Treatment Strategies for Chronic Coronary Heart Disease with Left Ventricular Systolic Dysfunction or Preserved Ejection Fraction—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
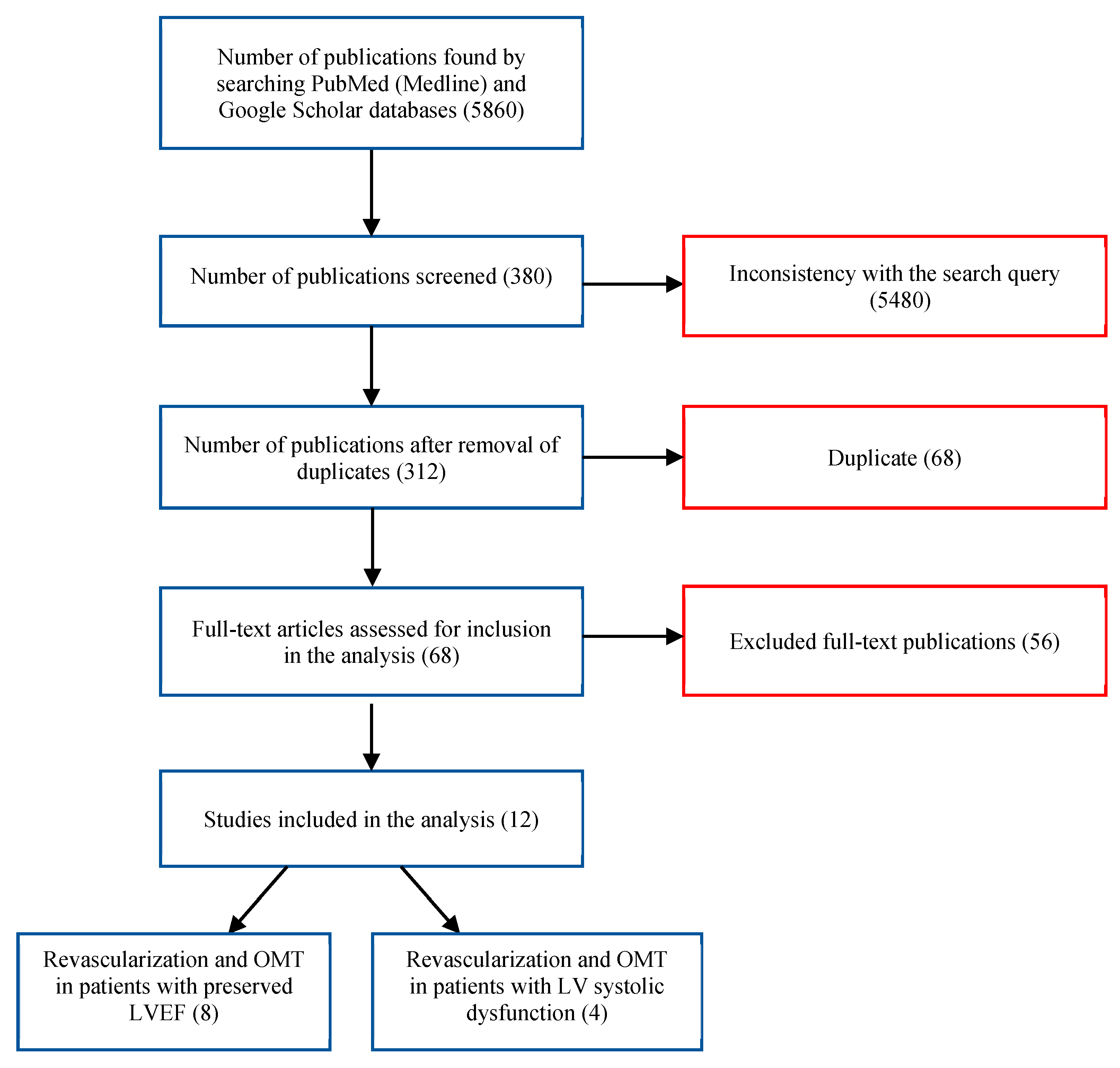
2. The Search Strategies and Study Selection
3. Inclusion/Exclusion Criteria
4. Extraction and Synthesis of Study Data
Statistical Analysis
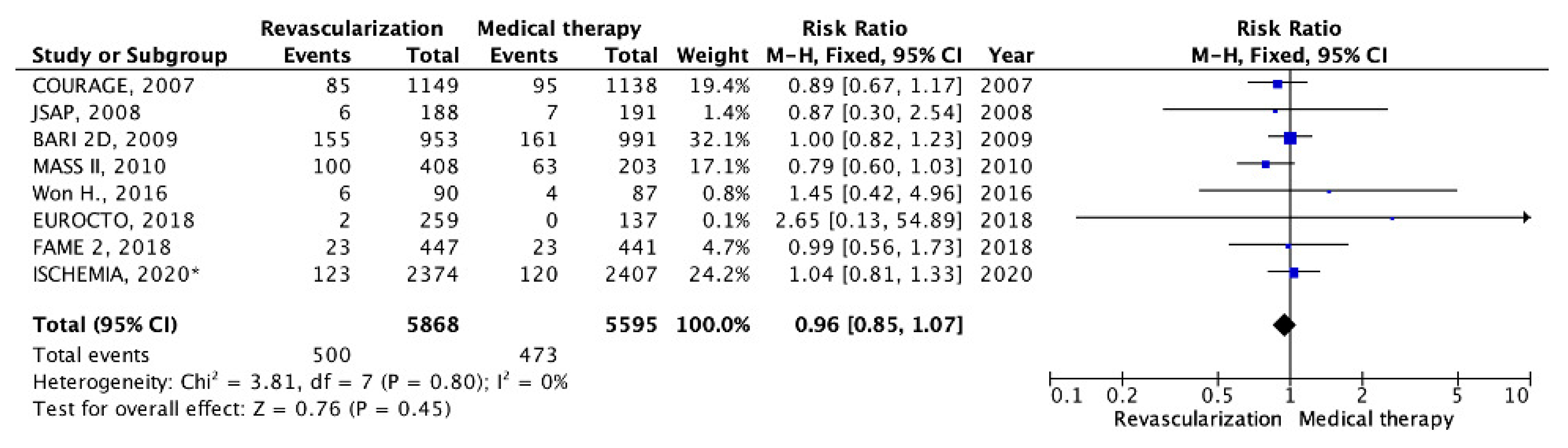
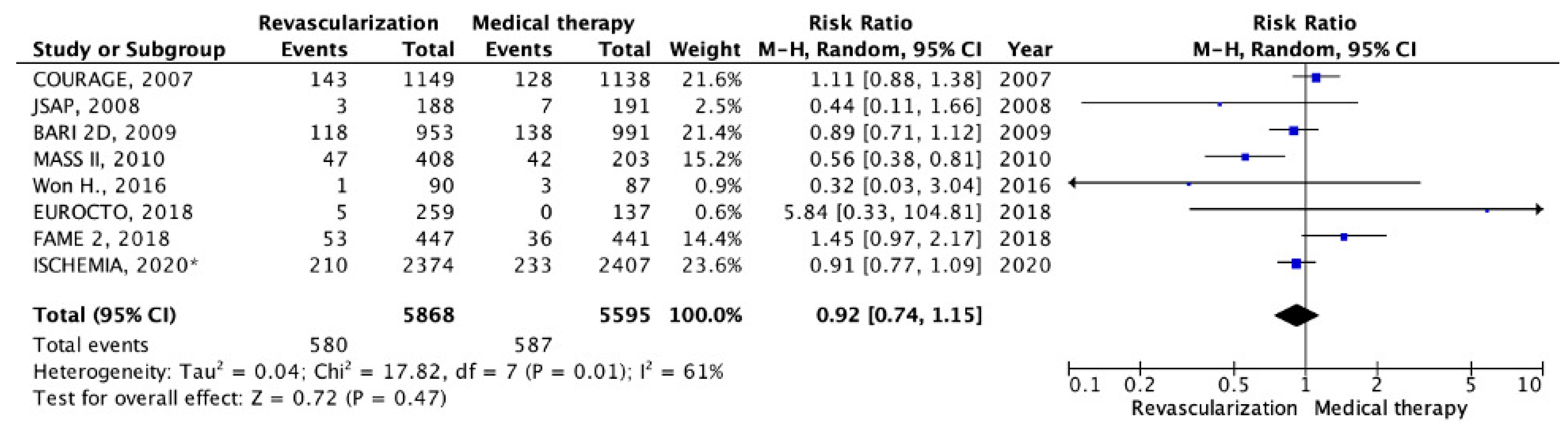
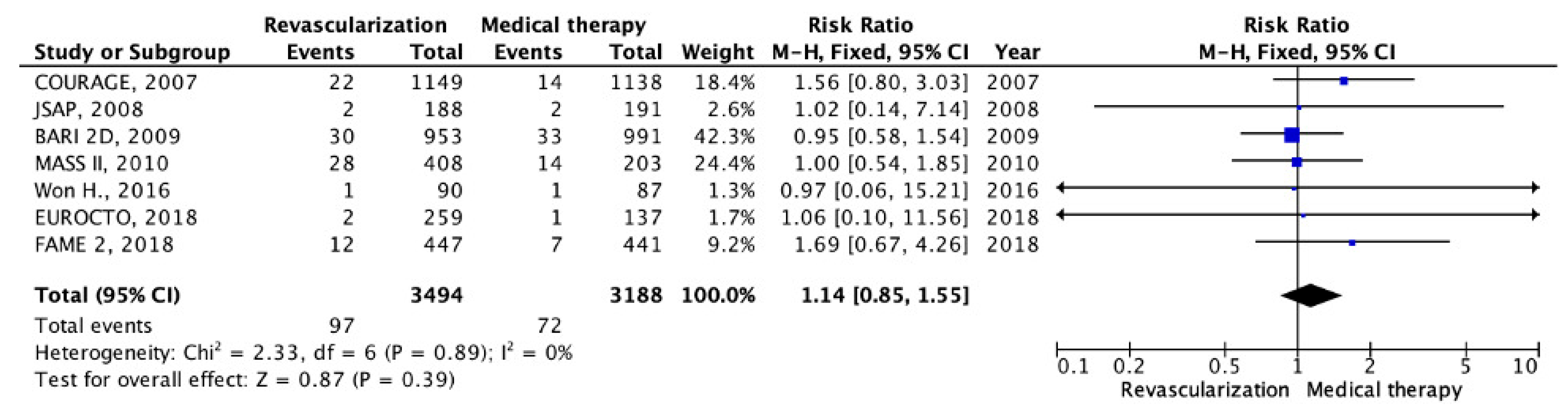
5. Results
5.1. Chronic CHD with Preserved LV EF
5.2. Chronic CHD with LV Systolic Dysfunction
6. Discussion
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Liga, R.; Boden, W.E. Myocardial revascularization in ischaemic cardiomyopathy: Routine practice vs scientific evidence. Eur. Heart J. 2022, 43, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Vassiliki Cousoumbas, G.; Casella, G.; Di Pasquale, G. What is the role of coronary revascularization to recover the contractility of the dysfunctional heart? Eur. Heart J. Suppl. 2023, 25 (Suppl. B), B75–B78. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Hameed, I.; Khan, F.M.; Tam, D.Y.; Rahouma, M.; Yongle, R.; Naik, A.; Di Franco, A.; Demetres, M.; Petrie, M.C. Treatment strategies in ischaemic left ventricular dysfunction: A network meta-analysis. Eur. J. Cardio-Thoracic Surg. 2021, 59, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Maron, D.J.; Stone, G.W.; Hochman, J.S. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Circulation 2020, 142, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Boden, W.E.; Hueb, W.; Brooks, M.M.; Vlachos, H.E.A.; O’Fee, K.; Hardi, A.; Brown, D.L. Death and Myocardial Infarction Following Initial Revascularization Versus Optimal Medical Therapy in Chronic Coronary Syndromes with Myocardial Ischemia: A Systematic Review and Meta-Analysis of Contemporary Randomized Controlled Trials. J. Am. Heart Assoc. 2021, 10, e019114. [Google Scholar] [CrossRef]
- Navarese, E.P.; Lansky, A.J.; Kereiakes, D.J.; Kubica, J.; Gurbel, P.A.; Gorog, D.A.; Valgimigli, M.; Curzen, N.; Kandzari, D.E.; Bonaca, M.P. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: A systematic review and meta-analysis. Eur. Heart J. 2021, 42, 4638–4651. [Google Scholar] [CrossRef]
- Wolff, G.; Dimitroulis, D.; Andreotti, F.; Kołodziejczak, M.; Jung, C.; Scicchitano, P.; Devito, F.; Zito, A.; Occhipinti, M.; Castiglioni, B. Survival Benefits of Invasive Versus Conservative Strategies in Heart Failure in Patients with Reduced Ejection Fraction and Coronary Artery Disease: A Meta-Analysis. Circ. Heart Fail. 2017, 10, e003255. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lopes, R.D.; Alexander, K.P.; Stevens, S.R.; Reynolds, H.R.; Stone, G.W.; Piña, I.L.; Rockhold, F.W.; Elghamaz, A.; Lopez-Sendon, J.L.; Farsky, P.S.; et al. Initial Invasive Versus Conservative fromgement of Stable Ischemic Heart Disease in Patients with a History of Heart Failure or Left Ventricular Dysfunction: Insights from the ISCHEMIA Trial. Circulation 2020, 142, 1725–1735, Erratum in Circulation 2020, 142, e236. [Google Scholar]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 10 September 2023).
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, K.; Yamazaki, T.; Kitabatake, A.; Yamaguchi, T.; Kanmatsuse, K.; Kodama, I.; Takekoshi, N.; Tomoike, H.; Hori, M.; Matsuzaki, M.; et al. Japanese Stable Angina Pectoris Study Investigators. Percutaneous coronary intervention plus medical therapy reduces the incidence of acute coronary syndrome more effectively than initial medical therapy only among patients with low-risk coronary artery disease a randomized, comparative, multicenter study. JACC Cardiovasc. Interv. 2008, 1, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.L.; August, P.; Brooks, M.M.; Hardison, R.M.; Kelsey, S.F.; MacGregor, J.M.; Orchard, T.J.; Chaitman, B.R.; Genuth, S.M.; Goldberg, S.H.; et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med. 2009, 360, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Hueb, W.; Lopes, N.; Gersh, B.J.; Soares, P.R.; Ribeiro, E.E.; Pereira, A.C.; Favarato, D.; Rocha, A.S.; Hueb, A.C.; Ramires, J.A. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): A randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2010, 122, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Won, H.; Her, A.Y.; Kim, B.K.; Kim, Y.H.; Shin, D.H.; Kim, J.S.; Ko, Y.G.; Choi, D.; Kwon, H.M.; Jang, Y.; et al. Percutaneous Coronary Intervention Is More Beneficial Than Optimal Medical Therapy in Elderly Patients with Angina Pectoris. Yonsei Med. J. 2016, 57, 382–387. [Google Scholar] [CrossRef]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.J.; Fearon, W.F.; Barbato, E.; Tonino, P.A.L.; Engstrøm, T.; Kääb, S.; Dambrink, J.H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef]
- Cleland, J.G.; Calvert, M.; Freemantle, N.; Arrow, Y.; Ball, S.G.; Bonser, R.S.; Chattopadhyay, S.; Norell, M.S.; Pennell, D.J.; Senior, R. The Heart Failure Revascularisation Trial (HEART). Eur. J. Heart Fail. 2011, 13, 227–233. [Google Scholar] [CrossRef]
- Petrie, M.C.; Jhund, P.S.; She, L.; Adlbrecht, C.; Doenst, T.; Panza, J.A.; Hill, J.A.; Lee, K.L.; Rouleau, J.L.; Prior, D.L.; et al. Ten-Year Outcomes After Coronary Artery Bypass Grafting According to Age in Patients with Heart Failure and Left Ventricular Systolic Dysfunction: An Analysis of the Extended Follow-Up of the STICH Trial (Surgical Treatment for Ischemic Heart Failure). Circulation 2016, 134, 1314–1324. [Google Scholar] [CrossRef]
- Vergallo, R.; Liuzzo, G. The REVIVED-BCIS2 trial: Percutaneous coronary intervention vs. optimal medical therapy for stable patients with severe ischaemic cardiomyopathy. Eur. Heart J. 2022, 43, 4775–4776. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, P.M.; Giacomini, J.C.; Folland, E.D.; Parisi, A.F. Two- to three-year follow-up of patients with single-vessel coronary artery disease randomized to PTCA or medical therapy (results of a VA cooperative study). Veterans Affairs Cooperative Studies Program. ACME Investigators. Angioplasty Compared to Medicine. Am. J. Cardiol. 1998, 82, 1445–1450. [Google Scholar] [PubMed]
- Hambrecht, R.; Walther, C.; Möbius-Winkler, S.; Gielen, S.; Linke, A.; Conradi, K.; Erbs, S.; Kluge, R.; Kendziorra, K.; Sabri, O.; et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: A randomized trial. Circulation 2004, 109, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Maron, D.J.; O’Brien, S.M.; Fleg, J.L.; Kretov, E.I.; Briguori, C.; Kaul, U.; Reynolds, H.R.; Mazurek, T.; Sidhu, M.S.; et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N. Engl. J. Med. 2020, 382, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Vij, A.; Kassab, K.; Chawla, H.; Kaur, A.; Kodumuri, V.; Jolly, N.; Doukky, R. Invasive therapy versus conservative therapy for patients with stable coronary artery disease: An updated meta-analysis. Clin. Cardiol. 2021, 44, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Anthopolos, R.; Reynolds, H.R.; Bangalore, S.; Xu, Y.; O’Brien, S.M.; Mavromichalis, S.; Chang, M.; Contreras, A.; Rosenberg, Y.; et al. Survival After Invasive or Conservative Management of Stable Coronary Disease. Circulation 2023, 147, 8–19. [Google Scholar] [CrossRef]
- Bytyci, I.; Morina, D.; Bytyqi, S.; Bajraktari, G.; Henein, M.Y. Percutaneous Coronary Intervention Is Not Superior to Optimal Medical Therapy in Chronic Coronary Syndrome: A Meta-Analysis. J. Clin. Med. 2023, 12, 1395. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef]
- Boden, W.E.; Marzilli, M.; Crea, F.; Mancini, G.B.J.; Weintraub, W.S.; Taqueti, V.R.; Pepine, C.J.; Escaned, J.; Al-Lamee, R.; Gowdak, L.H.W. Evolving Management Paradigm for Stable Ischemic Heart Disease Patients: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 505–514. [Google Scholar] [CrossRef]
- Reed, S.C.; Dhir, N.; Widmer, R.J. Optimal cardiovascular medical therapy: Current guidelines and new developments. Proc. Bayl. Univ. Med. Cent. 2022, 35, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Gomes, A.C.; Azevedo, L.; Ahn, J.M.; Park, S.J.; Hamza, T.H.; Farkouh, M.E.; Serruys, P.W.; Milojevic, M.; Kappetein, A.P.; Stone, G.W.; et al. Compliance with Guideline-Directed Medical Therapy in Contemporary Coronary Revascularization Trials. J. Am. Coll. Cardiol. 2018, 71, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Hameed, I.; Farkouh, M.E.; Rahouma, M.; Naik, A.; Robinson, N.B.; Ruan, Y.; Demetres, M.; Biondi-Zoccai, G.; Angiolillo, D.J.; et al. Overall and Cause-Specific Mortality in Randomized Clinical Trials Comparing Percutaneous Interventions with Coronary Bypass Surgery: A Meta-analysis. JAMA Intern. Med. 2020, 180, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Liga, R.; Colli, A.; Taggart, D.P.; Boden, W.E.; De Caterina, R. Myocardial Revascularization in Patients with Ischemic Cardiomyopathy: For Whom and How. J. Am. Heart Assoc. 2023, 12, e026943. [Google Scholar] [CrossRef]

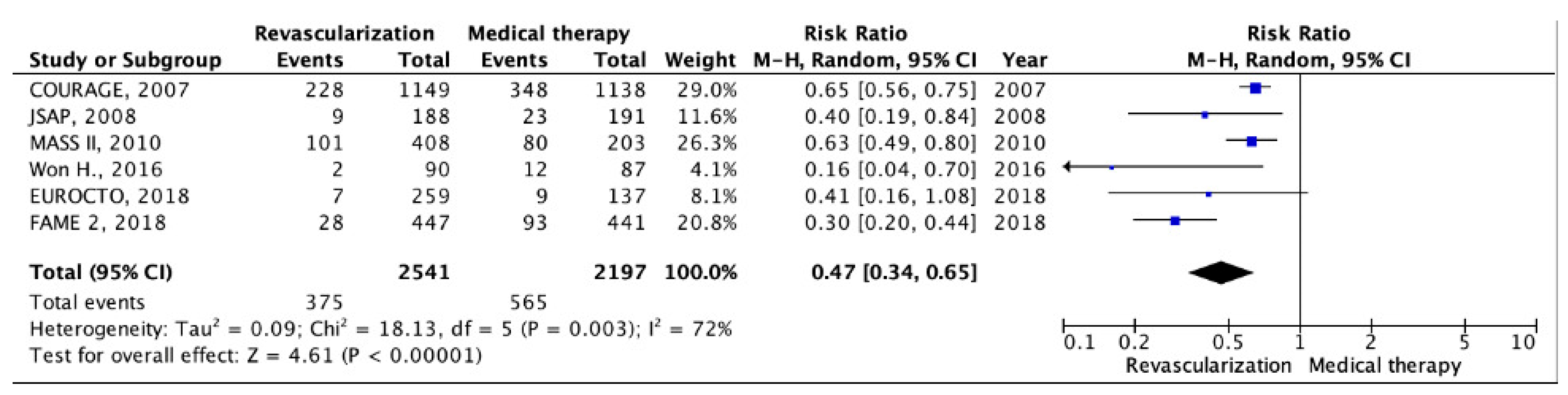
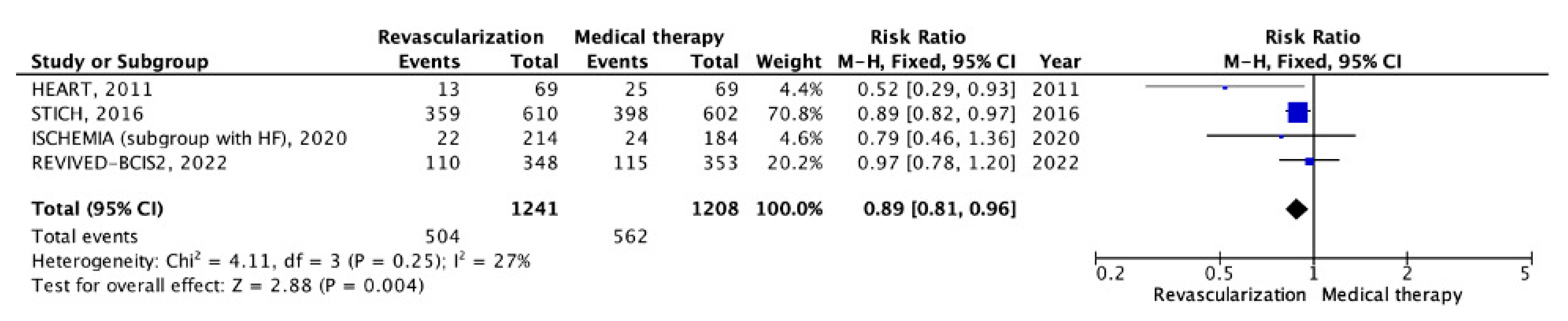

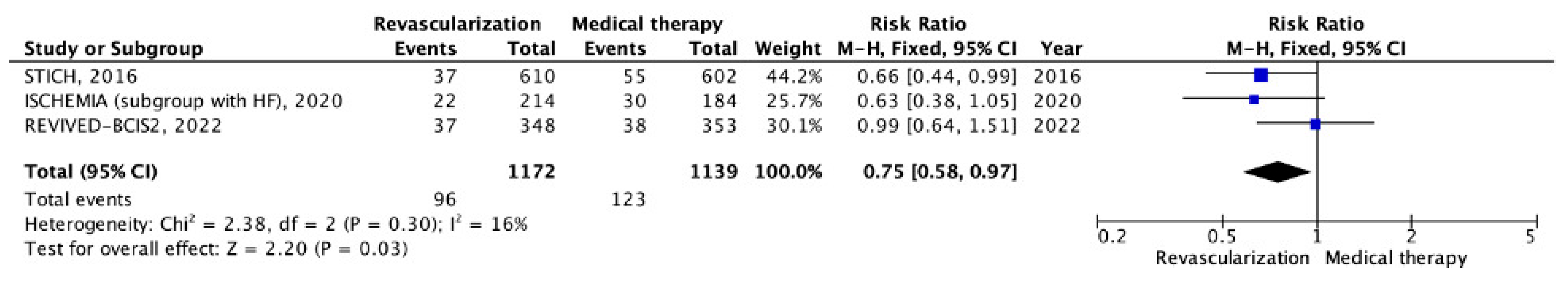
| Trials, Year Published (Region) | Study Years/Follow-Up | Study Group | Strategy Being Studied | Primary Endpoints | Secondary Endpoints | Number of Patients (n) |
|---|---|---|---|---|---|---|
| COURAGE, 2007 (North America), [13] | 1999–2004/ 4.6 | Chronic CHD, stenosis ≥70% in at least one proximal epicardial coronary artery and objective evidence of myocardial ischemia or at least one coronary stenosis of ≥80% and classic angina without provocative testing | OMT, PCI | Death from any cause and nonfatal myocardial infarction | Composite of death, MI, stroke, and hospitalization for unstable angina with negative biomarkers, quality of life | 2287 |
| JSAP, 2008 (Japan), [14] | 2002–2004/ 3.3 | Chronic CHD low-risk consisting of one- or two-vessel disease, stenosis ≥75%, and objective evidence of myocardial ischemia | OMT, PCI | Death (total death, cardiac death, and sudden death), acute coronary syndrome (MI or UAP), CVA (cerebral infarction or cerebral hemorrhage), and emergency hospitalization. | Evaluation of the angina severity grade 1 month, 6 months, 1 year, 2 years, and 3 years after registration and elective repeat revascularization. | 384 |
| BARI 2D, 2009 (USA, Europe), [15] | 2001–2005/ 5.3 | Both type 2 diabetes and coronary artery disease, ≥50% stenosis of a major epicardial coronary artery associated with a positive stress test or ≥70% stenosis of a major epicardial coronary artery and classic angina | OMT, PCI, CABG | Death from any cause | Composite of death, MI, or stroke | 2368 |
| MASS II, 2010 (Brazil), [16] | 1995–2000/ 10 | Chronic CHD, multivessel coronary stenosis of more than 70% by means of visual assessment and documented ischemia (class II or III) | OMT, PCI, CABG | Overall death, MI, and angina that required mechanical revascularization | Angina status, death due to a cardiac cause, and a cerebrovascular accident. | 611 |
| HEART, 2011 (United Kingdom), [20] | 2001–2004/ 4.9 | Heart failure, coronary artery disease, and LV EF < 35%, which had at least five viable segments with reduced contractility | OMT, PCI, CABG | Death from any cause | - | 138 |
| Won H., 2016 (Republic of Korea), [17] | 2010–2012/ 1 | Chronic CHD, stenosis in at least one proximal epicardial coronary artery (diameter stenosis of ≥70%) | OMT, PCI | Death from any cause, MI, stroke, repeat revascularization. | - | 177 |
| STICH, 2016 (USA, Canada, Europe), [21] | 2002–2007/ 10 | Chronic CHD that was amenable to CABG and LV EF of 35% or lower | OMT, CABG | Death from any cause | Death from cardiovascular causes, death from any cause or hospitalization for cardiovascular causes, death from any cause or hospitalization for heart failure, death from any cause or hospitalization for any cause, and death from any cause or revascularization. | 1212 |
| FAME 2, 2018 (Europe, North America), [19] | 2010–2012/ 5 | Chronic angina or documented silent ischemia that had at least one stenosis with 50% of its diameter in a large epicardial artery | OMT, PCI | Composite of death from any cause, MI, or urgent revascularization | Components of the primary endpoint as well as death from cardiac causes, any revascularization, stroke, and stent thrombosis | 888 |
| EUROCTO, 2018 (France), [18] | 2012–2015/ 1 | Chronic CHD, angina + ≥1 chronic coronary total occlusions | OMT, PCI | Change in health status subscales as assessed by SAQ | Changes from baseline to 12 months of EQ-5D and the Canadian Cardiology Society classification, and major cardiac adverse events, stent thrombosis, cerebrovascular events, and hospitalization for cardiac reasons | 396 |
| ISCHEMIA, 2020 (USA), [11] | 2012–2018/ 3.3 | Chronic CHD, stress testing showed moderate or severe reversible ischemia on imaging tests or severe ischemia on exercise tests without imaging | OMT, PCI, CABG | Composite of death from cardiovascular causes, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest | Composite of death from cardiovascular causes or MI and angina-related quality of life. | 5179 |
| ISCHEMIA, left ventricular dysfunction, 2020 (USA), [10] | 2012–2018/ 3.2 | Chronic CHD, LV EF 35–45% | OMT, PCI, CABG | Composite of cardiovascular death, MI, resuscitated cardiac arrest, or hospitalization for unstable angina or heart failure | All-cause death, cardiovascular death, MI, hospitalization for UAP, hospitalization for heart failure | 398 |
| REVIVED-BCIS2, 2022 (United Kingdom), [22] | 2013–2020/ 8.5 | Chronic CHD, multivessel coronary stenosis, LV EF of 35% or less | OMT, PCI | Composite outcome was death from any cause or hospitalization for heart failure | Components of the primary outcome, death from cardiovascular causes, appropriate ICD therapy (antitachycardia pacing or shocks, or both, for either ventricular tachycardia or ventricular fibrillation), MI, unplanned revascularization, serial NT-proBNP levels, the Canadian Cardiovascular Society angina class, and major bleeding | 700 |
| Name Research, Year | All-Cause Mortality | Cardiovascular Death | Myocardial Infarction | Cerebrovascular Accidents | Unplanned Revascularization | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OMT | INV | OMT | INV | OMT | INV | OMT | INV | OMT | INV | |
| COURAGE, 2007, [13] | 95 (8.3) | 85 (7.6) | 25 | 23 | 128 (12.3) | 143 (13.2) | 14 (1.8) | 22 (2.1) | 348 (32.6) | 228 (21.1) |
| JSAP, 2008, [14] | 7 (3.9) | 6 (2.9) | 3 | 2 | 7 (3.8) | 3 (1.6) | 2 (1.1) | 2 (0.6) | 23 (11.7) | 9 (5.0) |
| BARI 2D, 2009, [15] | 161 (13.5) | 155 (13.2) | nd | nd | 138 (11.6) | 118 (10) | 33 (2.8) | 30 (2.6) | - | - |
| MASS II, 2010, [16] | 63 (31) | PCI—49 (24.1) CABG—51 (25.1) | 42 (20.7) | PCI—29 (14.3) CABG—22 (10.8) | 42 (20.7) | PCI—27 (13.3) CABG—20 (10.3) | 14 (6.9) | PCI—11 (5.4) CABG—17 (8.4) | 80 (39.4) | PCI—86 (41.9) CABG—15 (17.4) |
| Won H., 2016, [17] | 4 (4.6) | 6 (6.7) | 2 (2.3) | 1 (1.1) | 3 (3.4) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 12 (13.8) | 2 (2.2) |
| FAME 2, 2018, [19] | 23 (5.2) | 23 (5.1) | 7 (1.6) | 11 (2.5) | 53 (12.0) | 36 (8.1) | 7 (1.6) | 12 (2.7) | 93 (21.1) | 28 (6.3) |
| EUROCTO, 2018, [18] | 0 | 2 (0.8) | 0 | 2 (0.8) | 0 | 5 (1.9) | 1 (0.7) | 2 (0.8) | 9 (6.7) | 7 (2.9) |
| ISCHEMIA, 2020, [11] | 144 (5.6) | 145 (5.6) | 111 | 92 | 233 (9.0) | 210 (8.1) | 38 | 45 | nd | nd |
| Name Research, Year | All-Cause Mortality | Cardiovascular Death | Myocardial Infarction | Cerebrovascular Accidents | Unplanned Revascularization | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OMT | INV | OMT | INV | OMT | INV | OMT | INV | OMT | INV | |
| HEART, 2011, [20] | 25 (37) | 13 (29) | nd | nd | nd | nd | nd | nd | nd | nd |
| STICH, 2016, [21] | 398 (66.1) | 359 (58.9) | 297 (49.3) | 247 (40.5) | 55 (9.1) | 37 (6.1) | 41 (6.8) | 47 (7.7) | 50 (8.3) | 43 (7.0) |
| ISCHEMIA, left ventricular dysfunction, 2020 [10] | 24 (13.3) | 22 (10.2) | 23 (12.7) | 14 (6.7) | 30 (16.5) | 22 (10.5) | nd | nd | nd | nd |
| REVIVED-BCIS2, 2022 [22] | 115 (32.6) | 110 (31.7) | 88 (24.9) | 76 (21.9) | 38 (10.8) | 37 (10.7) | nd | nd | 37 (10.5) | 10 (2.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golukhova, E.Z.; Slivneva, I.V.; Kozlova, O.S.; Berdibekov, B.S.; Skopin, I.I.; Merzlyakov, V.Y.; Baichurin, R.K.; Sigaev, I.Y.; Keren, M.A.; Alshibaya, M.D.; et al. Treatment Strategies for Chronic Coronary Heart Disease with Left Ventricular Systolic Dysfunction or Preserved Ejection Fraction—A Systematic Review and Meta-Analysis. Pathophysiology 2023, 30, 640-658. https://doi.org/10.3390/pathophysiology30040046
Golukhova EZ, Slivneva IV, Kozlova OS, Berdibekov BS, Skopin II, Merzlyakov VY, Baichurin RK, Sigaev IY, Keren MA, Alshibaya MD, et al. Treatment Strategies for Chronic Coronary Heart Disease with Left Ventricular Systolic Dysfunction or Preserved Ejection Fraction—A Systematic Review and Meta-Analysis. Pathophysiology. 2023; 30(4):640-658. https://doi.org/10.3390/pathophysiology30040046
Chicago/Turabian StyleGolukhova, Elena Zelikovna, Inessa Viktorovna Slivneva, Olga Sergeevna Kozlova, Bektur Shukurbekovich Berdibekov, Ivan Ivanovich Skopin, Vadim Yuryevich Merzlyakov, Renat Kamilyevich Baichurin, Igor Yuryevich Sigaev, Milena Abrekovna Keren, Mikhail Durmishkhanovich Alshibaya, and et al. 2023. "Treatment Strategies for Chronic Coronary Heart Disease with Left Ventricular Systolic Dysfunction or Preserved Ejection Fraction—A Systematic Review and Meta-Analysis" Pathophysiology 30, no. 4: 640-658. https://doi.org/10.3390/pathophysiology30040046
APA StyleGolukhova, E. Z., Slivneva, I. V., Kozlova, O. S., Berdibekov, B. S., Skopin, I. I., Merzlyakov, V. Y., Baichurin, R. K., Sigaev, I. Y., Keren, M. A., Alshibaya, M. D., Marapov, D. I., & Arzumanyan, M. A. (2023). Treatment Strategies for Chronic Coronary Heart Disease with Left Ventricular Systolic Dysfunction or Preserved Ejection Fraction—A Systematic Review and Meta-Analysis. Pathophysiology, 30(4), 640-658. https://doi.org/10.3390/pathophysiology30040046





