Neuromodulation and the Gut–Brain Axis: Therapeutic Mechanisms and Implications for Gastrointestinal and Neurological Disorders
Abstract
1. Introduction
2. Methodology
3. Discussion
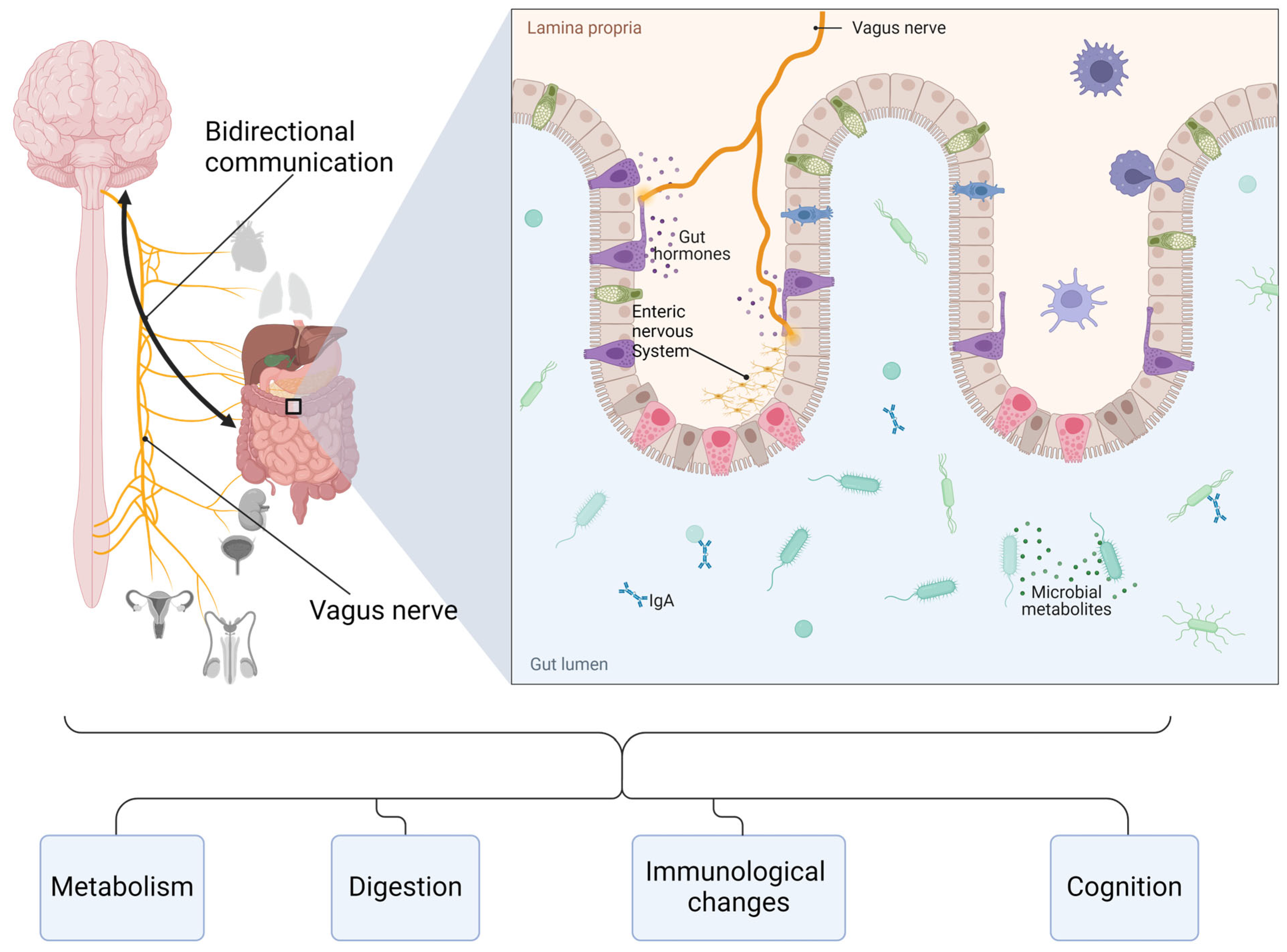
3.1. The Gut–Brain-Axis
3.1.1. Key Components of the Gut–Brain Axis
3.1.2. Physiology of the Gut–Brain Axis
3.1.3. Neural Communication between the Gut and the Brain
3.1.4. Pathophysiological Involvement of Brain–Gut Axis in Different Disorders
Irritable Bowel Syndrome (IBS)
Inflammatory Bowel Disease (IBD)
Gastroesophageal Reflux Disease (GERD)
Parkinson’s Disease
Neuropsychiatric Disorders
Autism Spectrum Disorder (ASD)
3.2. Effects of Neuromodulation on Gut–Brain Axis
3.2.1. Physiological Changes in the Gut–Brain Axis after Neuromodulation Including Changes in GI Physiology
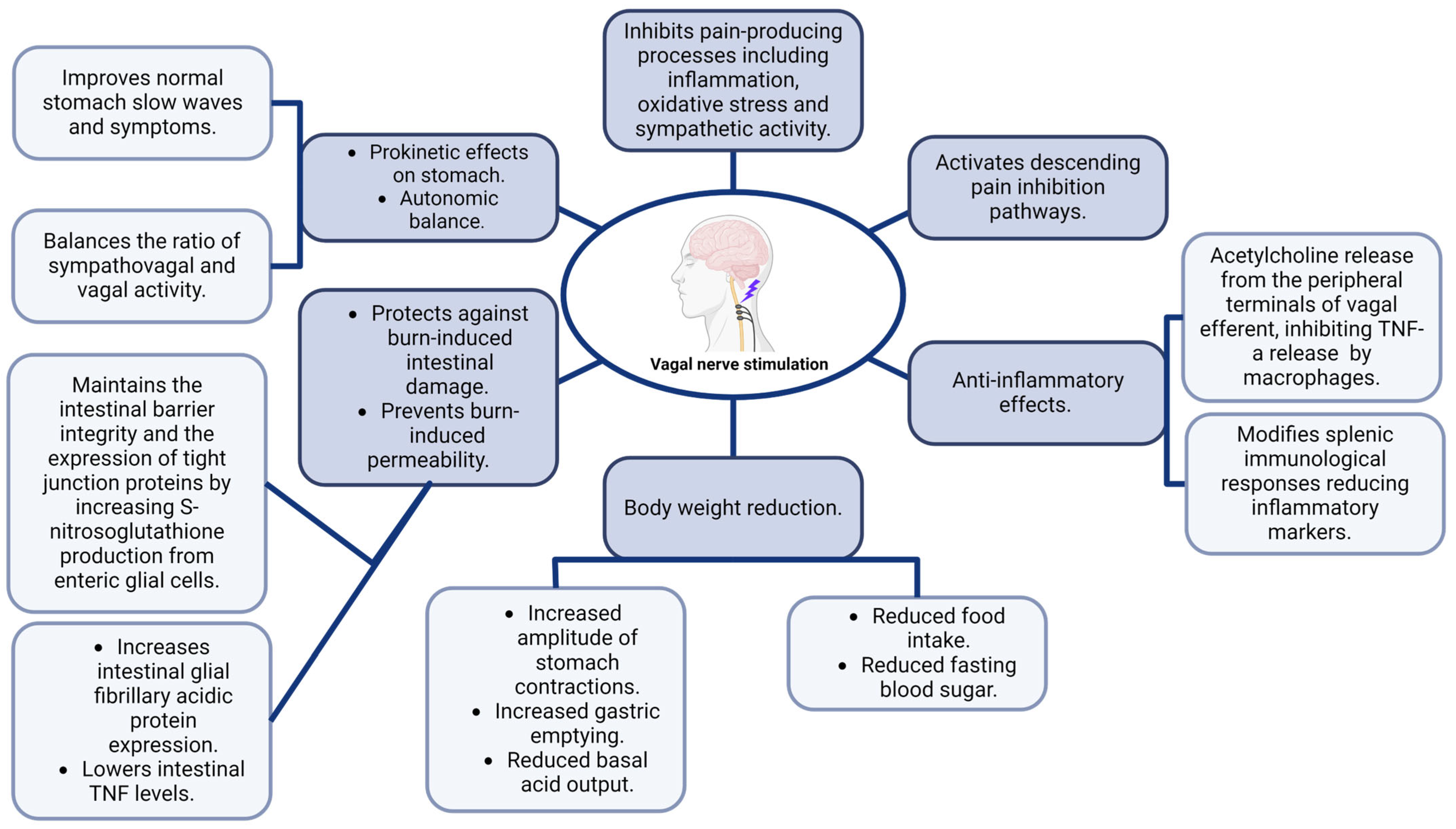
Neuromodulation Effect on Pain
Effects of Neuromodulation on GI Motility and Permeability
Effects of Neuromodulation on GI Inflammation and Immunity
Effects of Neuromodulation on Gut Microbiota
Effects of Neuromodulation on Weight Gain and Food Intake
Special Consideration: Microbiota-Induced Vagus Nerve Stimulation in Cerebral Ischemia
3.2.2. Effects of Neuromodulation on the Gut–Brain Axis Disorders
Irritable Bowel Syndrome (IBS)
Inflammatory Bowel Disease (IBD)
Gastroesophageal Reflux Disease (GERD)
Autism Spectrum Disorder
Parkinson’s Disease
Alzheimer’s Disease
Depression
4. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Ippolito, C.; Segnani, C.; Benvenuti, L.; D’Amati, A.; Errede, M.; Virgintino, D.; Fornai, M.; et al. Enteric Glia and Brain Astroglia: Complex Communication in Health and Disease along the Gut-Brain Axis. Neuroscientist 2023, 107385842311634. [Google Scholar] [CrossRef] [PubMed]
- Tait, C.; Sayuk, G.S. The Brain-Gut-Microbiotal Axis: A Framework for Understanding Functional GI Illness and Their Therapeutic Interventions. Eur. J. Intern. Med. 2021, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Zhang, J.-G. Neuromodulation: Past, Present, and Future. Sichuan Da Xue Xue Bao Yi Xue Ban 2022, 53, 559–563. [Google Scholar] [CrossRef]
- Rasche, D.; Rinaldi, P.C.; Young, R.F.; Tronnier, V.M. Deep Brain Stimulation for the Treatment of Various Chronic Pain Syndromes. Neurosurg. Focus 2006, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Ho, A.; Reddy, R.; Chen, J.; Castellanos, J. Narrative Review of Current Neuromodulation Modalities for Spinal Cord Injury. Front. Pain. Res. 2023, 4, 1143405. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Tagliati, M.; Alterman, R.L.; Lozano, A.M.; Volkmann, J.; Stefani, A.; Horak, F.B.; Okun, M.S.; Foote, K.D.; Krack, P.; et al. Deep Brain Stimulation for Parkinson Disease. Arch. Neurol. 2011, 68, 165. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.T.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A 5-Year Observational Study of Patients with Treatment-Resistant Depression Treated with Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation Technol. Neural Interface 2014, 17, 515–550. [Google Scholar] [CrossRef]
- George, M.S.; Taylor, J.J.; Short, E.B. The Expanding Evidence Base for RTMS Treatment of Depression. Curr. Opin. Psychiatry 2013, 26, 13–18. [Google Scholar] [CrossRef]
- Pires, M.; Severo, M.; Lopes, A.; Neves, S.; Matzel, K.; Povo, A. Sacral Neuromodulation for Low Anterior Resection Syndrome: Current Status—A Systematic Review and Meta-Analysis. Int. J. Color. Dis. 2023, 38, 189. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.B.; Rao, V.R. Responsive Neurostimulation: Candidates and Considerations. Epilepsy Behav. 2018, 88, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Fresnedo, A.; Perez-Vega, C.; Domingo, R.A.; Cheshire, W.P.; Middlebrooks, E.H.; Grewal, S.S. Motor Cortex Stimulation for Pain: A Narrative Review of Indications, Techniques, and Outcomes. Neuromodulation Technol. Neural Interface 2022, 25, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.D.; Hayek, S.M.; Rosenow, J.M.; Narouze, S.; Arle, J.E.; Pilitsis, J.G.; Schwalb, J.M.; Falowski, S.M.; Sweet, J.A. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Occipital Nerve Stimulation for the Treatment of Patients with Medically Refractory Occipital Neuralgia: Update. Neurosurgery 2023, 93, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Byczynski, G.; Vanneste, S. Modulating Motor Learning with Brain Stimulation: Stage-Specific Perspectives for Transcranial and Transcutaneous Delivery. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110766. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.M.D.; Cavalcanti, R.L.; Souza, C.G.; Chaves, G.; Macedo, L.B. Transcranial Direct Current Stimulation Combined with Peripheral Stimulation in Chronic Pain: A Systematic Review and Meta-Analysis. Expert. Rev. Med. Devices 2023, 20, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Nierenburg, H.; Stark-Inbar, A. Nerivio ® Remote Electrical Neuromodulation for Acute Treatment of Chronic Migraine. Pain Manag. 2022, 12, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L. Brain-Gut Communication: Vagovagal Reflexes Interconnect the Two “Brains”. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G576–G587. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Demirkan, A.; Twa, G.; Cohen, G.; Dean, M.N.; Standaert, D.G.; Sampson, T.R.; Payami, H. Metagenomics of Parkinson’s Disease Implicates the Gut Microbiome in Multiple Disease Mechanisms. Nat. Commun. 2022, 13, 6958. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Le, W. Intestinal Permeability, Dysbiosis, Inflammation and Enteric Glia Cells: The Intestinal Etiology of Parkinson’s Disease. Aging Dis. 2022, 13, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Chong, C.W.; Lim, S.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.Y. The Treatment of Chronic Hepatic Encephalopathy. Hepatogastroenterology 1991, 38, 377–387. [Google Scholar] [PubMed]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered Brain-Gut Axis in Autism: Comorbidity or Causative Mechanisms? BioEssays 2014, 36, 933–939. [Google Scholar] [CrossRef]
- Koloski, N.A.; Jones, M.; Kalantar, J.; Weltman, M.; Zaguirre, J.; Talley, N.J. The Brain–Gut Pathway in Functional Gastrointestinal Disorders Is Bidirectional: A 12-Year Prospective Population-Based Study. Gut 2012, 61, 1284–1290. [Google Scholar] [CrossRef]
- Sridhar, A. Let the Gut Do the Guiding. Nat. Rev. Microbiol. 2020, 18, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut–Brain Axis. J. Cell Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Zac-Varghese, S.; Tan, T.; Bloom, S.R. Hormonal Interactions between Gut and Brain. Discov. Med. 2010, 10, 543–552. [Google Scholar] [PubMed]
- Mudd, A.T.; Berding, K.; Wang, M.; Donovan, S.M.; Dilger, R.N. Serum Cortisol Mediates the Relationship between Fecal Ruminococcus and Brain N-Acetylaspartate in the Young Pig. Gut Microbes 2017, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut Peptides and the Microbiome: Focus on Ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Ruffle, J.K.; Aziz, Q. Brain Processing of Gastrointestinal Sensory Signaling. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 373–385. [Google Scholar]
- Primeaux, S.D.; Harrison-Bernard, L.M.; Barnes, M.J. Neurophysiology of the Hypothalamus. In Human Hypothalamus; Humana: Cham, Switzerland, 2021; pp. 33–52. [Google Scholar]
- Torsunova, Y.P.; Afanasieva, N.V. Morphology and Functioning of Limbic System: Literature Review. Perm. Med. J. 2023, 40, 61–77. [Google Scholar] [CrossRef]
- Grčić, A.; Varljen, J.; Bedoić, E.; Kučić, N.; Detel, D.; Batičić, L. Gut-Brain Axis. Med. Flum. 2022, 58, 4–19. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: What’s New for Rome IV? Lancet Gastroenterol. Hepatol. 2016, 1, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, K.; Smith, J. Irritable Bowel Syndrome: A Review and Update. Clin. Colon Rectal Surg. 2012, 25, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.L. Irritable Bowel Syndrome: A Clinical Review. World J. Gastroenterol. 2014, 20, 12144. [Google Scholar] [CrossRef]
- Drossman, D.A. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Boyce, P.M.; Talley, N.J.; Burke, C.; Koloski, N.A. Epidemiology of the Functional Gastrointestinal Disorders Diagnosed According to Rome II Criteria: An Australian Population-Based Study. Intern. Med. J. 2006, 36, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Heitkemper, M.; Crowell, M.D.; Emmanuel, A.; Halpert, A.; McRoberts, J.A.; Toner, B. Age, Gender, and Women’s Health and the Patient. Gastroenterology 2016, 150, 1332–1343.e4. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable Bowel Syndrome: A Disease Still Searching for Pathogenesis, Diagnosis and Therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Holtmann, G.; Walker, M.M. Therapeutic Strategies for Functional Dyspepsia and Irritable Bowel Syndrome Based on Pathophysiology. J. Gastroenterol. 2015, 50, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Irritable Bowel Syndrome: Towards Biomarker Identification. Trends Mol. Med. 2009, 15, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Roman, S.; Bredenoord, A.J.; Fox, M.; Keller, J.; Pandolfino, J.E.; Sifrim, D.; Tatum, R.; Yadlapati, R.; Savarino, E. Classification of Esophageal Motor Findings in Gastro-esophageal Reflux Disease: Conclusions from an International Consensus Group. Neurogastroenterol. Motil. 2017, 29, e13104. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Pandolfino, J.E. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology 2018, 154, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Mavroeidi, P.; Xilouri, M. Neurons and Glia Interplay in α-Synucleinopathies. Int. J. Mol. Sci. 2021, 22, 4994. [Google Scholar] [CrossRef] [PubMed]
- Lobo, B.; Tramullas, M.; Finger, B.-C.; Lomasney, K.W.; Beltran, C.; Clarke, G.; Santos, J.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. The Stressed Gut: Region-Specific Immune and Neuroplasticity Changes in Response to Chronic Psychosocial Stress. J. Neurogastroenterol. Motil. 2023, 29, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-X.; Gulam, M.Y.; Chia, N.S.J.; Feng, L.; Rotzschke, O.; Tan, E.-K. Gut–Brain Axis: Potential Factors Involved in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2020, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-Seeded α-Synuclein Fibrils Promote Gut Dysfunction and Brain Pathology Specifically in Aged Mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Lim, S.-Y.; Chong, K.K.; Azhan A Manap, M.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson’s Disease: A Randomized Placebo-Controlled Study. Neurology 2020, 96, e772–e782. [Google Scholar] [CrossRef]
- Gerhardt, S.; Mohajeri, M. Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson’s Disease. Neuroscience 2020, 432, 160–173. [Google Scholar] [CrossRef]
- Huang, T.-T.; Lai, J.-B.; Du, Y.-L.; Xu, Y.; Ruan, L.-M.; Hu, S.-H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The Microbiota and the Hypothalamus-Pituitary-Adrenocortical (HPA) Axis, Implications for Anxiety and Stress Disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tian, J.; Yang, B. Targeting Gut Microbiome: A Novel and Potential Therapy for Autism. Life Sci. 2018, 194, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.A.; Rocha-Guzmán, N.E.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Bravo-Muñoz, M. Ancestral Food Sources Rich in Polyphenols, Their Metabolism, and the Potential Influence of Gut Microbiota in the Management of Depression and Anxiety. J. Agric. Food Chem. 2022, 70, 944–956. [Google Scholar] [CrossRef]
- Platte, P.; Herbert, C.; Pauli, P.; Breslin, P.A.S. Oral Perceptions of Fat and Taste Stimuli Are Modulated by Affect and Mood Induction. PLoS ONE 2013, 8, e65006. [Google Scholar] [CrossRef] [PubMed]
- Le Port, A.; Gueguen, A.; Kesse-Guyot, E.; Melchior, M.; Lemogne, C.; Nabi, H.; Goldberg, M.; Zins, M.; Czernichow, S. Association between Dietary Patterns and Depressive Symptoms Over Time: A 10-Year Follow-Up Study of the GAZEL Cohort. PLoS ONE 2012, 7, e51593. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Ortuño Sánchez-Pedreño, F.; Van der Does, W.; Angel Martínez-González, M. Diet Quality and Depression Risk: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Dan, Z.; Mao, X.; Liu, Q.; Guo, M.; Zhuang, Y.; Liu, Z.; Chen, K.; Chen, J.; Xu, R.; Tang, J.; et al. Altered Gut Microbial Profile Is Associated with Abnormal Metabolism Activity of Autism Spectrum Disorder. Gut Microbes 2020, 11, 1246–1267. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism–Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Lozupone, C.; Kang, D.-W.; Adams, J.B. Gut Bacteria in Children with Autism Spectrum Disorders: Challenges and Promise of Studying How a Complex Community Influences a Complex Disease. Microb. Ecol. Health Dis. 2015, 26, 26914. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Windows: Implications for Brain Disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science (1979) 2019, 366, eaar2016. [Google Scholar] [CrossRef] [PubMed]
- Archie, E.A.; Tung, J. Social Behavior and the Microbiome. Curr. Opin. Behav. Sci. 2015, 6, 28–34. [Google Scholar] [CrossRef]
- Münger, E.; Montiel-Castro, A.J.; Langhans, W.; Pacheco-López, G. Reciprocal Interactions Between Gut Microbiota and Host Social Behavior. Front. Integr. Neurosci. 2018, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Sturm, K.; Wetter, T.C.; Lange, M.; Gabriels, L.; Mayer, E.A.; Schlaier, J. Deep Brain Stimulation for Obsessive Compulsive Disorder Reduces Symptoms of Irritable Bowel Syndrome in a Single Patient. Clin. Gastroenterol. Hepatol. 2015, 13, 1371–1374.e3. [Google Scholar] [CrossRef] [PubMed]
- Day, M. Sympathetic Blocks: The Evidence. Pain. Pract. 2008, 8, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Randich, A.; Gebhart, G.F. Vagal Afferent Modulation of Nociception. Brain Res. Rev. 1992, 17, 77–99. [Google Scholar] [CrossRef]
- De Couck, M.; Nijs, J.; Gidron, Y. You May Need a Nerve to Treat Pain. Clin. J. Pain. 2014, 30, 1099–1105. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, X.; Yan, N.; Chen, S.; Li, Y. Cholecystokinin Enhances Visceral Pain-Related Affective Memory via Vagal Afferent Pathway in Rats. Mol. Brain 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Wu, X.Y.; Cao, Z.J.; Fan, J.; Wang, M.; Owyang, C.; Li, Y. Subdiaphragmatic Vagal Afferent Nerves Modulate Visceral Pain. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G1441–G1449. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagal Tone: Effects on Sensitivity, Motility, and Inflammation. Neurogastroenterol. Motil. 2016, 28, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, F.; Sun, C.; Xu, W.; Li, M.; Gong, Y.; Rong, P.; Lin, L.; Chen, J.D.Z. Noninvasive Transcutaneous Auricular Vagal Nerve Stimulation Improves Gastric Slow Waves Impaired by Cold Stress in Healthy Subjects. Neuromodulation Technol. Neural Interface 2022, 26, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Costantini, T.W.; Bansal, V.; Krzyzaniak, M.; Putnam, J.G.; Peterson, C.Y.; Loomis, W.H.; Wolf, P.; Baird, A.; Eliceiri, B.P.; Coimbra, R. Vagal Nerve Stimulation Protects against Burn-Induced Intestinal Injury through Activation of Enteric Glia Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1308–G1318. [Google Scholar] [CrossRef]
- Bonaz, B. Anti-inflammatory Effects of Vagal Nerve Stimulation with a Special Attention to Intestinal Barrier Dysfunction. Neurogastroenterol. Motil. 2022, 34, e14456. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Costantini, T.; Ryu, S.Y.; Peterson, C.; Loomis, W.; Putnam, J.; Elicieri, B.; Baird, A.; Coimbra, R. Stimulating the Central Nervous System to Prevent Intestinal Dysfunction After Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2010, 68, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. Neural Circuitry and Immunity. Immunol. Res. 2015, 63, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Meregnani, J.; Clarençon, D.; Vivier, M.; Peinnequin, A.; Mouret, C.; Sinniger, V.; Picq, C.; Job, A.; Canini, F.; Jacquier-Sarlin, M.; et al. Anti-Inflammatory Effect of Vagus Nerve Stimulation in a Rat Model of Inflammatory Bowel Disease. Auton. Neurosci. 2011, 160, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Hoffmann, D.; Clarençon, D.; Mathieu, N.; Dantzer, C.; Vercueil, L.; Picq, C.; Trocmé, C.; Faure, P.; et al. Chronic Vagus Nerve Stimulation in Crohn’s Disease: A 6-Month Follow-up Pilot Study. Neurogastroenterol. Motil. 2016, 28, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, S.; Dantzer, C.; Canini, F.; Mathieu, N.; Bonaz, B. Psychological Adjustment and Autonomic Disturbances in Inflammatory Bowel Diseases and Irritable Bowel Syndrome. Psychoneuroendocrinology 2010, 35, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Korenblik, V.; Brouwer, M.E.; Korosi, A.; Denys, D.; Bockting, C.L.H.; Brul, S.; Lok, A. Are Neuromodulation Interventions Associated with Changes in the Gut Microbiota? A Systematic Review. Neuropharmacology 2023, 223, 109318. [Google Scholar] [CrossRef] [PubMed]
- Hey, G.; Nair, N.; Klann, E.; Gurrala, A.; Safarpour, D.; Mai, V.; Ramirez-Zamora, A.; Vedam-Mai, V. Therapies for Parkinson’s Disease and the Gut Microbiome: Evidence for Bidirectional Connection. Front. Aging Neurosci. 2023, 15, 1151850. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ding, W.; Dong, Y.; Chen, C.; Zeng, Y.; Jiang, Z.; Gan, S.; You, Z.; Zhao, Y.; Zhang, Y.; et al. Electroacupuncture Attenuates Surgical Pain-Induced Delirium-like Behavior in Mice via Remodeling Gut Microbiota and Dendritic Spine. Front. Immunol. 2022, 13, 955581. [Google Scholar] [CrossRef] [PubMed]
- Sobocki, J.; Królczyk, G.; Herman, R.M.; Matyja, A.; Thor, P.J. Influence of Vagal Nerve Stimulation on Food Intake and Body Weight--Results of Experimental Studies. J. Physiol. Pharmacol. 2005, 56 (Suppl. 6), 27–33. [Google Scholar] [PubMed]
- Díaz-Güemes, I.; Sánchez, F.M.; Luis, L.; Sun, F.; Pascual, S.; Usón, J. Continuous Vagus Nerve Stimulation Effects on the Gut-Brain Axis in Swine. Neuromodulation Technol. Neural Interface 2007, 10, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Krolczyk, G.; Zurowski, D.; Sobocki, J.; Słowiaczek, M.P.; Laskiewicz, J.; Matyja, A.; Zaraska, K.; Zaraska, W.; Thor, P.J. Effects of Continuous Microchip (MC) Vagal Neuromodulation on Gastrointestinal Function in Rats. J. Physiol. Pharmacol. 2001, 52, 705–715. [Google Scholar] [PubMed]
- Ni, S.; Yao, Z.; Wei, X.; Heng, X.; Qu, S.; Zhao, X.; Qi, Y.; Ge, P.; Xu, C.; Yang, N.; et al. Vagus Nerve Stimulated by Microbiota-derived Hydrogen Sulfide Mediates the Regulation of Berberine on Microglia in Transient Middle Cerebral Artery Occlusion Rats. Phytother. Res. 2022, 36, 2964–2981. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hu, Y.; Zhang, B.; Li, W.; Chen, J.D.; Liu, F. Ameliorating Effects and Mechanisms of Transcutaneous Auricular Vagal Nerve Stimulation on Abdominal Pain and Constipation. JCI Insight 2021, 6, e150052. [Google Scholar] [CrossRef]
- Palecek, J. The Role of Dorsal Columns Pathway in Visceral Pain. Physiol. Res. 2004, 53 (Suppl. 1), S125–S1230. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C.; Foreman, R.D.; Linderoth, B. Spinal Cord Stimulation Attenuates Visceromotor Reflexes in a Rat Model of Post-Inflammatory Colonic Hypersensitivity. Auton. Neurosci. 2005, 122, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Aulí, M.; Martínez, E.; Gallego, D.; Opazo, A.; Espín, F.; Martí-Gallostra, M.; Jiménez, M.; Clavé, P. Effects of Excitatory and Inhibitory Neurotransmission on Motor Patterns of Human Sigmoid Colon in Vitro. Br. J. Pharmacol. 2008, 155, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.A.; Welting, O.; van Hamersveld, H.P.; Meijer, S.L.; Folgering, J.H.A.; Darwinkel, H.; Witherington, J.; Sridhar, A.; Vervoordeldonk, M.J.; Seppen, J.; et al. Neuronal Control of Experimental Colitis Occurs via Sympathetic Intestinal Innervation. Neurogastroenterol. Motil. 2018, 30, e13163. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, G.; Gomez-Pinilla, P.J.; Nemethova, A.; Di Giovangiulio, M.; Cailotto, C.; van Bree, S.H.; Michel, K.; Tracey, K.J.; Schemann, M.; Boesmans, W.; et al. A Distinct Vagal Anti-Inflammatory Pathway Modulates Intestinal Muscularis Resident Macrophages Independent of the Spleen. Gut 2014, 63, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Y.; Shi, X.; Li, W.; Zeng, X.; Liu, F.; Chen, J.D.Z.; Xie, W.-F. Integrative Effects and Vagal Mechanisms of Transcutaneous Electrical Acustimulation on Gastroesophageal Motility in Patients with Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2021, 116, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, R.; Liu, Z.; Xu, J.; Xu, C.; Chen, J.D.Z. Ameliorating Effects of Transcutaneous Electrical Acustimulation Combined With Deep Breathing Training on Refractory Gastroesophageal Reflux Disease Mediated via the Autonomic Pathway. Neuromodulation Technol. Neural Interface 2019, 22, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, Diagnosis and Therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Follesa, P.; Biggio, F.; Gorini, G.; Caria, S.; Talani, G.; Dazzi, L.; Puligheddu, M.; Marrosu, F.; Biggio, G. Vagus Nerve Stimulation Increases Norepinephrine Concentration and the Gene Expression of BDNF and BFGF in the Rat Brain. Brain Res. 2007, 1179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R. Neural Plasticity; Harvard University Press: Cambridge, MA, USA, 2009; ISBN 9780674038936. [Google Scholar]
- Barahona-Corrêa, J.B.; Velosa, A.; Chainho, A.; Lopes, R.; Oliveira-Maia, A.J. Repetitive Transcranial Magnetic Stimulation for Treatment of Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Integr. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Enticott, P.G.; Kirkovski, M.; Oberman, L.M. Transcranial Magnetic Stimulation in Autism Spectrum Disorder. Neurotechnol. Brain Stimul. Pediatr. Psychiatr. Neurodev. Disord. 2019, 83–113. [Google Scholar] [CrossRef]
- Oberman, L.M.; Enticott, P.G.; Casanova, M.F.; Rotenberg, A.; Pascual-Leone, A.; McCracken, J.T. Transcranial Magnetic Stimulation in Autism Spectrum Disorder: Challenges, Promise, and Roadmap for Future Research. Autism Res. 2016, 9, 184–203. [Google Scholar] [CrossRef] [PubMed]
- Rizzone, M.G.; Fasano, A.; Daniele, A.; Zibetti, M.; Merola, A.; Rizzi, L.; Piano, C.; Piccininni, C.; Romito, L.M.; Lopiano, L.; et al. Long-Term Outcome of Subthalamic Nucleus DBS in Parkinson’s Disease: From the Advanced Phase towards the Late Stage of the Disease? Park. Relat. Disord. 2014, 20, 376–381. [Google Scholar] [CrossRef]
- Shirota, Y.; Ohtsu, H.; Hamada, M.; Enomoto, H.; Ugawa, Y. Supplementary Motor Area Stimulation for Parkinson Disease. Neurology 2013, 80, 1400–1405. [Google Scholar] [CrossRef]
- Mann, A.; Gondard, E.; Tampellini, D.; Milsted, J.A.T.; Marillac, D.; Hamani, C.; Kalia, S.K.; Lozano, A.M. Chronic Deep Brain Stimulation in an Alzheimer’s Disease Mouse Model Enhances Memory and Reduces Pathological Hallmarks. Brain Stimul. 2018, 11, 435–444. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Ling, Z.; Yu, X. Repetitive Transcranial Magnetic Stimulation for Cognitive Impairment in Alzheimer’s Disease: A Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2020, 267, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, Y.; Liu, C.; Meng, Z. Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex for Treatment of Neuropsychiatric Disorders. Front. Behav. Neurosci. 2022, 16, 893955. [Google Scholar] [CrossRef] [PubMed]
- Brunelin, J.; Mondino, M.; Gassab, L.; Haesebaert, F.; Gaha, L.; Suaud-Chagny, M.F.; Saoud, M.; Mechri, A.; Poulet, E. Examining Transcranial Direct-Current Stimulation (TDCS) as a Treatment for Hallucinations in Schizophrenia. Am. J. Psychiatry 2012, 169, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, A.; Rushby, J.A.; Loo, C.; Short, B.; Weickert, C.S.; Weickert, T.W. Transcranial Direct Current Stimulation Influences Probabilistic Association Learning in Schizophrenia. Schizophr. Res. 2011, 131, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable Bowel Syndrome. JAMA 2015, 313, 949. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.; Spohn, S. Non-Conventional Features of Peripheral Serotonin Signalling—The Gut and Beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar]
- Qin, H.-Y.; Zang, K.-H.; Zuo, X.; Wu, X.-A.; Bian, Z.-X. Quercetin Attenuates Visceral Hypersensitivity and 5-Hydroxytryptamine Availability in Postinflammatory Irritable Bowel Syndrome Rats: Role of Enterochromaffin Cells in the Colon. J. Med. Food 2019, 22, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D. Parasympathetic Control of Gastrointestinal Motility and Cross-Branch Actions of Parasympathetic Neuromodulation. Chin. Med. J. 2023, 136, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, T.; Meng, Y.; Maisiyiti, A.; Dong, Y.; Li, S.; Chen, Y.; Yin, J.; Chen, J.D.Z. Auricular Vagal Nerve Stimulation Improves Constipation by Enhancing Colon Motility via the Central-Vagal Efferent Pathway in Opioid-Induced Constipated Rats. Neuromodulation Technol. Neural Interface 2021, 24, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Coffin, B. Alteration of the Spinal Modulation of Nociceptive Processing in Patients with Irritable Bowel Syndrome. Gut 2004, 53, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, A.; Di Cesare Mannelli, L.; Ghelardini, C. Analgesic and Antineuropathic Drugs Acting Through Central Cholinergic Mechanisms. Recent. Pat. CNS Drug Discov. 2011, 6, 119–140. [Google Scholar] [CrossRef]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The Liver–Brain–Gut Neural Arc Maintains the Treg Cell Niche in the Gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, B.; Shi, X.; Ning, B.; Shi, J.; Zeng, X.; Liu, F.; Chen, J.D.; Xie, W.-F. Ameliorating Effects and Autonomic Mechanisms of Transcutaneous Electrical Acustimulation in Patients with Gastroesophageal Reflux Disease. Neuromodulation Technol. Neural Interface 2020, 23, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Dickman, R.; Levy, S.; Perets, T.T.; Hazani-Pauker, M.; Boltin, D.; Schmilovitz-Weiss, H.; Nidal, I.; Siterman, M.; Carter, D.; Fass, R.; et al. Effect of the Transcutaneous Electrical Stimulation System on Esophageal-Acid Exposure in Patients Non-Responsive to Once-Daily Proton-Pump Inhibitor: Proof-of-Concept Study. Gastroenterol. Rep. 2021, 9, 323–328. [Google Scholar] [CrossRef]
- Lin, T.C.; Lo, Y.C.; Lin, H.C.; Li, S.J.; Lin, S.H.; Wu, H.F.; Chu, M.C.; Lee, C.W.; Lin, I.C.; Chang, C.W.; et al. MR Imaging Central Thalamic Deep Brain Stimulation Restored Autistic-like Social Deficits in the Rat. Brain Stimul. 2019, 12, 1410–1420. [Google Scholar] [CrossRef]
- Engineer, C.T.; Hays, S.A.; Kilgard, M.P. Vagus Nerve Stimulation as a Potential Adjuvant to Behavioral Therapy for Autism and Other Neurodevelopmental Disorders. J. Neurodev. Disord. 2017, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, W.P. Highlights in Clinical Autonomic Neuroscience: New Insights into Autonomic Dysfunction in Autism. Auton. Neurosci. 2012, 171, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Levy, K.M.; Hoff, D.; Amar, A.P.; Park, M.S.; Conklin, J.M.; Baird, L.; Apuzzo, M.L.J. Vagus Nerve Stimulation Therapy in Patients with Autism Spectrum Disorder and Intractable Epilepsy: Results from the Vagus Nerve Stimulation Therapy Patient Outcome Registry. J. Neurosurg. Pediatr. 2010, 5, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical Excitation/Inhibition Balance in Information Processing and Social Dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Khan, S.; Michmizos, K.; Tommerdahl, M.; Ganesan, S.; Kitzbichler, M.G.; Zetino, M.; Garel, K.-L.A.; Herbert, M.R.; Hämäläinen, M.S.; Kenet, T. Somatosensory Cortex Functional Connectivity Abnormalities in Autism Show Opposite Trends, Depending on Direction and Spatial Scale. Brain 2015, 138, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Hulsey, D.R.; Riley, J.R.; Loerwald, K.W.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Parametric Characterization of Neural Activity in the Locus Coeruleus in Response to Vagus Nerve Stimulation. Exp. Neurol. 2017, 289, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Businaro, R.; Ippoliti, F.; Lo Vasco, V.R.; Massoni, F.; Onofri, E.; Troili, G.M.; Pontecorvi, V.; Morelli, M.; Rapp Ricciardi, M.; et al. Altered Cytokine and BDNF Levels in Autism Spectrum Disorder. Neurotox. Res. 2013, 24, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial Magnetic Stimulation and the Human Brain. Nature 2000, 406, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Tormos, J.M.; Keenan, J.; Tarazona, F.; Cañete, C.; Catalá, M.D. Study and Modulation of Human Cortical Excitability with Transcranial Magnetic Stimulation. J. Clin. Neurophysiol. 1998, 15, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical Research with Transcranial Direct Current Stimulation (TDCS): Challenges and Future Directions. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2012, 5, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Herrmann, C.S. Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plast. 2016, 2016, 3616807. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.V.; Yun, K. Transcranial Alternating Current Stimulation (TACS) Mechanisms and Protocols. Front. Cell Neurosci. 2017, 11, 280666. [Google Scholar] [CrossRef]
- Moisa, M.; Polania, R.; Grueschow, M.; Ruff, C.C. Brain Network Mechanisms Underlying Motor Enhancement by Transcranial Entrainment of Gamma Oscillations. J. Neurosci. 2016, 36, 12053–12065. [Google Scholar] [CrossRef] [PubMed]
- Hoy, K.E.; Bailey, N.; Arnold, S.; Windsor, K.; John, J.; Daskalakis, Z.J.; Fitzgerald, P.B. The Effect of γ-TACS on Working Memory Performance in Healthy Controls. Brain Cogn. 2015, 101, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, M.C.; Sharma, K.; Migdadi, H.A.; Cucca, A. Non-Invasive Neuromodulation Therapies for Parkinson’s Disease. In Parkinson’s Disease-Understanding Pathophysiology and Developing Therapeutic Strategies; InTech: Houston, TX, USA, 2018. [Google Scholar]
- Manenti, R.; Brambilla, M.; Benussi, A.; Rosini, S.; Cobelli, C.; Ferrari, C.; Petesi, M.; Orizio, I.; Padovani, A.; Borroni, B.; et al. M Ild Cognitive Impairment in Parkinson’s Disease Is Improved by Transcranial Direct Current Stimulation Combined with Physical Therapy. Mov. Disord. 2016, 31, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Schüpbach, M.; Knudsen, K.; Pinsker, M.O.; Cornu, P.; Rau, J.; Agid, Y.; Schade-Brittinger, C. Stimulation of the Subthalamic Nucleus at an Earlier Disease Stage of Parkinson’s Disease: Concept and Standards of the EARLYSTIM-Study. Park. Relat. Disord. 2013, 19, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Elder, C.M.; Okun, M.S.; Patrick, S.K.; Vitek, J.L. Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons. J. Neurosci. 2003, 23, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Grill, W.M.; Sherman, D.L.; Thakor, N.V. Cellular Effects of Deep Brain Stimulation: Model-Based Analysis of Activation and Inhibition. J. Neurophysiol. 2004, 91, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Chervyakov, A.V.; Chernyavsky, A.Y.; Sinitsyn, D.O.; Piradov, M.A. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front. Hum. Neurosci. 2015, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. J. Neurosci. 2001, 21, RC157. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Simon, D.K.; Wu, A.; Pascual-Leone, A. Non-Invasive Brain Stimulation for Parkinson’s Disease: A Systematic Review and Meta-Analysis of the Literature. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; Wilson, R.S.; Evans, D.A. Prevalence and Incidence of Clinically Diagnosed Alzheimer’s Disease Dementia from 1994 to 2012 in a Population Study. Alzheimers Dement 2019, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mantzavinos, V.; Alexiou, A. Biomarkers for Alzheimer’s Disease Diagnosis. Curr. Alzheimer Res. 2017, 14, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Laxton, A.W.; Lozano, A.M. Deep Brain Stimulation for the Treatment of Alzheimer Disease and Dementias. World Neurosurg. 2013, 80, S28.e1–S28.e8. [Google Scholar] [CrossRef]
- Huang, C.; Chu, H.; Ma, Y.; Zhou, Z.; Dai, C.; Huang, X.; Fang, L.; Ao, Q.; Huang, D. The Neuroprotective Effect of Deep Brain Stimulation at Nucleus Basalis of Meynert in Transgenic Mice with Alzheimer’s Disease. Brain Stimul. 2019, 12, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Scharre, D.W.; Weichart, E.; Nielson, D.; Zhang, J.; Agrawal, P.; Sederberg, P.B.; Knopp, M.V.; Rezai, A.R. Deep Brain Stimulation of Frontal Lobe Networks to Treat Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Yiu, A.; Stone, S.S.D.; Oh, S.; Lozano, A.M.; Josselyn, S.A.; Frankland, P.W. Entorhinal Cortical Deep Brain Stimulation Rescues Memory Deficits in Both Young and Old Mice Genetically Engineered to Model Alzheimer’s Disease. Neuropsychopharmacology 2017, 42, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Li, C.T.; Brunoni, A.R.; Yang, F.C.; Tseng, P.T.; Tu, Y.K.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Rajji, T.K.; et al. Cognitive Effects and Acceptability of Non-Invasive Brain Stimulation on Alzheimer’s Disease and Mild Cognitive Impairment: A Component Network Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, H.A.; Harris, M.G.; McKeon, G.; Baxter, A.; Pennell, C.; Barendregt, J.J.; Wang, J. Estimating Remission from Untreated Major Depression: A Systematic Review and Meta-Analysis. Psychol. Med. 2013, 43, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Moffa, A.; Nikolin, S.; Bennabi, D.; Brunoni, A.R.; Flannery, W.; Haffen, E.; McClintock, S.M.; Moreno, M.L.; Padberg, F.; et al. Cognitive Effects of Transcranial Direct Current Stimulation Treatment in Patients with Major Depressive Disorder: An Individual Patient Data Meta-Analysis of Randomised, Sham-Controlled Trials. Neurosci. Biobehav. Rev. 2018, 90, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Demirtas-Tatlidede, A.; Vahabzadeh-Hagh, A.M.; Pascual-Leone, A. Can Noninvasive Brain Stimulation Enhance Cognition in Neuropsychiatric Disorders? Neuropharmacology 2013, 64, 566–578. [Google Scholar] [CrossRef] [PubMed]

| Disease | Study | Type of the Study (Human or Animal) | Reported Effects of Neuromodulation |
|---|---|---|---|
| Irritable bowel syndrome (IBS) | [107] | Human (42 patients) | tVNS increases bowel movements, alleviates abdominal discomfort, enhances IBS symptoms and quality of life, and improves rectal sensation and rectal distention-induced relaxation of the internal anal sphincter. |
| [108,109] | Animal | Spinal Cord Stimulation alleviates IBS pain, potentially by suppressing dorsal column pain pathways and selectively suppressing the response to visceronoxious stimulus. | |
| [110] | Human tissue | Direct electric stimulation activates inhibitory neurons releasing nitric oxide and purine; stimulates excitatory neurons releasing acetylcholine and tachykinins in human intestinal strips. | |
| Inflammatory bowel disease (IBD) | [111] | Animal | VNS reduces inflammation in the gut. |
| [112] | Animal | VNS decreases inflammation in the small intestine across normal, spleen-denervated, and T-cell-deficient mice. It mediates effects through targeting resident macrophages. | |
| Gastroesophageal reflux disease (GERD) | [113] | Human | Acute TEA (at bilateral ST36 and PC6 acupoints) enhances stomach accommodation and pace-making activity and reduces post-prandial dyspepsia in GERD. |
| [114] | Human | Four-week TEA (at ST36 and PC6) alleviates reflux symptoms, increases distal esophageal motility, enhances stomach accommodation, and reduces inefficient esophageal contractions. | |
| [115] | Human | Transcutaneous abdominal electrical stimulation reduces acid exposure and DeMeester score by over 50% in PPI-resistant GERD patients. | |
| Autism spectrum disorder (ASD) | [116,117] | Human | VNS in people with autism improves mood after 12 months compared to non-autistic individuals. VNS significantly increases BDNF levels, enhancing neural plasticity in people with autism. |
| [118,119,120] | Human | rTMS (of DLPFC) reduces repetitive behaviors and irritability and enhances neurophysiological perception. SMA stimulation improves movement-related potentials. Premotor cortex stimulation enhances sensorimotor integration. | |
| Parkinson’s disease | [121] | Human | STN DBS provides long-term symptom relief in neurodegenerative disorders, despite cognitive and gait decline over time. |
| [122] | Human | TMS (for SMA in PD) reduces UPDRS part III scores by 6.84 points at 12 weeks post-intervention. | |
| Alzheimer’s disease | [123] | Animal | Entorhinal cortex stimulation reduces memory impairments in spatial and recognition tasks in young and old animals. |
| [124] | Human | TMS for AD improves short-term cognitive function and has enhanced benefits with multi-site, long-term, high-frequency stimulation. | |
| Depression | [125] | Human | Anodic tDCS (of the left DLPFC) may reduce depression scores for up to 30 days. |
| Schizophrenia | [126] | Human | Anodic (excitatory) stimulation of the left DLPFC in conjunction with cathodic (inhibitory) stimulation of the left temporal–parietal junction was found to significantly reduce auditory verbal hallucinations in schizophrenia. |
| [127] | Human | Anodic tDCS to left DLPFC improves probabilistic association learning in schizophrenia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljeradat, B.; Kumar, D.; Abdulmuizz, S.; Kundu, M.; Almealawy, Y.F.; Batarseh, D.R.; Atallah, O.; Ennabe, M.; Alsarafandi, M.; Alan, A.; et al. Neuromodulation and the Gut–Brain Axis: Therapeutic Mechanisms and Implications for Gastrointestinal and Neurological Disorders. Pathophysiology 2024, 31, 244-268. https://doi.org/10.3390/pathophysiology31020019
Aljeradat B, Kumar D, Abdulmuizz S, Kundu M, Almealawy YF, Batarseh DR, Atallah O, Ennabe M, Alsarafandi M, Alan A, et al. Neuromodulation and the Gut–Brain Axis: Therapeutic Mechanisms and Implications for Gastrointestinal and Neurological Disorders. Pathophysiology. 2024; 31(2):244-268. https://doi.org/10.3390/pathophysiology31020019
Chicago/Turabian StyleAljeradat, Baha’, Danisha Kumar, Sulaiman Abdulmuizz, Mrinmoy Kundu, Yasser F. Almealawy, Dima Ratib Batarseh, Oday Atallah, Michelle Ennabe, Muath Alsarafandi, Albert Alan, and et al. 2024. "Neuromodulation and the Gut–Brain Axis: Therapeutic Mechanisms and Implications for Gastrointestinal and Neurological Disorders" Pathophysiology 31, no. 2: 244-268. https://doi.org/10.3390/pathophysiology31020019
APA StyleAljeradat, B., Kumar, D., Abdulmuizz, S., Kundu, M., Almealawy, Y. F., Batarseh, D. R., Atallah, O., Ennabe, M., Alsarafandi, M., Alan, A., & Weinand, M. (2024). Neuromodulation and the Gut–Brain Axis: Therapeutic Mechanisms and Implications for Gastrointestinal and Neurological Disorders. Pathophysiology, 31(2), 244-268. https://doi.org/10.3390/pathophysiology31020019







