The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characteristics
2.2. Evaluation of Cognitive Function
2.3. Evaluation of Neuropathy and Foot Alterations
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
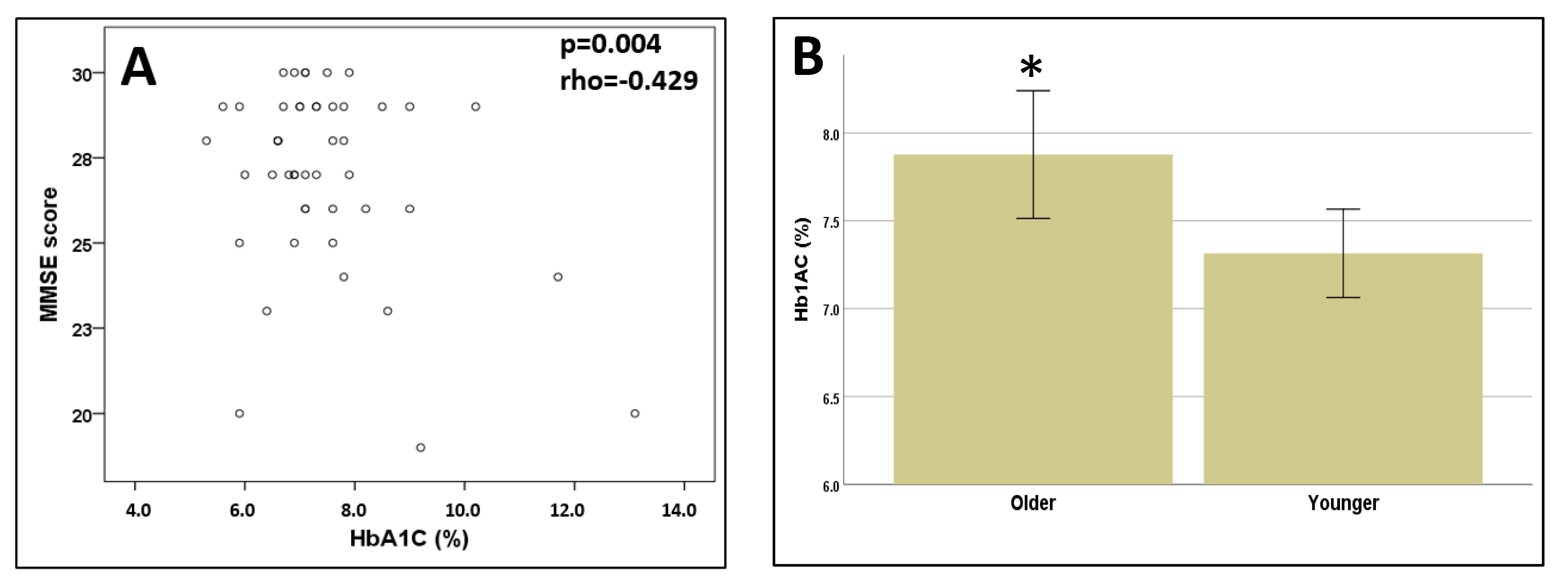
3.2. Cognitive Function
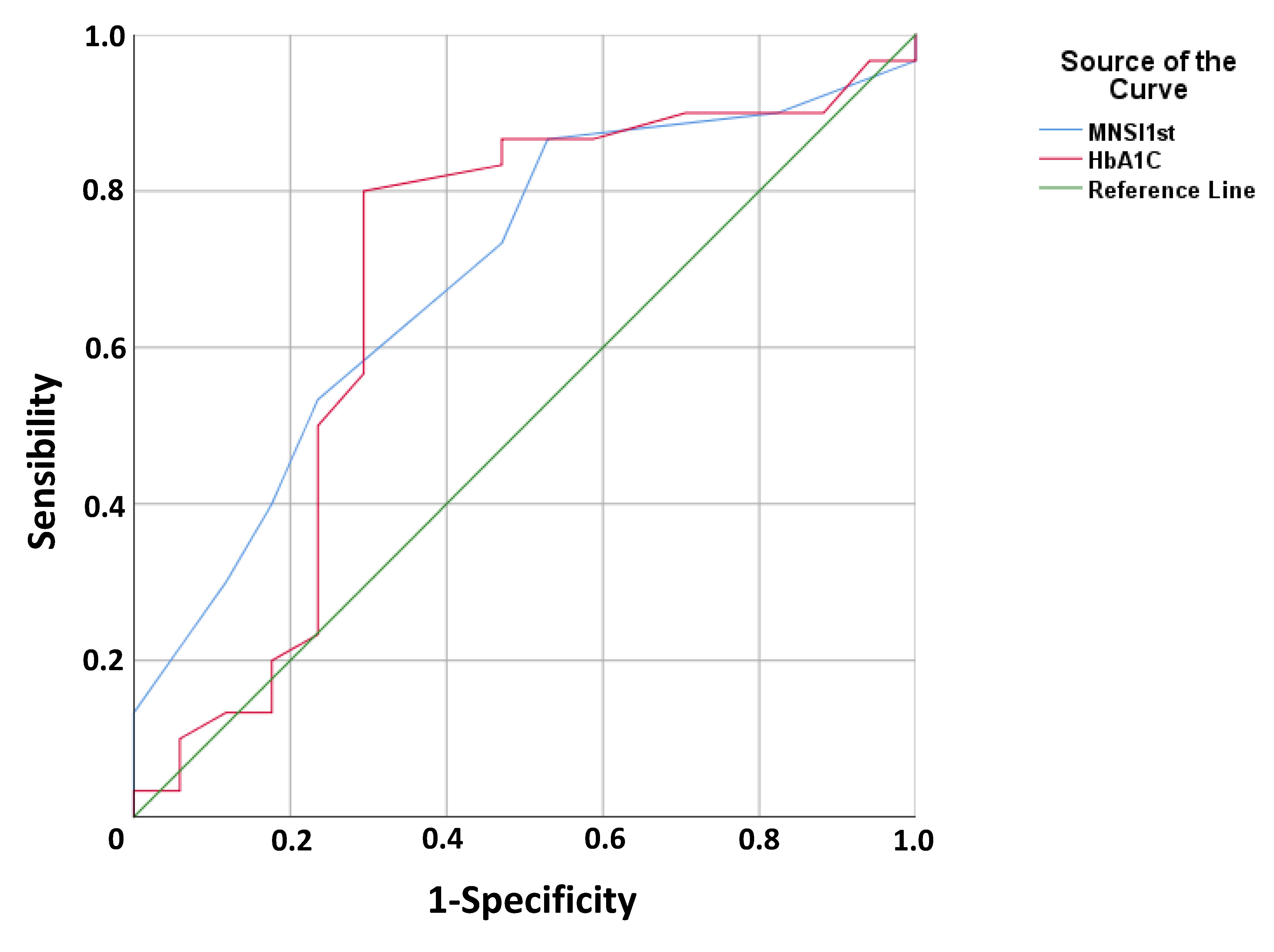
3.3. Relationship between Foot Alterations and Cognitive Function
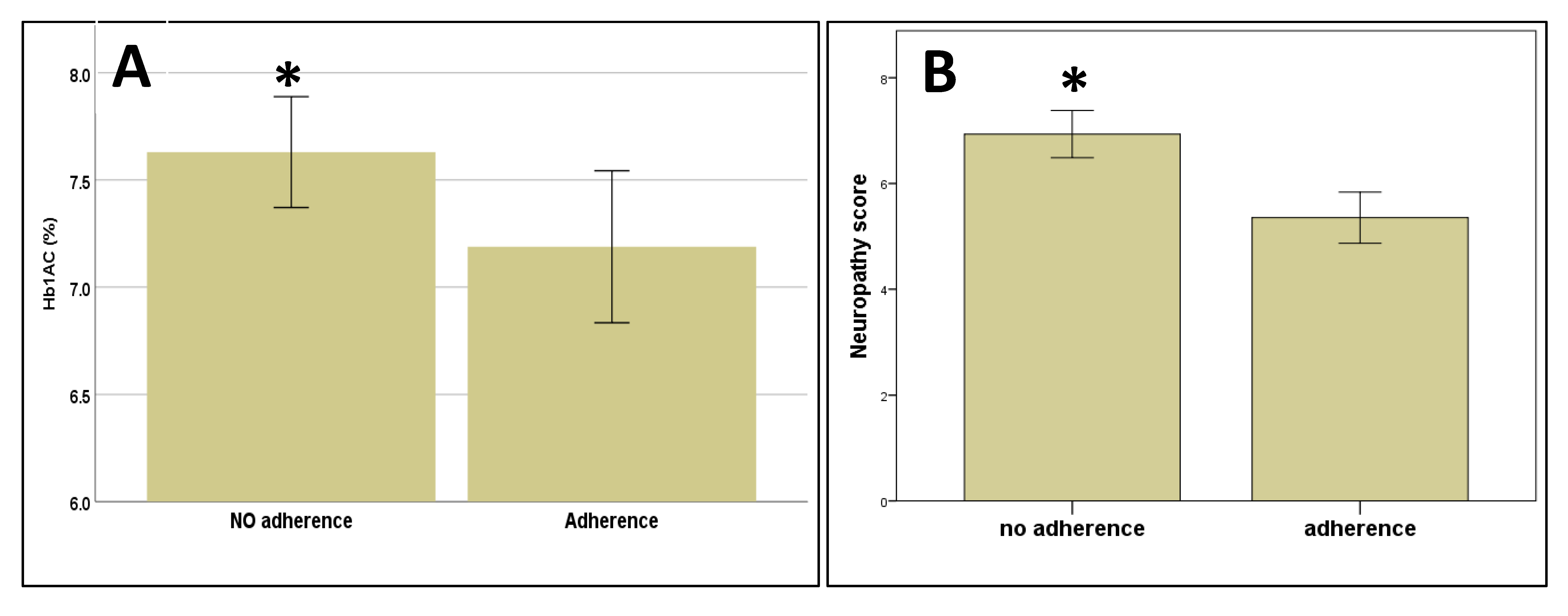
3.4. Adherence to Treatment at the Follow-Up Visit
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Katsilambros, N. Atlas of the Diabetic Foot; Wiley: Hoboken, NJ, USA, 2003; p. 231. [Google Scholar]
- Lavery, L.A.; Hunt, N.A.; Ndip, A.; Lavery, D.C.; Van Houtum, W.; Boulton, A.J.M. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 2010, 33, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, K.; Berhane, T.; Hamilton, M.; Chandra, A.P.; Falhammar, H. Mortality in patients with diabetic foot ulcer: A retrospective study of 513 cases from a single Centre in the Northern Territory of Australia. BMC Endocr. Disord. 2019, 19, 1. [Google Scholar] [CrossRef]
- Bus, S.A. Priorities in offloading the diabetic foot. Diabetes Metab. Res. Rev. 2012, 28, 54–59. [Google Scholar] [CrossRef]
- Taub, L.F.M. Concordance of provider recommendations with American Diabetes Association’s Guidelines. J. Am. Acad. Nurse Pract. 2006, 18, 124–133. [Google Scholar] [CrossRef]
- Natovich, R.; Harman-Boehm, I.; Margalit, D.; Cukierman-Yaffe, T.; Kushnir, T. Adherence to self-care among individuals with diabetes with and without diabetic foot complications: Objective and self-report measures. Diabetes Manag. 2017, 7, 234. [Google Scholar]
- Bus, S.A.; van Netten, J.J. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 195–200. [Google Scholar] [CrossRef]
- Dubský, M.; Jirkovská, A.; Bem, R.; Fejfarová, V.; Skibová, J.; Schaper, N.C.; Lipsky, B.A. Risk factors for recurrence of diabetic foot ulcers: Prospective follow-up analysis in the Eurodiale subgroup. Int. Wound J. 2013, 10, 555–561. [Google Scholar] [CrossRef]
- Bakker, K.; Apelqvist, J.; Lipsky, B.A.; Van Netten, J.J.; Schaper, N.C. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: Development of an evidence-based global consensus. Diabetes Metab. Res. Rev. 2016, 32, 2–6. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Pérez-Ros, P.; Marténez-Arnau, F.M.; Julían-Rochina, I.; Cauli, O.; Fm, M.-A. Neuro-Psychiatric Alterations in Patients with Diabetic Foot Syndrome. CNS Neurol. Disord. Drug Targets 2019, 18, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Schneider, A.L.; Biessels, G.J.; Touradji, P.; Hill-Briggs, F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. JINS 2014, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Flores, E.; Cauli, O. Quality of life in individuals with diabetic foot syndrome. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Pozzessere, G.; Rizzo, P.A.; Valle, E.; Mollica, M.A.; Meccia, A.; Morano, S.; Di Mario, U.; Andreani, D.; Morocutti, C.; Mario, U.D. Early detection of neurological involvement in IDDM and NIDDM. Multimodal evoked potentials versus metabolic control. Diabetes Care 1988, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Haroon, E.; Darwin, C.; Pham, D.; Ajilore, O.; Rodriguez, G.; Mintz, J. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J. Magn. Reson. Imaging 2008, 27, 14–19. [Google Scholar] [CrossRef]
- De Bresser, J.; Tiehuis, A.M.; Van Den Berg, E.; Reijmer, Y.D.; Jongen, C.; Kappelle, L.J.; Mali, W.P.; Viergever, M.A.; Biessels, G.J. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010, 33, 1309–1314. [Google Scholar] [CrossRef]
- Manschot, S.M.; Biessels, G.J.; De Valk, H.; Algra, A.; Rutten, G.E.H.M.; Van Der Grond, J.; Kappelle, L.J.; Utrecht Diabetic Encephalopathy Study Group. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia 2007, 50, 2388–2397. [Google Scholar] [CrossRef]
- Van Duinkerken, E.; Klein, M.; Schoonenboom, N.S.M.; Hoogma, R.P.L.M.; Moll, A.C.; Snoek, F.J.; Stam, C.J.; Diamant, M. Functional brain connectivity and neurocognitive functioning in patients with long-standing type 1 diabetes with and without microvascular complications: A magnetoencephalography study. Diabetes 2009, 58, 2335–2343. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet 2012, 379, 2291–2299. [Google Scholar] [CrossRef]
- Ryan, C.; Vega, A.; Drash, A. Cognitive Deficits in Adolescents Who Developed Diabetes Early in Life. Pediatrics 1985, 75, 921–927. [Google Scholar]
- Ryan, C.M. Diabetes and brain damage: More (or less) than meets the eye? Diabetologia 2006, 49, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, A.; Xu, W.; Rizzuto, D.; Ferrari, C.; Whisstock, C.; Brocco, E.; Maggi, S. Cognitive functioning among patients with diabetic foot. J. Diabetes Its Complicat. 2014, 28, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Corbett, C.; Jolley, J.; Barson, E.; Wraight, P.; Perrin, B.; Fisher, C. Cognition and understanding of neuropathy of inpatients admitted to a specialized tertiary diabetic foot unit with diabetes-related foot ulcers. Int. J. Low. Extrem. Wounds 2019, 18, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Yaffe, K.; Cauley, J.A.; Rolka, D.B.; Blackwell, T.L.; Narayan, K.V.; Cummings, S.R. Is Diabetes Associated With Cognitive Impairment and Cognitive Decline Among Older Women? Arch. Intern. Med. 2000, 160, 174–180. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Tang, M.X.; Stern, Y.; Shea, S.; Mayeux, R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 2001, 154, 635–641. [Google Scholar] [CrossRef]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased Risk of Type 2 Diabetes in Alzheimer Disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef]
- Huntley, A.C. Cutaneous Manifestations of Diabetes Mellitus. Dermatol. Clin. 1989, 7, 531–546. [Google Scholar] [CrossRef]
- Perez, M.I.; Kohn, S.R. Cutaneous manifestations of diabetes mellitus. J. Am. Acad. Dermatol. 1994, 30, 519–531. [Google Scholar] [CrossRef]
- Stechmiller, J.K.; Lyon, D.; Schultz, G.; Gibson, D.J.; Weaver, M.T.; Wilkie, D.; Ferrell, A.V.; Whitney, J.; Kim, J.; Millan, S.B. Biobehavioral Mechanisms Associated with Nonhealing Wounds and Psychoneurologic Symptoms (Pain, Cognitive Dysfunction, Fatigue, Depression, and Anxiety) in Older Individuals with Chronic Venous Leg Ulcers. Biol. Res. Nurs. 2019, 21, 407–419. [Google Scholar] [CrossRef]
- Fried, R.G.; Gupta, M.A.; Gupta, A.K. Depression and skin disease. Dermatol. Clin. 2005, 23, 657–664. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K. Psychodermatology: An update. J. Am. Acad. Dermatol. 1996, 34, 1030–1046. [Google Scholar] [CrossRef]
- Niwa, H.; Koumoto, C.; Shiga, T.; Takeuchi, J.; Mishima, S.; Segawa, T.; Atsumi, T.; Shimizu, C.; Koike, T.; Yoshioka, N. Clinical analysis of cognitive function in diabetic patients by MMSE and SPECT. Diabetes Res. Clin. Pract. 2006, 72, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Zheng, Z.; Goo, B.; Cho, S.B. Antifungal effects of a 1444-nm neodymium:Yttrium-aluminum-garnet laser on onychomycosis: A pilot study. J. Dermatol. Treat. 2014, 25, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Laosa, O.; Mañas, L.R. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef]
- Dash, S.K. Cognitive impairment and diabetes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.R.; Wong, C.K.; Yerovinkina, M.; Spindler, S.J.; See, A.S.; Panjaki, S.; Loven, S.L.; D’Andrea, R.F.; Nowygrod, R. A Meta-analysis of Long-term Mortality and Associated Risk Factors following Lower Extremity Amputation. Ann. Vasc. Surg. 2017, 42, 322–327. [Google Scholar] [CrossRef]
- Guerchet, M.; Aboyans, V.; Nubukpo, P.; Lacroix, P.; Clément, J.-P.; Preux, P.-M. Ankle-brachial index as a marker of cognitive impairment and dementia in general population. A systematic review. Atherosclerosis 2011, 216, 251–257. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Barrett-Connor, E.; Gildengorin, G.; Yaffe, K. Change in cognitive function by glucose tolerance status in older adults: A 4-year prospective study of the Rancho Bernardo Study cohort. Arch. Intern. Med. 2004, 164, 1327–1333. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch. Clin. Neuropsychol. 2008, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Molloy, D.W.; Alemayehu, E.; Roberts, R. Reliability of a standardized mini-mental state examination compared with the traditional mini-mental state examination. Am. J. Psychiatry 1991, 148, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, E.; Monami, M.; Dicembrini, I.; Piselli, A.; Porta, M. Achieving HbA1c targets in clinical trials and in the real world: A systematic review and meta-analysis. J. Endocrinol. Investig. 2014, 37, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, B. Assessment scales in dementia. Ther. Adv. Neurol. Disord. 2012, 5, 349–358. [Google Scholar] [CrossRef]
- Feldman, E.L.; Stevens, M.J.; Thomas, P.K.; Brown, M.B.; Canal, N.; Greene, D.A. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994, 17, 1281–1289. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA J. Am. Med. Assoc. 1993, 269, 2239–2386. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.X.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Papandonatos, G.D.; Ott, B.R.; Davis, J.D.; Barco, P.P.; Carr, D.B. Clinical utility of the trail-making test as a predictor of driving performance in older adults. J. Am. Geriatr. Soc. 2015, 63, 2358–2364. [Google Scholar] [CrossRef]
- Sastre, A.A.; Vernooij, R.W.; Harmand, M.G.-C.; Martínez, G. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst. Rev. 2017, 6, CD003804. [Google Scholar] [CrossRef]
- Iversen, M.M.; Midthjell, K.; Tell, G.S.; Moum, T.; Østbye, T.; Nortvedt, M.W.; Uhlving, S.; Hanestad, B.R. The association between history of diabetic foot ulcer, perceived health and psychological distress: The Nord-Trøndelag Health Study. BMC Endocr. Disord. 2009, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Kloos, C.; Hagen, F.; Lindloh, C.; Braun, A.; Leppert, K.; Müller, N.; Wolf, G.; Müller, U.A. Cognitive function is not associated with recurrent foot ulcers in patients with diabetes and neuropathy. Diabetes Care 2009, 32, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Soldera, A.L.; Cury, B.; Meireles, C.; Kupfer, R. Is cognitive impairment associated with the presence and severity of peripheral neuropathy in patients with type 2 diabetes mellitus? Diabetol. Metab. Syndr. 2015, 7, 51. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Morales-Asencio, J.M.; Cervera-Marín, J.A.; Labajos-Manzanares, M.T.; Gijon-Nogueron, G. Development, validation and psychometric analysis of the diabetic foot self-care questionnaire of the University of Malaga, Spain (DFSQ-UMA). J. Tissue Viability 2015, 24, 24–34. [Google Scholar] [CrossRef]
- Faria, C.D.A.; Alves, H.V.D.; Charchat-Fichman, H. The most frequently used tests for assessing executive functions in aging. Dement. Neuropsychol. 2015, 9, 149–155. [Google Scholar] [CrossRef]
- Pappas, C.; Small, B.J.; Andel, R.; Laczó, J.; Parizkova, M.; Lerch, O.; Hort, J. Blood Glucose Levels May Exacerbate Executive Function Deficits in Older Adults with Cognitive Impairment. J. Alzheimer’s Dis. 2019, 67, 81–89. [Google Scholar] [CrossRef]
- Aung, P.P.; Strachan, M.W.J.; Frier, B.M.; Butcher, I.; Deary, I.J.; Price, J.F. Severe hypoglycaemia and late-life cognitive ability in older people with Type2 diabetes: The Edinburgh Type2 Diabetes Study. Diabet. Med. 2012, 29, 328–336. [Google Scholar] [CrossRef]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 2005, 26, 11–16. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; di Sciacca, R.; Pinto, A.; Licata, G. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008, 14, 3574–3589. [Google Scholar] [CrossRef]
- Zhou, H.; Al-Ali, F.; Rahemi, H.; Kulkarni, N.; Hamad, A.; Ibrahim, R.; Talal, T.; Najafi, B. Hemodialysis Impact on Motor Function beyond Aging and Diabetes-Objectively Assessing Gait and Balance by Wearable Technology. Sensors 2018, 18, 3939. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, D.; Tovaruela-Carrión, N.; López-López, D.; Palomo-López, P.; Romero-Morales, C.; Navarro-Flores, E.; Calvo-Lobo, C. Foot disorders in the elderly: A mini-review. Disease-a-Month 2018, 64, 64–91. [Google Scholar] [CrossRef] [PubMed]
- Chicharro-Luna, E.; Pomares-Gómez, F.J.; Ortega-Ávila, A.B.; Marchena-Rodríguez, A.; Blanquer-Gregori, J.F.J.; Navarro-Flores, E. Predictive model to identify the risk of losing protective sensibility of the foot in patients with diabetes mellitus. Int. Wound J. 2020, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Flores, E.; Gijón-Noguerón, G.; Cervera-Marín, J.A.; Labajos-Manzanares, M.T. Assessment of Foot Self-Care in Patients With Diabetes: Retrospective Assessment (2008–2014). Foot Ankle Spec. 2015, 8, 406–412. [Google Scholar] [CrossRef]
- Uckay, I.; Gariani, K.; Pataky, Z.; Lipsky, B.A. Diabetic foot infections: State-of-the-art. Diabetes Obes. Metab. 2014, 16, 305–316. [Google Scholar] [CrossRef]
- Koga, M.; Matsumoto, S.; Saito, H.; Kasayama, S. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr. J. 2006, 53, 387–391. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38, 8–16. [Google Scholar] [CrossRef]
- Ribu, L.; Hanestad, B.R.; Moum, T.; Birkeland, K.; Rustoen, T. Health-related quality of life among patients with diabetes and foot ulcers: Association with demographic and clinical characteristics. J. Diabetes Complicat. 2007, 21, 227–236. [Google Scholar] [CrossRef]
- Apelqvist, J.; Bakker, K.; Van Houtum, W.H.; Nabuurs-Franssen, M.H.; Schaper, N.C.; on behalf of the International Working Group on the Diabetic Foot. International consensus and practical guidelines on the management and the prevention of the diabetic foot. Diabetes/Metabolism Res. Rev. 2000, 16, S84–S92. [Google Scholar] [CrossRef]
- Sodi, R.; McKay, K.; Dampetla, S.; Pappachan, J.M. Monitoring glycaemic control in patients with diabetes mellitus. BMJ 2018, 363, k4723. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Al Derwish, M.; Ouizi, S.; Youssef, A.M.; Subhani, S.N.; Ibrahim, H.M.; Alamri, B.N. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS ONE 2015, 10, e0124446. [Google Scholar] [CrossRef] [PubMed]
- Iwase, M.; Fujii, H.; Nakamura, U.; Ohkuma, T.; Ide, H.; Jodai-Kitamura, T.; Sumi, A.; Komorita, Y.; Yoshinari, M.; Kitazono, T. Incidence of diabetic foot ulcer in Japanese patients with type 2 diabetes mellitus: The Fukuoka diabetes registry. Diabetes Res. Clin. Pract. 2018, 137, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Grüneberg, C.; Thiel, C. German translation, cross-cultural adaptation and diagnostic test accuracy of three frailty screening tools: PRISMA-7, FRAIL scale and Groningen Frailty Indicator. Z. Gerontol. Geriatr. 2018, 51, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Fabrício-Wehbe, S.C.C.; Schiaveto, F.V.; Vendrusculo, T.R.P.; Haas, V.J.; Dantas, R.A.S.; Rodrigues, R.A.P. Adaptación cultural y validez de la Edmonton frail scale—EFS en una muestra de ancianos Brasileños. Rev. Lat. Am. Enferm. 2009, 17, 1043–1049. [Google Scholar] [CrossRef]
- Fabrício-Wehbe, S.C.C.; Cruz, I.R.; Haas, V.J.; Diniz, M.A.; Dantas, R.A.S.; Rodrigues, R.A.P. Reprodutibilidade da versão Brasileira adaptada da Edmonton Frail Scale para idosos residentes na comunidade. Rev. Lat. Am. Enferm. 2013, 21, 1330–1336. [Google Scholar] [CrossRef]
| Cutaneous Impairment and Appearance of Feet According to the Michigan Neuropathy Screening Instrument | |
|---|---|
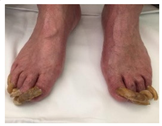 | Nail diseases: diseases of the nail plate and tissues surrounding it. |
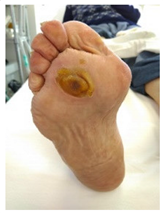 | Hyperkeratosis: hyperkeratosis is caused by excessive mechanical loading. The hyperkeratosis (or callus) is thickened skin leading to a further increase in the loading of the foot, often with subcutaneous hemorrhage and eventually skin ulceration. |
 | Skin care and dryness (xerosis): abnormal dryness of foot tissues caused by a lack of moisture in the skin (which can be treated by emollient therapy). |
 | Amputation: resection of a segment of a limb through a bone or through a joint. |
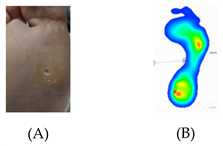 | Overload areas: (A) a mechanical stress in some areas, the response to which is usually represented by a thickened skin callus; (B) peak pressures evaluated with baropodometric platform. |
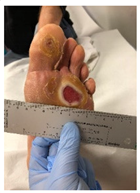 | Foot ulceration: a break in the skin of the foot that involves at least the epidermis and part of the dermis. We considered the foot ulceration in people with currently or previously diagnosed diabetes mellitus. |
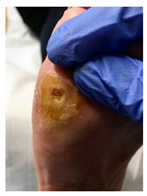 | Pre ulcerative lesions: a foot lesion with a high risk of developing into a foot ulcer, such as intra- or subcutaneous hemorrhage, blister, or skin fissure not penetrating the dermis. |
| Age | Mean ± SD: 70.8 ± 1.7 years (Minimum 42–Maximum 88) |
| Sex | 27 male; 27 female |
| Educational level: number of individuals. | Primary school: 40 Secondary school: 6 University: 1 |
| Type of Diabetes | Type I: 3 individuals Type II: 44 individuals |
| Years diagnosed with diabetes | Mean ± SD: 18.8 ± 1.7 years (Minimum 2–Maximum 47) |
| Glycated hemoglobin (HbA1C; %) | Mean ± SE: 7.5% ± 0.2% (Minimum 5.3–Maximum 13.1) |
| BMI | Mean ± SD: 27.8 ± 0.7 (Minimum 20.0–Maximum 37.9) |
| Type of foot alterations | Nail diseases: 31 of 47 individuals (64.6%) Hyperkeratosis: 22 of 47 individuals (45.8%) Skin care and dryness (xerosis): 37 of 47 individuals (77.1%) Amputation: 10 of 47 individuals (20.8%) Overload areas: 23 of 47 individuals (47.9%) Foot ulceration: 5 of 47 individuals (10.4%) Pre-ulcerative lesions: 3 of 47 individuals (6.3%) |
| Neuropathy score | Mean ± SD: 6.4 ± 0.4 (Minimum 2–Maximum 13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brognara, L.; Volta, I.; Cassano, V.M.; Navarro-Flores, E.; Cauli, O. The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin. Pathophysiology 2020, 27, 14-27. https://doi.org/10.3390/pathophysiology27010003
Brognara L, Volta I, Cassano VM, Navarro-Flores E, Cauli O. The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin. Pathophysiology. 2020; 27(1):14-27. https://doi.org/10.3390/pathophysiology27010003
Chicago/Turabian StyleBrognara, Lorenzo, Iacopo Volta, Vito Michele Cassano, Emmanuel Navarro-Flores, and Omar Cauli. 2020. "The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin" Pathophysiology 27, no. 1: 14-27. https://doi.org/10.3390/pathophysiology27010003
APA StyleBrognara, L., Volta, I., Cassano, V. M., Navarro-Flores, E., & Cauli, O. (2020). The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin. Pathophysiology, 27(1), 14-27. https://doi.org/10.3390/pathophysiology27010003








