The Pathophysiological Mechanisms and Pattern of Dyslipidemia Associated with Iodine Deficiency and Subclinical Hypothyroidism in Pregnant Normotensive and Preeclamptic Central African Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. The Relationship Between Iodine Nutrition Status, TSH, Nitric Oxide, and Serum Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| NO | Nitric Oxide |
| oxLDL | Oxidized low-density lipoprotein |
| ONOO- | Peroxynitrite |
| PlGF | Placental Growth Factor |
| sFlt-1 | Soluble fms-like tyrosine kinase-1 |
| sdLDL-C | Small-dense low-density lipoprotein cholesterol |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| Trigli | Triglycerides |
| TSH | Thyroid-stimulating hormone |
| UIC | Urinary Iodine Concentration |
| VEGF | Vascular Endothelial Growth Factor |
| VLDL-C | Very low-density lipoprotein cholesterol |
References
- Bohn, M.K.; Adeli, K. Physiological and metabolic adaptations in pregnancy: Importance of trimester-specific reference intervals to investigate maternal health and complications. Crit. Rev. Clin. Lab. Sci. 2022, 59, 76–92. [Google Scholar]
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. Ann. Nutr. Metab. 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Bashir, M.; Navti, O.B.; Ahmed, B.; Konje, J.C. Hyperlipidaemia and severe hypertriglyceridaemia in pregnancy. Obstet. Gynaecol. 2023, 25, 196–209. [Google Scholar]
- Poornima, I.G.; Indaram, M.; Ross, J.D.; Agarwala, A.; Wild, R.A. Hyperlipidemia and risk for preclampsia. J. Clin. Lipidol. 2022, 16, 253–260. [Google Scholar] [CrossRef]
- Lewek, J.; Bielecka-Dąbrowa, A.; Toth, P.P.; Banach, M. Dyslipidaemia management in pregnant patients: A 2024 update. Eur. Heart J. Open 2024, 4, oeae032. [Google Scholar]
- Vekic, J.; Zeljkovic, A.; Cicero, A.F.G.; Janez, A.; Stoian, A.P.; Sonmez, A. Atherosclerosis Development and Progression: The Role of Atherogenic Small, Dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal Fatty Acid Metabolism in Pregnancy and Its Consequences in the Feto-Placental Development. Front. Physiol. 2021, 12, 787848. [Google Scholar] [CrossRef] [PubMed]
- Formisano, E.; Proietti, E.; Perrone, G.; Demarco, V.; Galoppi, P.; Stefanutti, C. Characteristics, Physiopathology and Management of Dyslipidemias in Pregnancy: A Narrative Review. Nutrients 2024, 16, 2927. [Google Scholar] [CrossRef]
- Baumfeld, Y.; Novack, L.; Wiznitzer, A.; Sheiner, E.; Henkin, Y.; Sherf, M. Pre-Conception Dyslipidemia Is Associated with Development of Preeclampsia and Gestational Diabetes Mellitus. PLoS ONE 2015, 10, e0139164. [Google Scholar]
- Retnakaran, R.; Shah, B.R. The adverse cardiovascular risk factor profile of women with pre-eclampsia develops over time in the years before pregnancy. BJOG 2022, 129, 1512–1520. [Google Scholar]
- Graves, M.; Howse, K.; Pudwell, J.; Smith, G.N. Pregnancy-related cardiovascular risk indicators: Primary care approach to postpartum management and prevention of future disease. Can. Fam. Physician 2019, 65, 883–889. [Google Scholar]
- Tabacu, C.; Manolea, M.M.; Novac, L.; Dijmarescu, A.L.; Boldeanu, M.V. Maternal Lipid Profile as a Risk Factor for Gestational Diabetes Mellitus in Obese Women. Curr. Health Sci. J. 2021, 47, 209–214. [Google Scholar]
- Sharami, H.S.; Ranjbar, A.Z.; Alizadeh, F.; Kazemnejad, E. The relationship of hyperlipidemia with maternal and neonatal outcomes in pregnancy: A cross-sectional study. Int. J. Reprod. Biomed. 2019, 17, 739–748. [Google Scholar] [CrossRef]
- Lee, K.W.; Shin, D.; Song, W.O. Low Urinary Iodine Concentrations Associated with Dyslipidemia in US Adults. Nutrients 2016, 8, 171. [Google Scholar] [CrossRef]
- Wang, X.; Xian, T.; Zhang, L.; Jia, X.; Man, F.; Liu, L. Associations between urinary iodine concentration, lipid profile and other cardiometabolic risk factors in adolescents: A cross-sectional, population-based analysis. Br. J. Nutr. 2019, 121, 1039–1048. [Google Scholar] [CrossRef]
- Herter-Aeberli, I.; Cherkaoui, M.; El Ansari, N.; Rohner, R.; Stinca, S.; Chabaa, L. Iodine Supplementation Decreases Hypercholesterolemia in Iodine-Deficient, Overweight Women: A Randomized Controlled Trial. J. Nutr. 2015, 145, 2067–2075. [Google Scholar] [CrossRef]
- Shin, D.; Shim, S.R.; Wu, Y.; Hong, G.; Jeon, H.; Kim, C.-G. How Do Brown Seaweeds Work on Biomarkers of Dyslipidemia? A Systematic Review with Meta-Analysis and Meta-Regression. Mar. Drugs 2023, 21, 220. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Z.; Li, Y.; Teng, D.; Shi, X.; Ba, J. U-Shaped Associations Between Urinary Iodine Concentration and the Prevalence of Metabolic Disorders: A Cross-Sectional Study. Thyroid 2020, 30, 1053–1065. [Google Scholar] [CrossRef]
- Wang, D.; Wan, S.; Liu, P.; Meng, F.; Ren, B.; Qu, M. Associations between water iodine concentration and the prevalence of dyslipidemia in Chinese adults: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 208, 111682. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Iodine nutrition in pregnancy and lactation. Endocrinol. Metab. Clin. N. Am. 2011, 40, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Delshad, H. Iodine nutrition in pregnancy. Ann. Thyroid. 2018, 3, 20. [Google Scholar] [CrossRef]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global iodine nutrition: Where do we stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef]
- Sharami, S.H.; Tangestani, A.; Faraji, R.; Zahiri, Z.; Amiri, A. Role of dyslipidemia in preeclamptic overweight pregnant women. Iran. J. Reprod. Med. 2012, 10, 105–112. [Google Scholar]
- Hosier, H.; Lipkind, H.S.; Rasheed, H.; DeWan, A.T.; Rogne, T. Dyslipidemia and Risk of Preeclampsia: A Multiancestry Mendelian Randomization Study. Hypertension 2023, 80, 1067–1076. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Nirupama, R.; Divyashree, S.; Janhavi, P.; Muthukumar, S.P.; Ravindra, P.V. Preeclampsia: Pathophysiology and management. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101975. [Google Scholar] [CrossRef]
- Abbas, R.A.; Ghulmiyyah, L.; Hobeika, E.; Usta, I.M.; Mirza, F.; Nassar, A.H. Preeclampsia: A Review of Early Predictors. Matern. Fetal Med. 2021, 3, 197–202. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Lu, J.; Li, Y. Global, regional, and national burdens of common micronutrient deficiencies from 1990 to 2019: A secondary trend analysis based on the Global Burden of Disease 2019 study. EClinicalMedicine 2022, 44, 101299. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2022, 27, 148–169. [Google Scholar] [PubMed]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef]
- Andersson, M.; de Benoist, B.; Delange, F.; Zupan, J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007, 10, 1606–1611. [Google Scholar] [PubMed]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-González, E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int. J. Mol. Sci. 2021, 22, 1228. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Aeberli, I.; Melse-Boonstra, A.; Grimci, L.; Bridson, J.; Chaouki, N. Iodine treatment in children with subclinical hypothyroidism due to chronic iodine deficiency decreases thyrotropin and C-peptide concentrations and improves the lipid profile. Thyroid 2009, 19, 1099–1104. [Google Scholar] [CrossRef]
- Asvold, B.O.; Bjøro, T.; Vatten, L.J. Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11-year follow-up of the HUNT study. Eur. J. Endocrinol. 2013, 169, 73–82. [Google Scholar] [CrossRef]
- Al-Odat, I.; Al-Fawaeir, S.; Al-Mahmoud, M.H. Study of the association between thyroid dysfunction and serum lipid abnormalities. Biomed. Rep. 2024, 21, 138. [Google Scholar] [CrossRef]
- WHO Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007.
- Pearce, E.N. Update in lipid alterations in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Ren, B.; He, Y.; Li, F.; Wang, L. Alteration of Lipid Profile Between Subclinical Hypothyroidism and Well-Matched Controls: A Meta-Analysis. Horm. Metab. Res. 2023, 55, 479–486. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef]
- Winkler, R. Iodine—A Potential Antioxidant and the Role of Iodine/Iodide in Health and Disease. Nat. Sci. 2015, 7, 548–557. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Wankasi, M.M.; Odumoson, N.C.; Alabrah, W.P.; Agoro, E.-Y.S. Lipid Profile and Some Parameters of Lipid Peroxidation in Pregnancy Trimesters. Asian Pac. J. Health Sci. 2024, 11, 12–16. [Google Scholar]
- Bassi, R.; Kaur, M.; Sharma, S. Study of Changes in Lipid Profile, Lipid Peroxidation and Superoxide Dismutase during Normal Pregnancy. Indian J. Fundam. Appl. Life Sci. 2011, 1, 249–254. [Google Scholar]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Li, S.; Cui, L.; Zhao, J.; Liao, L. Selenium and thyroid diseases. Front. Endocrinol. 2023, 14, 1133000. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Gluvic, Z.M.; Obradovic, M.M.; Sudar-Milovanovic, E.M.; Zafirovic, S.S.; Radak, D.J.; Essack, M.M. Regulation of nitric oxide production in hypothyroidism. Biomed. Pharmacother. 2020, 124, 109881. [Google Scholar] [CrossRef]
- Dardano, A.; Ghiadoni, L.; Plantinga, Y.; Caraccio, N.; Bemi, A.; Duranti, E. Recombinant human thyrotropin reduces endothelium-dependent vasodilation in patients monitored for differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 4175–4178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, H.; Bao, S.; Zhou, H.; Zhang, Y.; Yan, Y. Thyrotropin Regulates eNOS Expression in the Endothelium by PGRN Through Akt Pathway. Front. Endocrinol. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, C.B.; Gao, L.; Zhao, J.J. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction. Exp. Ther. Med. 2015, 9, 3–10. [Google Scholar] [CrossRef]
- Taddei, S.; Caraccio, N.; Virdis, A.; Dardano, A.; Versari, D.; Ghiadoni, L. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: Beneficial effect of levothyroxine therapy. J. Clin. Endocrinol. Metab. 2003, 88, 3731–3737. [Google Scholar]
- Cabral, M.D.; Teixeira, P.; Soares, D.; Leite, S.; Salles, E.; Waisman, M. Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. Clinics 2011, 66, 1321–1327. [Google Scholar]
- Razvi, S.; Jabbar, A.; Pingitore, A.; Danzi, S.; Biondi, B.; Klein, I. Thyroid Hormones and Cardiovascular Function and Diseases. J. Am. Coll. Cardiol. 2018, 71, 1781–1796. [Google Scholar] [PubMed]
- Tian, L.; Zhang, L.; Liu, J.; Guo, T.; Gao, C.; Ni, J. Effects of TSH on the function of human umbilical vein endothelial cells. J. Mol. Endocrinol. 2014, 52, 215–222. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; Linares Sánchez, M.; López Alvarez, F.; Suárez Nieto, C.; Mäkitie, A.A.; Olsen, K.D. Evaluation of Intima-Media Thickness and Arterial Stiffness as Early Ultrasound Biomarkers of Carotid Artery Atherosclerosis. Cardiol. Ther. 2022, 11, 231–247. [Google Scholar] [CrossRef]
- Darabian, S.; Hormuz, M.; Latif, M.A.; Pahlevan, S.; Budoff, M.J. The role of carotid intimal thickness testing and risk prediction in the development of coronary atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 306. [Google Scholar] [CrossRef]
- da Silva, W.A.; Pinheiro, A.M.; Lima, P.H.; Malbouisson, L.M.S. Renal and cardiovascular repercussions in preeclampsia and their impact on fluid management: A literature review. Braz. J. Anesthesiol. 2021, 71, 421–428. [Google Scholar] [CrossRef]
- Wikerholmen, M.B.; Rosendahl-Riise, H.; Børresen, K.; Haugsgjerd, T.R.; Gerdts, E.; Brantsæter, A.L. Low maternal iodine intake and subsequent risk of pharmacologically treated hypertension: A population-based prospective cohort study. Clin. Nutr. 2025, 45, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns1,2. Am. J. Clin. Nutr. 2016, 104, 918S–923S. [Google Scholar] [CrossRef]
- Manousou, S.; Eggertsen, R.; Hulthén, L.; Filipsson Nyström, H. A randomized, double-blind study of iodine supplementation during pregnancy in Sweden: Pilot evaluation of maternal iodine status and thyroid function. Eur. J. Nutr. 2021, 60, 3411–3422. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Lixian, Z. Stem Cell Therapy for Thyroid Diseases: Progress and Challenges. Curr. Ther. Res. 2022, 96, 100665. [Google Scholar] [CrossRef]
- Medenica, S.; Abazovic, D.; Ljubić, A.; Vukovic, J.; Begovic, A.; Cucinella, G. The Role of Cell and Gene Therapies in the Treatment of Infertility in Patients with Thyroid Autoimmunity. Int. J. Endocrinol. 2022, 2022, 4842316. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Shab-Bidar, S.; Kord-Varkaneh, H.; Khorshidi, M.; Djafarian, K. Effect of alpha-lipoic acid supplementation on lipid profile: A systematic review and meta-analysis of controlled clinical trials. Nutrition 2019, 59, 121–130. [Google Scholar] [CrossRef]
- Mahmoudinezhad, M.; Farhangi, M.A. Alpha-lipoic acid supplementation affects serum lipids in a dose and duration-dependent manner in different health status. Int. J. Vitam. Nutr. Res. 2023, 93, 352–361. [Google Scholar] [CrossRef]
- Laganà, A.S.; Monti, N.; Fedeli, V.; Gullo, G.; Bizzarri, M. Does Alpha-lipoic acid improve effects on polycystic ovary syndrome? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1241–1247. [Google Scholar]
- Lete, I.; Ainara, M.; Irene, L.; Eva, C.; Vesga, A. Update on the combination of myo-inositol/d-chiro-inositol for the treatment of polycystic ovary syndrome. Gynecol. Endocrinol. 2024, 40, 2301554. [Google Scholar] [CrossRef]
- Gullo, G.; Carlomagno, G.; Unfer, V.; D’Anna, R. Myo-inositol: From induction of ovulation to menopausal disorder management. Minerva Ginecol. 2015, 67, 485–486. [Google Scholar] [PubMed]
- Pasta, V.; Gullo, G.; Giuliani, A.; Harrath, A.H.; Alwasel, S.H.; Tartaglia, F. An association of boswellia, betaine and myo-inositol (Eumastós) in the treatment of mammographic breast density: A randomized, double-blind study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4419–4426. [Google Scholar] [PubMed]
- Coldebella, D.; Buzzaccarini, G.; Sleiman, Z.; Ferrari, J.; D’Alterio, M.; Della Corte, L. Inositols administration: Further insights on their biological role. Gynaecol. Obstet. Ital. J. Narrat. Rev. 2023, 35, 30–36. [Google Scholar] [CrossRef]
- Babakr, A.T.; Eldein, M.M.N. Assessment of lipid peroxidation and total antioxidant capacity in patients with breast cancer. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002284. [Google Scholar] [CrossRef]
- Chauhan, A.; Thacker, H.; Bhalerao-Gandhi, A.; Khanna, R.; Gopinath, P.M.; Coelho, K. Expert consensus on the role of nutraceuticals in women’s health: Menarche to menopause. Int. J. Clin. Obstet. Gynaecol. 2022, 6, 24–31. [Google Scholar] [CrossRef]
| Preeclamptic | Normotensive | ||
|---|---|---|---|
| Variable | Mean ± SD or Median (IQR) | Mean ± SD or Median (IQR) | p |
| UIC µg/L | 109.2 ± 31.1 | 161.8 ± 24.1 | <0.0001 |
| TSH mIU/L | 5.3 ± 2.6 | 4.0 ± 3.2 | <0.0001 |
| T3 ng/mL | 1.46 ± 0.40 | 1.15 ± 0.28 | <0.0001 |
| T4 µg/mL | 11.21 ± 2.56 | 9.70 ± 2.32 | <0.0001 |
| BMI Kg/m2 | 25.3 ± 6.2 | 22.2 ± 5.4 | <0.0001 |
| HDL-C mg/dL | 21.3 ± 12.7 | 52.6 ± 2.9 | <0.0001 |
| LDL-C mg/dL | 45.9 ± 11.0 | 56.5 ± 3.1 | <0.0001 |
| oxLDL-C UI/L | 189.0 (98.0, 222.0) | 87.5 (21.2, 189.0) | <0.0001 |
| Trigl mg/dL | 30.75 (20.00, 102.72) | 19.83(19.82, 19.83) | <0.0001 |
| NO µmol/L | 3.0 (2.0, 7.0) | 7.0 (1.0, 32.0) | <0.0001 |
| Preeclamptic Participants | Normotensive Participants | |||||
|---|---|---|---|---|---|---|
| TSH ≤ 3 mIU/L n = 60 | TSH > 3 mIU/L n = 180 | TSH ≤ 3 mIU/L n = 55 | TSH > 3 mIU/L n = 65 | |||
| Variable | Mean ± SD or Median (IQR) | Mean ± SD or Median (IQR) | p-Value | Mean ± SD or Median (IQR) | Mean ± SD or Median (IQR) | p-Value |
| HDL-C mg/dL | 20.5 (10.0, 30.9) | 20.0 (10.0, 28.6) | 0.763 | 50.3 ± 2.0 | 52.2 ± 3.7 | 0.313 |
| LDL-C mg/dL | 46.0 ± 11.3 | 45.8 ± 11.0 | 0.934 | 56.9 ± 2.1 | 56.2 ± 3.9 | 0.189 |
| oxLDL-C UI/L | 93.5 (19.9, 222.8) | 190.0 (110.3, 222.0) | <0.001 | 29.9 (9.0, 58.0) | 108.4 (46.9, 211.0) | <0.001 |
| Trigl mg/dL | 52.1 ± 11.3 | 52 ± 11.0 | 0.933 | 63.0 ± 2.0 | 63.0 ± 4.0 | 0.213 |
| NO µmol/L | 7.0 (4.0, 27.0) | 2.0 (1.0, 6.0) | <0.001 | 32.0 (20.3, 44.0) | 2.0 (1.0, 3.0) | <0.001 |
| Preeclamptic Participants | Normotensive Participants | |||||
|---|---|---|---|---|---|---|
| UIC > 150 µg/L n = 31 | UIC < 150 µg/L n = 209 | UIC > 150 µg/L n = 88 | UIC < 150 µg/L n = 32 | |||
| Variable | Mean ± SD or Median (IQR) | Mean ± SD or Median (IQR) | p-Value | Mean ± SD or Median (IQR) | Mean ± SD or Median (IQR) | p-Value |
| HDL-C mg/dL | 35.0 (28.0, 45.0) | 14.0 (10.0, 28.0) | <0.001 | 52.9 ± 1.9 | 51.6 ± 4.6 | 0.036 |
| LDL-C mg/dL | 42.0 ± 8.0 | 47.0 ± 11.0 | 0.027 | 56.9 ± 2.1 | 55.3 ± 4.9 | 0.036 |
| oxLDL-C UI/L | 191.2 (89.0, 234.0) | 189.0 (95.6, 222.0) | 0.436 | 87.5 (21.2, 189.0) | 87.5 (18.7, 186.3) | 0.808 |
| Trigl mg/dL | 48.0 ± 10.0 | 53.0 ± 11.0 | 0.027 | 63.2 ± 2.1 | 61.8 ± 5.0 | 0.360 |
| NO µmol/L | 3.0 (1.0, 5.0) | 3.0 (2.0, 7.5) | 0.472 | 3.5 (1.0, 32) | 16 (2.0, 39) | 0.132 |
| Pearson | UIC | HDL | LDL | Trigl | TSH | NO | oxLDL-C | |
|---|---|---|---|---|---|---|---|---|
| UIC | correlation | 0.729 ** | 0.239 ** | 0.239 ** | −0.133 * | 0.207 ** | −0.215 ** | |
| p-value | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | <0.001 | ||
| HDL-C | correlation | 0.729 ** | 0.214 ** | 0.214 ** | −0.182 ** | 0.269 ** | −0.246 ** | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| LDL-C | correlation | 0.239 ** | 0.214 ** | 1.000 ** | −0.144 ** | 0.143 ** | −0.165 ** | |
| p-value | <0.001 | <0.001 | <0.001 | 0.006 | 0.007 | 0.002 | ||
| Trigl | correlation | 0.239 ** | 0.214 ** | 1.000 ** | −0.144 ** | 0.143 ** | −0.165 ** | |
| p-value | <0.001 | <0.001 | <0.001 | 0.006 | 0.007 | 0.002 | ||
| TSH | correlation | −0.133 * | −0.182 ** | −0.144 ** | −0.144 ** | −0.674 ** | 0.316 ** | |
| p-value | 0.011 | <0.001 | 0.006 | 0.006 | <0.001 | <0.001 | ||
| NO | correlation | 0.207 ** | 0.269 ** | 0.143 ** | 0.143 ** | −0.674 ** | −0.445 ** | |
| p-value | <.0.001 | <0.001 | 0.007 | 0.007 | <0.001 | <0.001 |
| Variable | Univariable Beta Coefficient (95% CI) | p Value | Multivariable Beta Coefficient (95% CI) | p Value |
|---|---|---|---|---|
| Preeclampsia | 1.131 (0.973–1.309) | 0.102 | 194.66 (55.15–687.09) | <0.001 |
| TSH > 3 UI/L | 1.347 (1.191–1.524) | <0.001 | 3.98 (1.89–8.40) | <0.001 |
| UIC > 150 µg/L | 0.644 (0.566–0.733) | <0.001 | 0.067 (0.032–0.140) | <0.001 |
| BMI > 25 Kg/m2 | 1.403 (1.212–1.610) | <0.001 | 1.24 (0.58–2.63) | 0.580 |
| Variables in the Rotated Matrix | Components ‡ | ||
|---|---|---|---|
| 1 | 2 | ||
| UIC | 0.686 | ||
| Triglycerides | −0.734 | ||
| HDL | 0.832 | ||
| LDL | −0.628 | ||
| Oxidized LDL | 0.688 | ||
| Nitric Oxide | −0.863 | ||
| TSH | 0.766 | ||
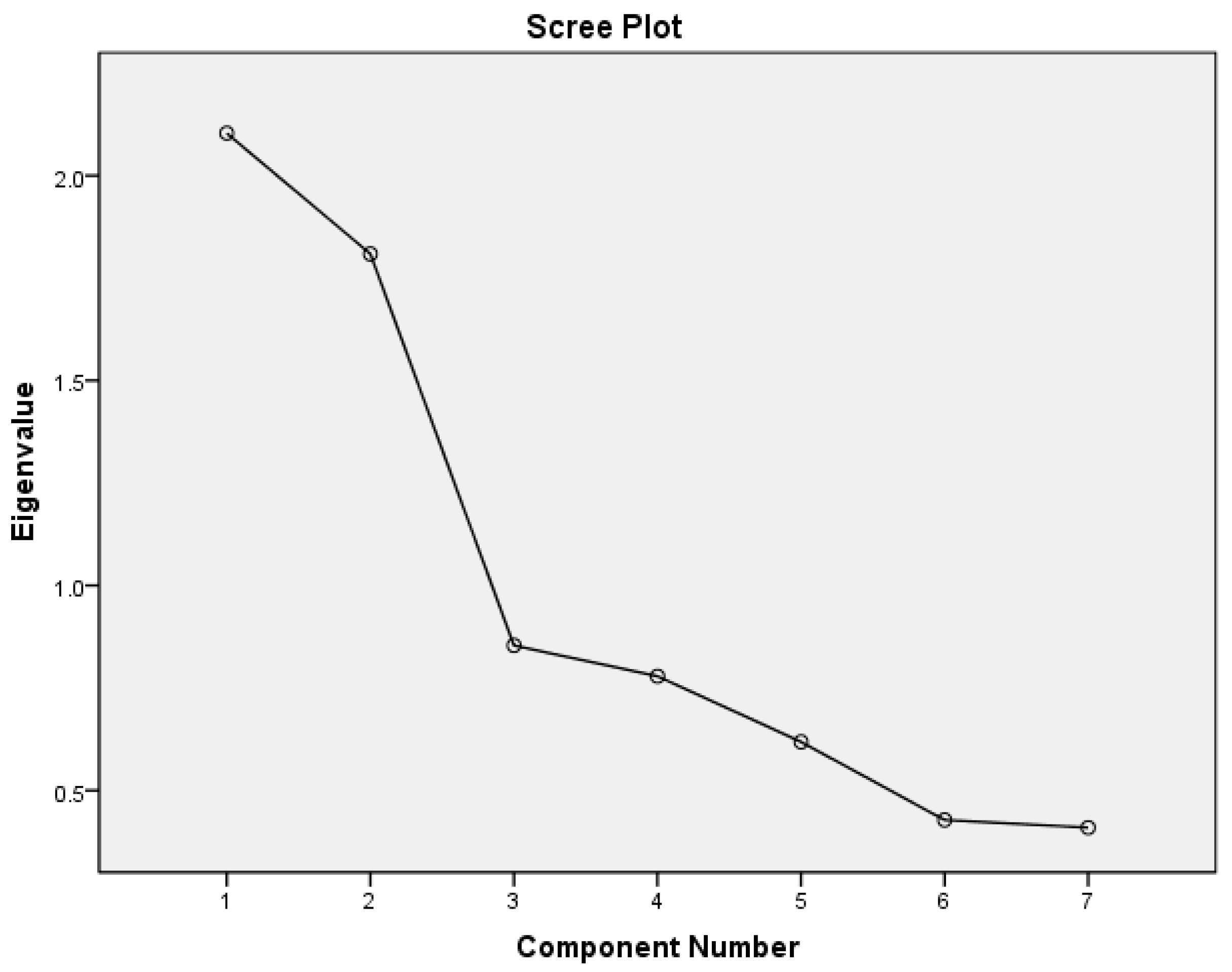
| Component | Eigenvalues | % variance | Total variance |
| 1 | 2.103 | 30.05 | 30.04 |
| 2 | 1.809 | 25.84 | 55.89 |
| All five components with Eigenvalues < 1 | 2.864 | 44.11 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Businge, C.B.; Longo-Mbenza, B. The Pathophysiological Mechanisms and Pattern of Dyslipidemia Associated with Iodine Deficiency and Subclinical Hypothyroidism in Pregnant Normotensive and Preeclamptic Central African Women. Pathophysiology 2025, 32, 18. https://doi.org/10.3390/pathophysiology32020018
Businge CB, Longo-Mbenza B. The Pathophysiological Mechanisms and Pattern of Dyslipidemia Associated with Iodine Deficiency and Subclinical Hypothyroidism in Pregnant Normotensive and Preeclamptic Central African Women. Pathophysiology. 2025; 32(2):18. https://doi.org/10.3390/pathophysiology32020018
Chicago/Turabian StyleBusinge, Charles Bitamazire, and Benjamin Longo-Mbenza. 2025. "The Pathophysiological Mechanisms and Pattern of Dyslipidemia Associated with Iodine Deficiency and Subclinical Hypothyroidism in Pregnant Normotensive and Preeclamptic Central African Women" Pathophysiology 32, no. 2: 18. https://doi.org/10.3390/pathophysiology32020018
APA StyleBusinge, C. B., & Longo-Mbenza, B. (2025). The Pathophysiological Mechanisms and Pattern of Dyslipidemia Associated with Iodine Deficiency and Subclinical Hypothyroidism in Pregnant Normotensive and Preeclamptic Central African Women. Pathophysiology, 32(2), 18. https://doi.org/10.3390/pathophysiology32020018






