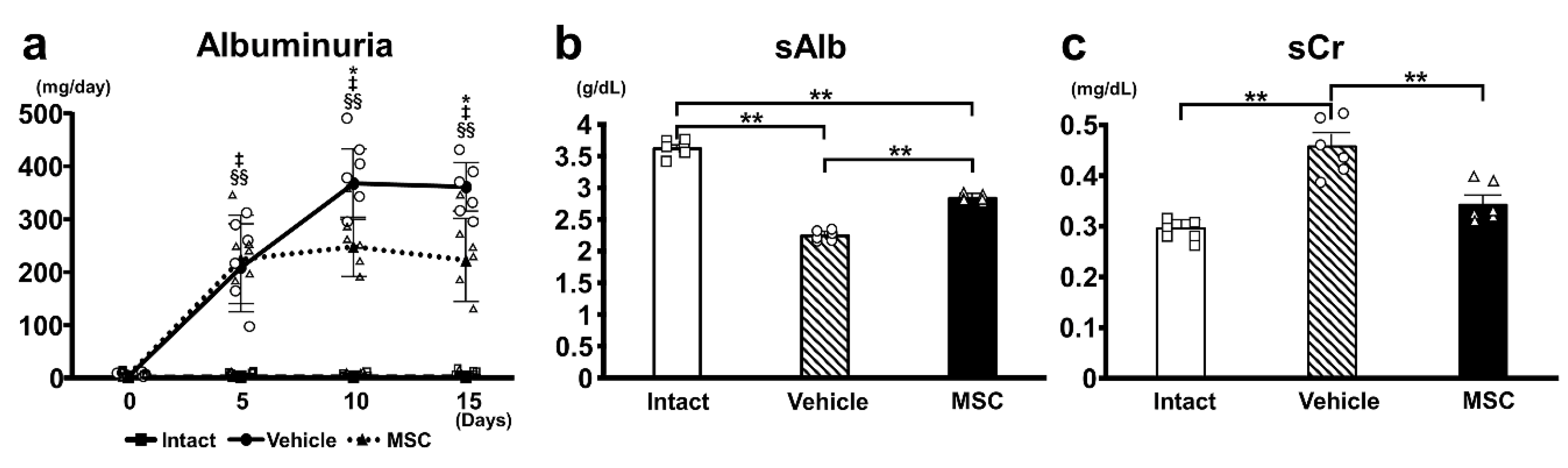
3.1. Urine Albumin and Kidney Function
3.2. Light Microscopy with PAS Staining
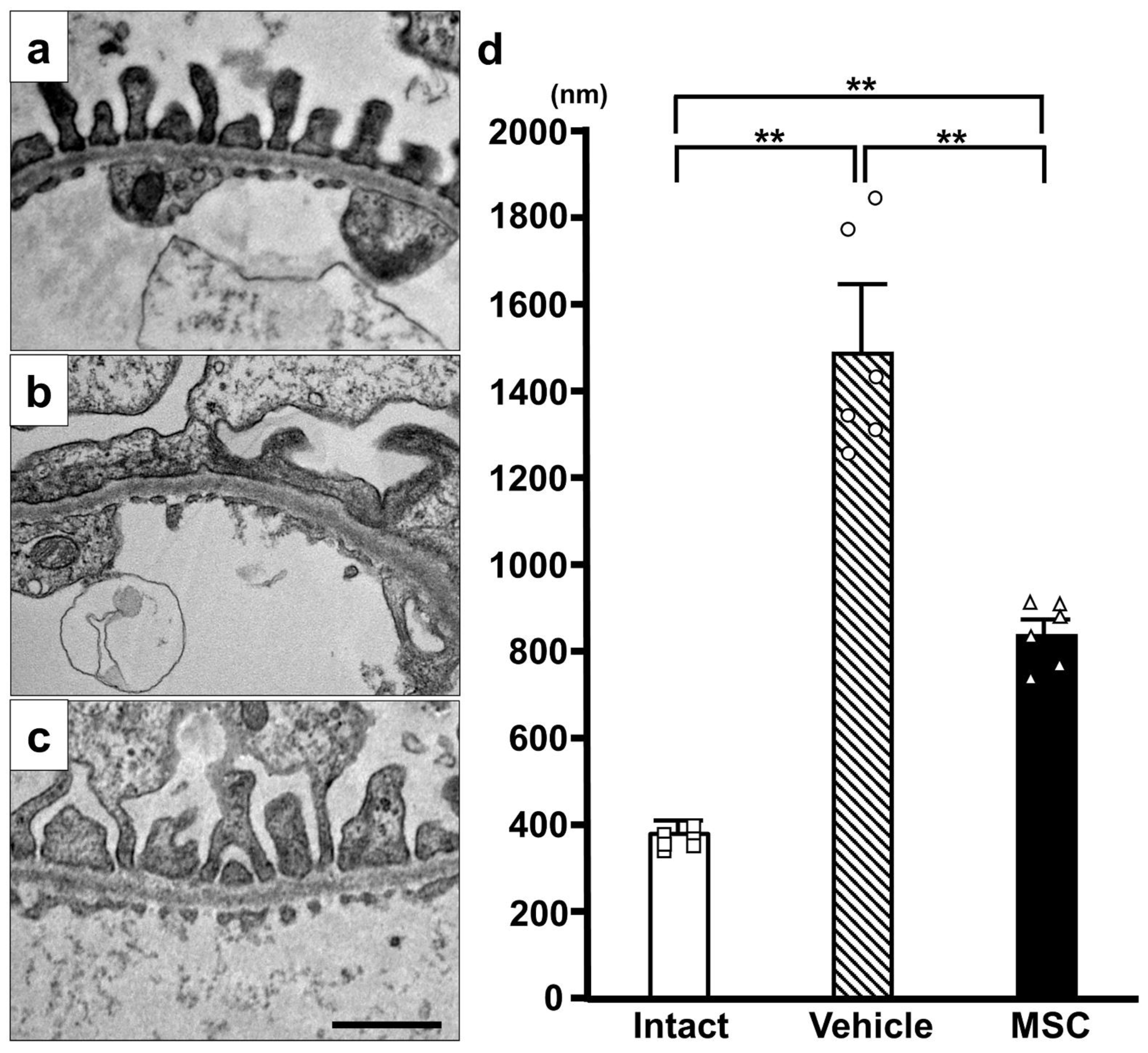
3.3. Electron Microscopy
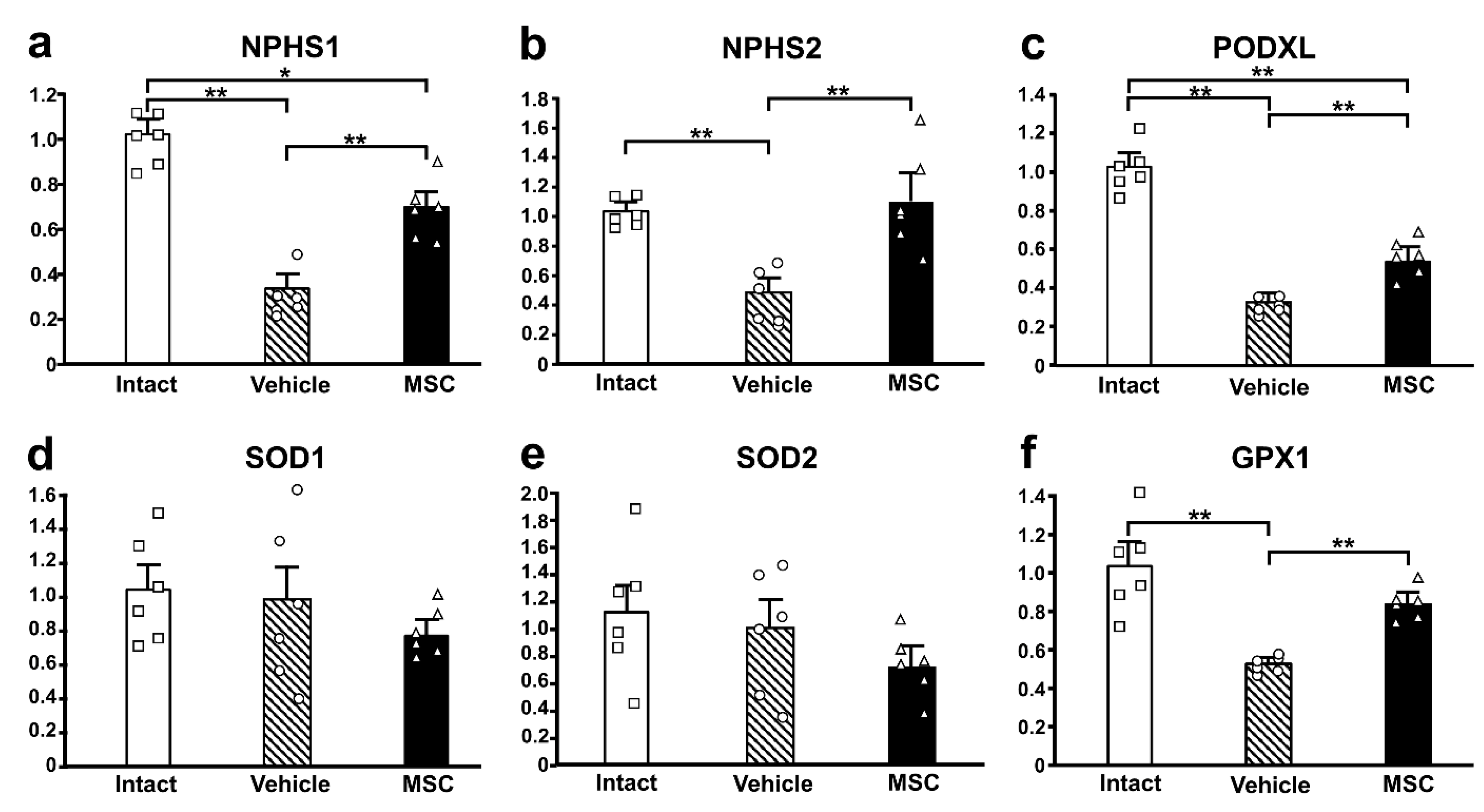
3.4. Expression of Podocyte-Associated Proteins
3.5. Antioxidant Enzyme Activity
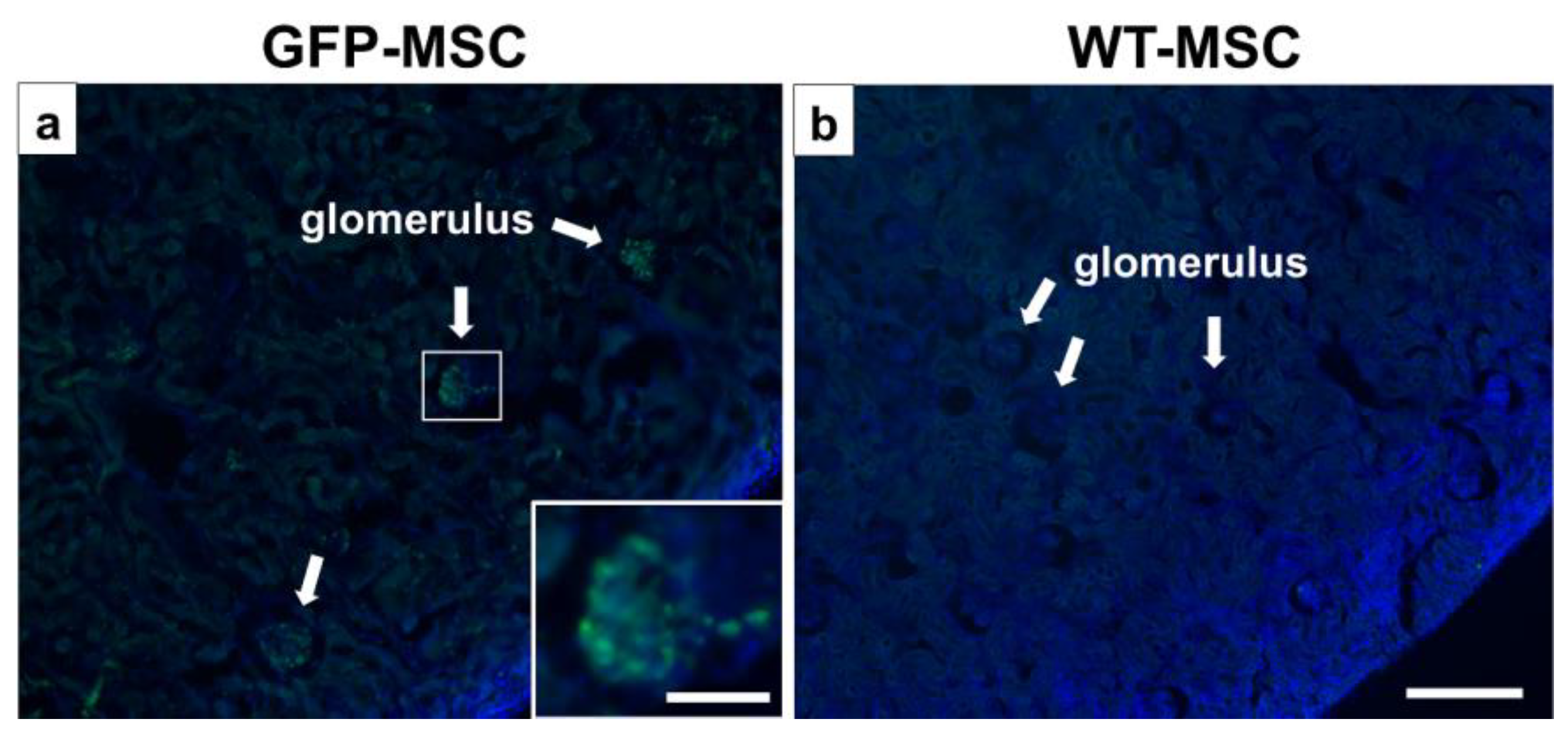
3.6. Detection of GFP-MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Jin, Q.; Ren, F.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Li, P.; Zhan, Y. Potential therapeutic effects of natural compounds targeting autophagy to alleviate podocyte injury in glomerular diseases. Biomed. Pharmacother. 2022, 155, 113670. [Google Scholar] [CrossRef] [PubMed]
- Vöing, K.; Michgehl, U.; Mertens, N.D.; Picciotto, C.; Maywald, M.L.; Goretzko, J.; Waimann, S.; Gilhaus, K.; Rogg, M.; Schell, C.; et al. Disruption of the Rab7-Dependent Final Common Pathway of Endosomal and Autophagic Processing Results in a Severe Podocytopathy. J. Am. Soc. Nephrol. 2023, 34, 1191–1206. [Google Scholar] [CrossRef]
- Kopp, J.B.; Anders, H.-J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef]
- Eddy, A.A.; Symons, J.M. Nephrotic syndrome in childhood. Lancet 2003, 362, 629–639. [Google Scholar] [CrossRef]
- Purohit, S.; Piani, F.; Ordoñez, F.A.; de Lucas-Collantes, C.; Bauer, C.; Cara-Fuentes, G. Molecular Mechanisms of Proteinuria in Minimal Change Disease. Front. Med. 2021, 8, 761600. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ishibe, S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am. J. Physiol. Ren. Physiol. 2015, 309, F398–F405. [Google Scholar] [CrossRef] [PubMed]
- Vega-Warner, V.; Ransom, R.F.; Vincent, A.M.; Brosius, F.C.; Smoyer, W.E. Induction of antioxidant enzymes in murine podocytes precedes injury by puromycin aminonucleoside. Kidney Int. 2004, 66, 1881–1889. [Google Scholar] [CrossRef]
- Kamireddy, R.; Kavuri, S.; Devi, S.; Vemula, H.; Chandana, D.; Harinarayanan, S.; James, R.; Rao, A. Oxidative stress in pediatric nephrotic syndrome. Clin. Chim. Acta 2002, 325, 147–150. [Google Scholar] [CrossRef]
- Bruno, V.; Mühlig, A.K.; Oh, J.; Licht, C. New insights into the immune functions of podocytes: The role of complement. Mol. Cell. Pediatr. 2023, 10, 3. [Google Scholar] [CrossRef]
- Marshall, C.B.; Pippin, J.W.; Krofft, R.D.; Shankland, S.J. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 2006, 70, 1962–1973. [Google Scholar] [CrossRef]
- Simões, E.S.A.C.; Oliveira, E.A.; Cheung, W.W.; Mak, R.H. Redox Signaling in Chronic Kidney Disease-Associated Cachexia. Antioxid 2023, 12, 945. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Rezaee, R.; Sahebkar, A. Oxidative stress induces renal failure: A review of possible molecular pathways. J. Cell. Biochem. 2018, 119, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Beaman, M.; Birtwistle, R.; Howie, A.J.; Michael, J.; Adu, D. The role of superoxide anion and hydrogen peroxide in glomerular injury induced by puromycin aminonucleoside in rats. Clin. Sci. 1987, 73, 329–332. [Google Scholar] [CrossRef]
- Ornellas, F.M.; Ramalho, R.J.; Fanelli, C.; Garnica, M.R.; Malheiros, D.; Martini, S.V.; Morales, M.M.; Noronha, I.L. Mesenchymal Stromal Cells Induce Podocyte Protection in the Puromycin Injury Model. Sci. Rep. 2019, 9, 19604. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Sharma, M.; Yew, K.H.; Sharma, R.; Duncan, R.S.; Saleem, M.A.; McCarthy, E.T.; Kats, A.; Cudmore, P.A.; Alon, U.S.; et al. LPS and PAN-induced podocyte injury in an in vitro model of minimal change disease: Changes in TLR profile. J. Cell Commun. Signal. 2013, 7, 49–60. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsumura, H.; Yamazaki, S.; Shirasu, A.; Nakakura, H.; Ogihara, T.; Ashida, A. Efficacy of a mitochondrion-targeting agent for reducing the level of urinary protein in rats with puromycin aminonucleoside-induced minimal-change nephrotic syndrome. PLoS ONE 2020, 15, e0227414. [Google Scholar] [CrossRef]
- Terada, K.; Sasaki, M.; Nagahama, H.; Kataoka-Sasaki, Y.; Oka, S.; Ukai, R.; Yokoyama, T.; Iizuka, Y.; Sakai, T.; Fukumura, S.; et al. Therapeutic efficacy of intravenous infusion of mesenchymal stem cells in rat perinatal brain injury. Pediatr. Res. 2023, 94, 1921–1928. [Google Scholar] [CrossRef]
- Kim, S.; Honmou, O.; Kato, K.; Nonaka, T.; Houkin, K.; Hamada, H.; Kocsis, J. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006, 1123, 27–33. [Google Scholar] [CrossRef]
- Takemura, M.; Sasaki, M.; Kataoka-Sasaki, Y.; Kiyose, R.; Nagahama, H.; Oka, S.; Ukai, R.; Yokoyama, T.; Kocsis, J.D.; Ueba, T.; et al. Repeated intravenous infusion of mesenchymal stem cells for enhanced functional recovery in a rat model of chronic cerebral ischemia. J. Neurosurg. 2021, 137, 1–10. [Google Scholar] [CrossRef]
- Frenk, S.; Antonowicz, I.; Craig, J.M.; Metcoff, J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc. Soc. Exp. Biol. Med. 1955, 89, 424–427. [Google Scholar] [CrossRef]
- Hosoyamada, M.; Yan, K.; Nishibori, Y.; Takiue, Y.; Kudo, A.; Kawakami, H.; Shibasaki, T.; Endou, H. Nephrin and podocin expression around the onset of puromycin aminonucleoside nephrosis. J. Pharmacol. Sci. 2005, 97, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, X.; Cai, X.; Wang, K.; Chen, Y.; Deng, Y. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS ONE 2014, 9, e85690. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Shimomura, Y.; Nishida, O.; Maeda, M.; Kato, Y.; Nakamura, T.; Kuriyama, N.; Komura, H. Effects of recombinant human soluble thrombomodulin on neutrophil extracellular traps in the kidney of a mouse model of endotoxin shock. Fujita Med. J. 2023, 9, 225–230. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kwon, T.; Kim, D.J.; Huh, W.; Kim, Y.G.; Oh, H.Y.; Kawachi, H. Ultrastructural study on nephrin expression in experimental puromycin aminonucleoside nephrosis. Nephrol. Dial. Transpl. 2004, 19, 2981–2986. [Google Scholar] [CrossRef]
- Tabata, H.; Sasaki, M.; Kataoka-Sasaki, Y.; Shinkai, N.; Ichihara, K.; Masumori, N.; Kocsis, J.D.; Honmou, O. Possible role of intravenous administration of mesenchymal stem cells to alleviate interstitial cystitis/bladder pain syndrome in a Toll-like receptor-7 agonist-induced experimental animal model in rat. BMC Urol. 2021, 21, 156. [Google Scholar] [CrossRef]
- Sato, Y.; Wharram, B.L.; Lee, S.K.; Wickman, L.; Goyal, M.; Venkatareddy, M.; Chang, J.W.; Wiggins, J.E.; Lienczewski, C.; Kretzler, M.; et al. Urine podocyte mRNAs mark progression of renal disease. J. Am. Soc. Nephrol. 2009, 20, 1041–1052. [Google Scholar] [CrossRef]
- Lima-Posada, I.; Fontana, F.; Pérez-Villalva, R.; Berman-Parks, N.; Bobadilla, N.A. Pirfenidone prevents acute kidney injury in the rat. BMC Nephrol. 2019, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Basgen, J.M.; Wong, J.S.; Ray, J.; Nicholas, S.B.; Campbell, K.N. Podocyte Foot Process Effacement Precedes Albuminuria and Glomerular Hypertrophy in CD2-Associated Protein Deficient Mice. Front. Med. 2021, 8, 745319. [Google Scholar] [CrossRef]
- Lannigan, R.; Kark, R.; Pollak, V.E. The effect of a single intravenous injection of aminonucleoside of puromycin on the rat kidney: A light- and electron-microscope study. J. Pathol. Bacteriol. 1962, 83, 357–362. [Google Scholar] [CrossRef]
- Gwinner, W.; Landmesser, U.; Brandes, R.P.; Kubat, B.; Plasger, J.; Eberhard, O.; Koch, K.M.; Olbricht, C.J. Reactive oxygen species and antioxidant defense in puromycin aminonucleoside glomerulopathy. J. Am. Soc. Nephrol. 1997, 8, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Guh, J.Y.; Lai, Y.H. Alterations of glomerular and extracellular glutathione peroxidase levels in patients and rats with focal segmental glomerulosclerosis. J. Lab. Clin. Med. 2001, 137, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxid 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.R.; Bonventre, J.V.; Karnovsky, M.J. A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int. 1986, 29, 478–483. [Google Scholar] [CrossRef]
- Yamanaka, M.; Tamura, Y.; Kuribayashi-Okuma, E.; Uchida, S.; Shibata, S. Nicorandil protects podocytes via modulation of antioxidative capacity in acute puromycin aminonucleoside-induced nephrosis in rats. Am. J. Physiol. Ren. Physiol. 2023, 324, F168–F178. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iizuka, Y.; Sasaki, M.; Terada, K.; Sakai, T.; Nagaoka, Y.; Fukumura, S.; Kocsis, J.D.; Tsugawa, T.; Honmou, O. Therapeutic Efficacy of Mesenchymal Stem Cells in Modulating Oxidative Stress in Puromycin-Induced Nephropathy. Pathophysiology 2025, 32, 19. https://doi.org/10.3390/pathophysiology32020019
Iizuka Y, Sasaki M, Terada K, Sakai T, Nagaoka Y, Fukumura S, Kocsis JD, Tsugawa T, Honmou O. Therapeutic Efficacy of Mesenchymal Stem Cells in Modulating Oxidative Stress in Puromycin-Induced Nephropathy. Pathophysiology. 2025; 32(2):19. https://doi.org/10.3390/pathophysiology32020019
Chicago/Turabian StyleIizuka, Yusuke, Masanori Sasaki, Kojiro Terada, Takuro Sakai, Yoshinobu Nagaoka, Shinobu Fukumura, Jeffery D. Kocsis, Takeshi Tsugawa, and Osamu Honmou. 2025. "Therapeutic Efficacy of Mesenchymal Stem Cells in Modulating Oxidative Stress in Puromycin-Induced Nephropathy" Pathophysiology 32, no. 2: 19. https://doi.org/10.3390/pathophysiology32020019
APA StyleIizuka, Y., Sasaki, M., Terada, K., Sakai, T., Nagaoka, Y., Fukumura, S., Kocsis, J. D., Tsugawa, T., & Honmou, O. (2025). Therapeutic Efficacy of Mesenchymal Stem Cells in Modulating Oxidative Stress in Puromycin-Induced Nephropathy. Pathophysiology, 32(2), 19. https://doi.org/10.3390/pathophysiology32020019





