Histologic Analysis of ‘Distraction Vaginogenesis’ in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
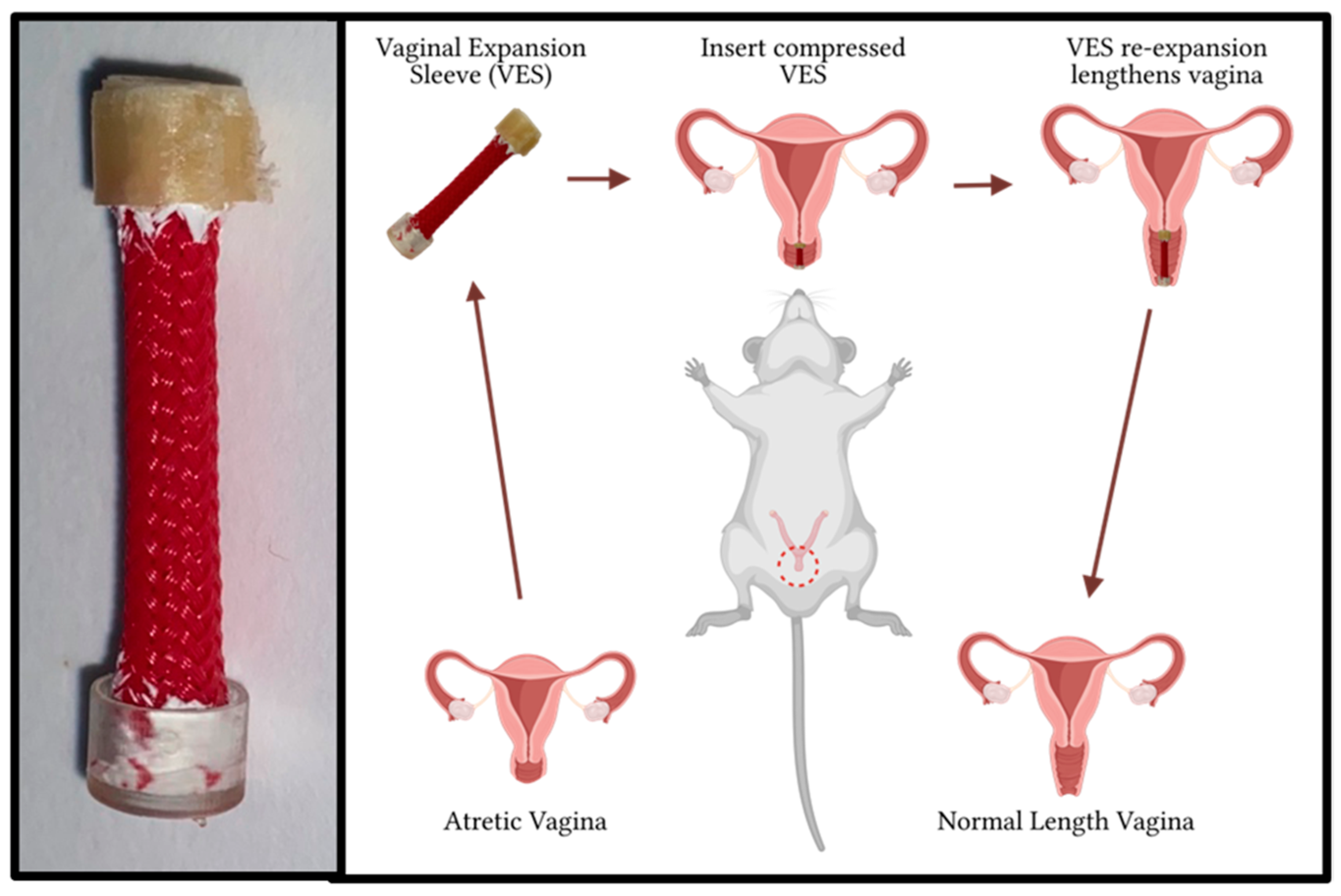
2.1. VES Design
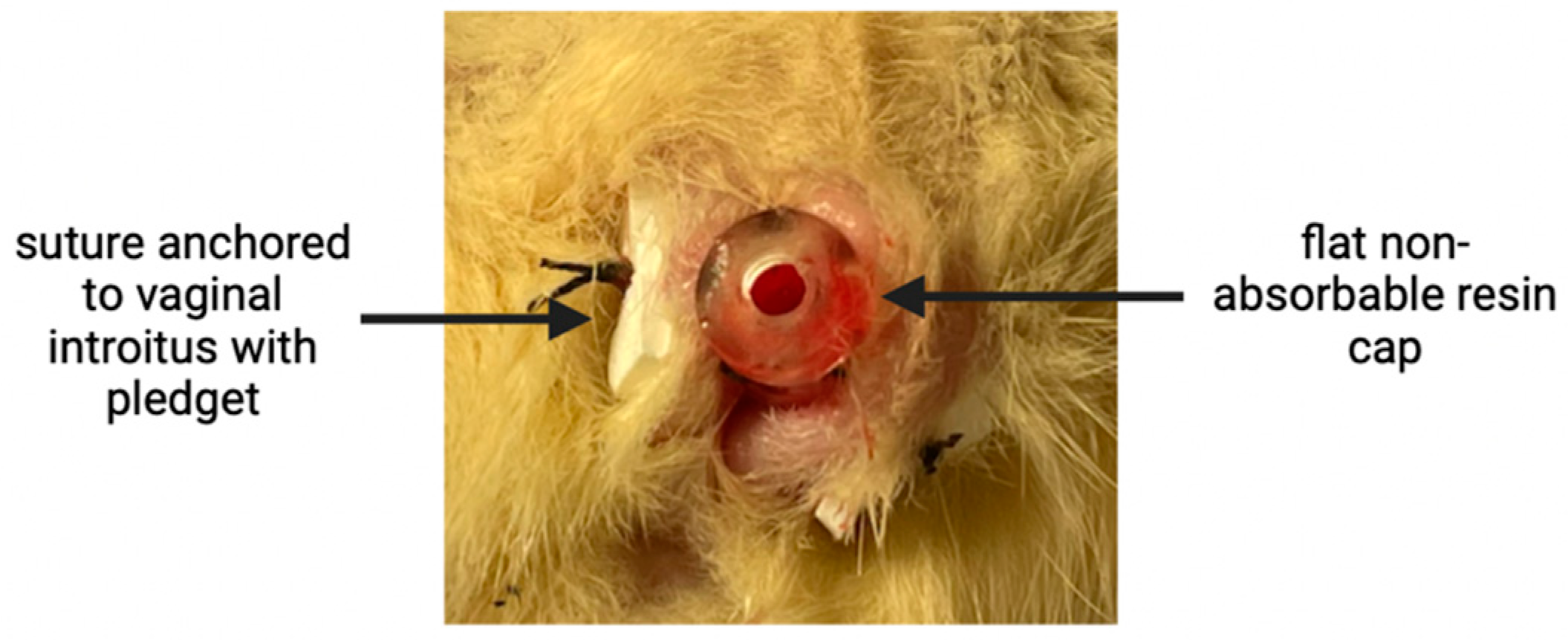
2.2. Perioperative and Vaginal Lengthening Periods
2.3. Histological Analysis
3. Results
3.1. Vaginal Lengthening with VES
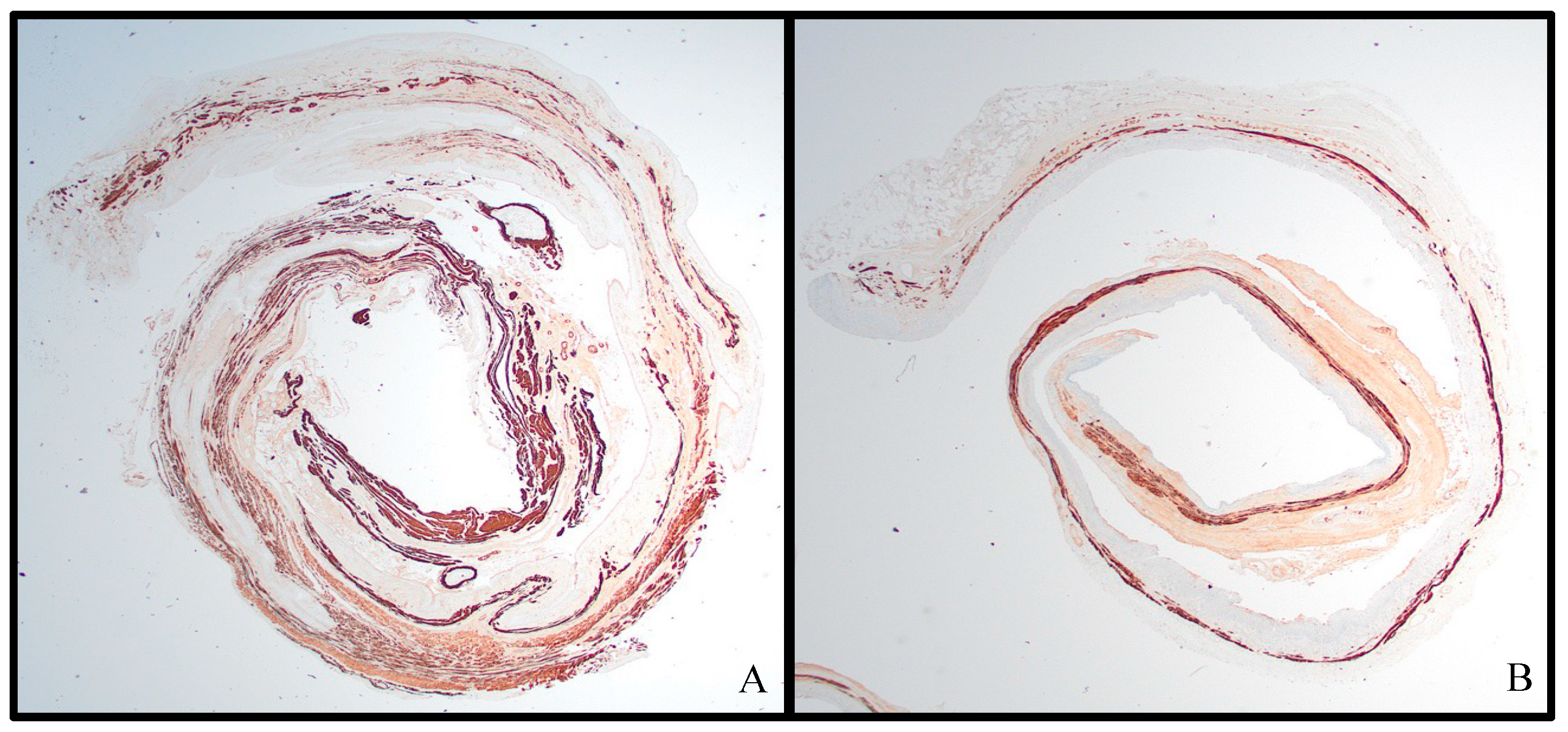
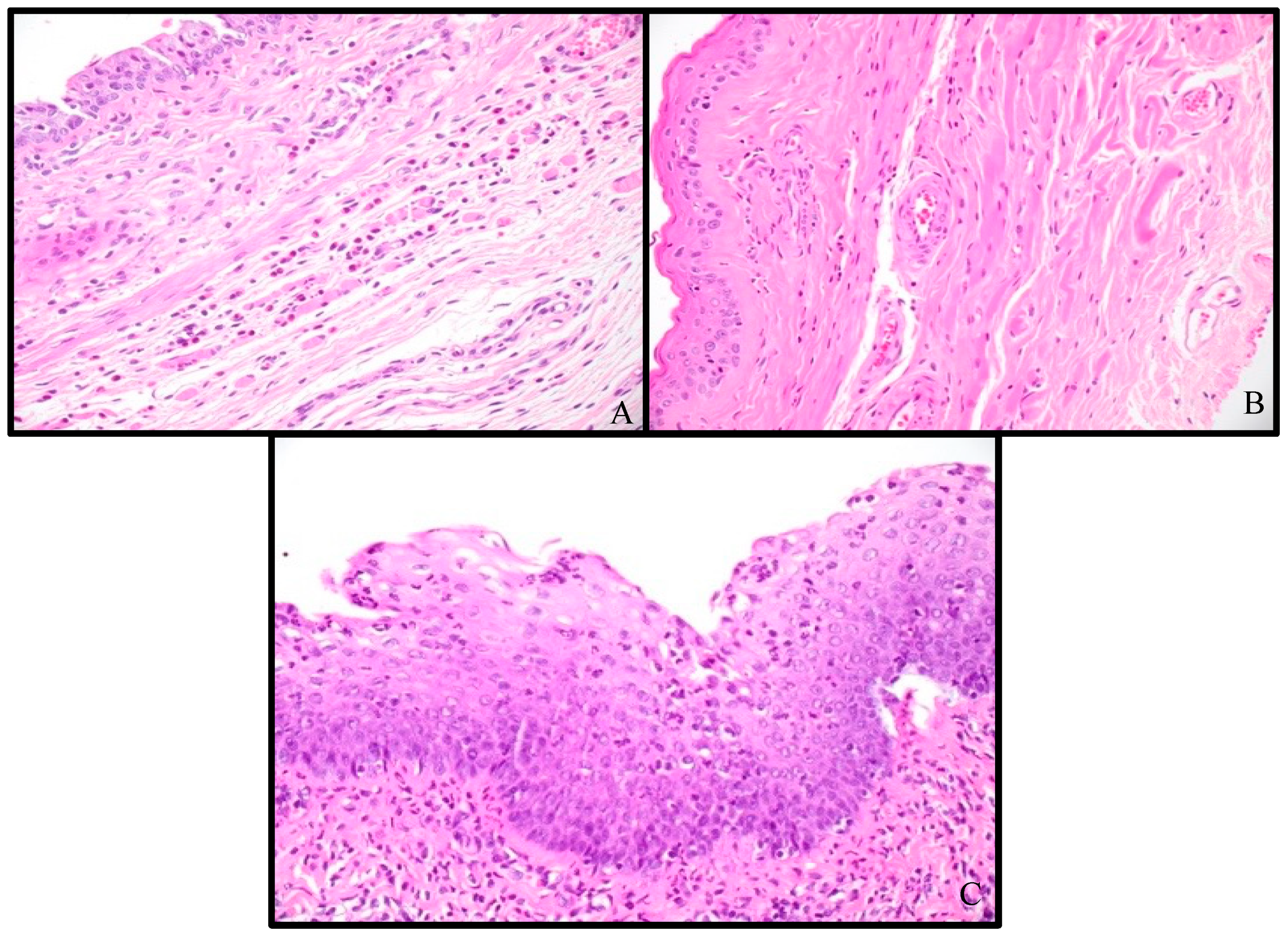
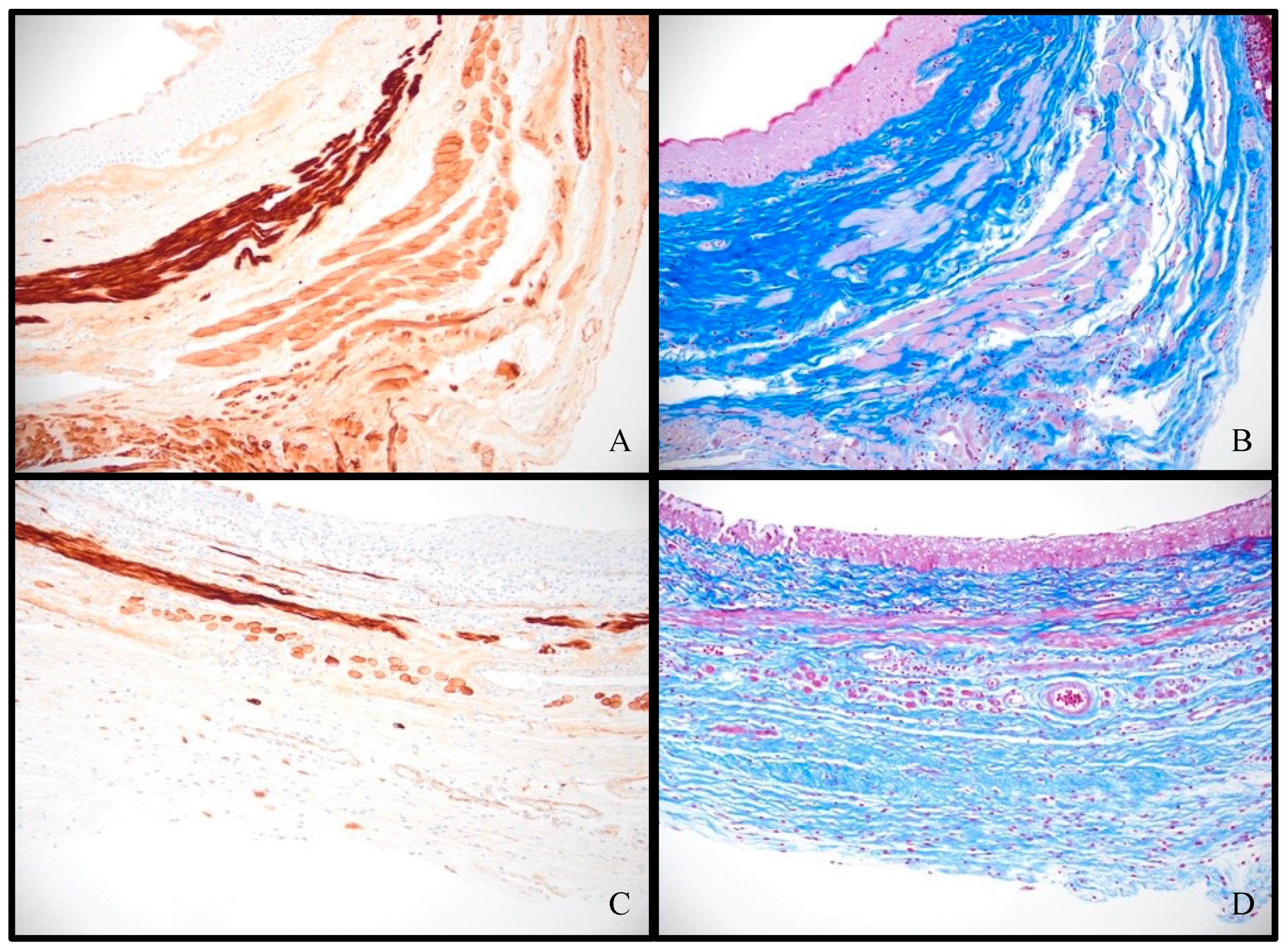
3.2. Histologic Analysis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakhal, R.S.; Creighton, S.M. Management of vaginal agenesis. J. Pediatr. Adolesc. Gynecol. 2012, 25, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Oelschlager, A.M.; Debiec, K.; Appelbaum, H. Primary vaginal dilation for vaginal agenesis: Strategies to anticipate challenges and optimize outcomes. Curr. Opin. Obstet. Gynecol. 2016, 28, 345–349. [Google Scholar] [CrossRef]
- Committee on Adolescent Health Care. ACOG Committee Opinion No. 728 Summary: Müllerian Agenesis: Diagnosis, Management, and Treatment. Obstet. Gynecol. 2018, 131, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Acién, P.; Acién, M. Malformations of the female genital tract and embryological bases. Curr. Women’s Health Rev. 2007, 3, 248–288. [Google Scholar] [CrossRef]
- Edmonds, D.K.; Rose, G.L.; Lipton, M.G.; Quek, J. Mayer-Rokitansky-Küster-Hauser syndrome: A review of 245 consecutive cases managed by a multidisciplinary approach with vaginal dilators. Fertil. Steril. 2012, 97, 686–690. [Google Scholar] [CrossRef]
- Herlin, M.K.; Petersen, M.B.; Brännström, M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: A comprehensive update. Orphanet J. Rare Dis. 2020, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Fulare, S.; Deshmukh, S.; Gupta, J. Androgen Insensitivity Syndrome: A rare genetic disorder. Int. J. Surg. Case Rep. 2020, 71, 371–373. [Google Scholar] [CrossRef]
- Hughes, I.A.; Davies, J.D.; Bunch, T.I.; Pasterski, V.; Mastroyannopoulou, K.; MacDougall, J. Androgen insensitivity syndrome. Lancet 2012, 380, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chen, N.; Song, S.; Zhang, Y.; Ma, C.; Ma, Y.; Zhu, L. Sexual function and quality of life after the creation of a neovagina in women with Mayer-Rokitansky-Küster-Hauser syndrome: Comparison of vaginal dilation and surgical procedures. Fertil. Steril. 2020, 113, 1024–1031. [Google Scholar] [CrossRef]
- Callens, N.; De Cuypere, G.; De Sutter, P.; Monstrey, S.; Weyers, S.; Hoebeke, P.; Cools, M. An update on surgical and non-surgical treatments for vaginal hypoplasia. Hum. Reprod. Update 2014, 20, 775–801. [Google Scholar] [CrossRef]
- Ozkan, O.; Erman Akar, M.; Ozkan, O.; Doğan, N.U. Reconstruction of vaginal agenesis. Ann. Plast. Surg. 2011, 66, 673–678. [Google Scholar] [CrossRef]
- Abbe, R. New method of creating a vagina in a case of congenital absence. Med. Rec. 1898, 54, 836. [Google Scholar]
- McIndoe, A. The treatment of congenital absence and obliterative condition of the vagina. Br. J. Plast. Surg. 1950, 2, 254–267. [Google Scholar]
- Davydov, S.N. Colpopoiesis from the peritoneum of the uterorectal space. Obstet. Gynecol. 1969, 12, 255–257. [Google Scholar]
- Davydov, S.N.; Zhvitiashvili, O.D. Formation of vagina from peritoneum of Douglas pouch. Acta Chir. Plast. 1974, 16, 35–41. [Google Scholar] [CrossRef]
- Vecchietti, G. Neovagina nella sindrome di Rokitansky-Kuster-Hauser. Attual. Ostet. Ginecol. 1965, 11, 131–147. [Google Scholar] [PubMed]
- Vecchietti, G. The neovagina in the Robitansky-Kuster-Hauser syndrome. Rev. Medicale Suisse Rom. 1979, 99, 593–601. [Google Scholar]
- Bach, F.; Glanville, J.M.; Balen, A.H. An observational study of women with müllerian agenesis and their need for vaginal dilator therapy. Fertil. Steril. 2011, 96, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Frank, R. The formation of an artificial vagina without operation. Am. J. Obstet. Gynecol. 1938, 35, 1053–1055. [Google Scholar] [CrossRef]
- Ismail-Pratt, I.S.; Bikoo, M.; Liao, L.M.; Conway, G.S.; Creighton, S.M. Normalization of the vagina by dilator treatment alone in complete androgen insensitivity syndrome and Mayer-Rokitansky-Kuster-Hauser syndrome. Hum. Reprod. 2007, 22, 2020–2024. [Google Scholar] [CrossRef]
- Passos, I.D.M.P.E.; Britto, R.L. Diagnosis and treatment of müllerian malformations. Taiwan. J. Obstet. Gynecol. 2020, 59, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gargollo, P.C.; Cannon, G.M., Jr.; Diamond, D.A.; Thomas, P.; Burke, V.; Laufer, M.R. Should progressive perineal dilation be considered first line therapy for vaginal agenesis? J. Urol. 2009, 182, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.P.; Haber, M.J.; Rock, J.A. Vaginal creation for müllerian agenesis. Am. J. Obstet. Gynecol. 2001, 185, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, S.; Quek, J.; Rose, G.L.; Edmonds, D.K. Sexual function in women treated with dilators for vaginal agenesis. J. Pediatr. Adolesc. Gynecol. 2005, 18, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.A.; Reeves, L.A.; Retto, H.; Baramki, T.A.; Zacur, H.A.; Jones, H.W., Jr. Success following vaginal creation for Müllerian agenesis. Fertil. Steril. 1983, 39, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Trosclair, L.; Clayton, S.D.; O’Quin, C.; Connelly, Z.; Rieger, R.; Sorrells, D. ‘Distraction Vaginogenesis’: Preliminary Results Using a Novel Method for Vaginal Canal Expansion in Rats. Bioengineering 2023, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.; Alexander, J.S.; Solitro, G.; White, L.; Villalba, S.; Winder, E.; Boudreaux, M.; Veerareddy, P.; Dong, E.; Minagar, A.; et al. Self-expanding intestinal expansion sleeves (IES) for short gut syndrome. Pediatr. Surg. Int. 2022, 38, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Sun, X.; Yang, H.; Nose, K.; Somara, S.; Bitar, K.N.; Owyang, C.; Okawada, M.; Teitelbaum, D.H. Distraction-induced intestinal enterogenesis: Preservation of intestinal function and lengthening after reimplantation into normal jejunum. Ann. Surg. 2012, 255, 302–310. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Moolenbeek, C.; Ruitenberg, E.J. The ‘Swiss roll’: A simple technique for histological studies of the rodent intestine. Lab. Anim. 1981, 15, 57–60. [Google Scholar] [CrossRef]
- Daly, T.J.M.; Kramer, B. Alterations in rat vaginal histology by exogenous gonadotrophins. J. Anat. 1998, 193, 469–472. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.A.; Monclova, J.L.; Coariti, A.C.S.; Stine, C.A.; Toussaint, K.C., Jr.; Munson, J.M.; De Vita, R. Tear propagation in vaginal tissue under inflation. Acta Biomater. 2021, 127, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Moalli, P.A.; Howden, N.S.; Lowder, J.L.; Navarro, J.; Debes, K.M.; Abramowitch, S.D.; Woo, S.L. A rat model to study the structural properties of the vagina and its supportive tissues. Am. J. Obstet. Gynecol. 2005, 192, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Acién, P.; Nohales-Alfonso, F.J.; Sánchez-Ferrer, M.L.; Sánchez-Lozano, M.; Navarro-Lillo, V.; Acién, M. Clinical pilot study to evaluate the neovaginal PACIENA prosthesis® for vaginoplasty without skin grafts in women with vaginal agenesis. BMC Women’s Health 2019, 19, 144. [Google Scholar] [CrossRef]
- Samat, A.; Abdul Hamid, Z.A.; Jaafar, M.; Ong, C.C.; Yahaya, B.H. Investigation of the in vitro and in vivo biocompatibility of a Three-Dimensional printed thermoplastic Polyurethane/Polylactic Acid blend for the development of tracheal scaffolds. Bioengineering 2023, 10, 394. [Google Scholar]
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| VES Length (mm) | 25 | 27 | 27 | 27 | 25 | 30 |
| Pre-insertion VL (mm) | 18 | 23 | 18 | 18 | 20 | 23 |
| Post-insertion VL (mm) | 25 | 24 | 22 | 24 | 23 | 25 |
| Expansion (%) | 38.9 | 4.3 | 22.2 | 33.3 | 15.0 | 8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, H.; Trosclair, L.; Clayton, S.D.; O’Quin, C.; Crochet, C.; Colvin, J.C.; Welch, V.; Alhaque, A.; Solitro, G.; Shah-Bruce, M.; et al. Histologic Analysis of ‘Distraction Vaginogenesis’ in a Rat Model. Pathophysiology 2024, 31, 298-308. https://doi.org/10.3390/pathophysiology31020022
Meyer H, Trosclair L, Clayton SD, O’Quin C, Crochet C, Colvin JC, Welch V, Alhaque A, Solitro G, Shah-Bruce M, et al. Histologic Analysis of ‘Distraction Vaginogenesis’ in a Rat Model. Pathophysiology. 2024; 31(2):298-308. https://doi.org/10.3390/pathophysiology31020022
Chicago/Turabian StyleMeyer, Hannah, Lexus Trosclair, Sean D. Clayton, Collyn O’Quin, Carol Crochet, Joshua C. Colvin, Valerie Welch, Ahmed Alhaque, Giovanni Solitro, Mila Shah-Bruce, and et al. 2024. "Histologic Analysis of ‘Distraction Vaginogenesis’ in a Rat Model" Pathophysiology 31, no. 2: 298-308. https://doi.org/10.3390/pathophysiology31020022
APA StyleMeyer, H., Trosclair, L., Clayton, S. D., O’Quin, C., Crochet, C., Colvin, J. C., Welch, V., Alhaque, A., Solitro, G., Shah-Bruce, M., Alexander, J. S., & Sorrells, D. L. (2024). Histologic Analysis of ‘Distraction Vaginogenesis’ in a Rat Model. Pathophysiology, 31(2), 298-308. https://doi.org/10.3390/pathophysiology31020022










