Tetrahydrobiopterin (BH4) Supplementation Prevents the Cardiorenal Effects of Diabetes in Mice by Reducing Oxidative Stress, Inflammation and Fibrosis
Abstract
1. Introduction
2. Methods
2.1. Experimental Model and Protocol
2.2. Sample Collection and Storage
2.3. Plasma Biochemical Measurements
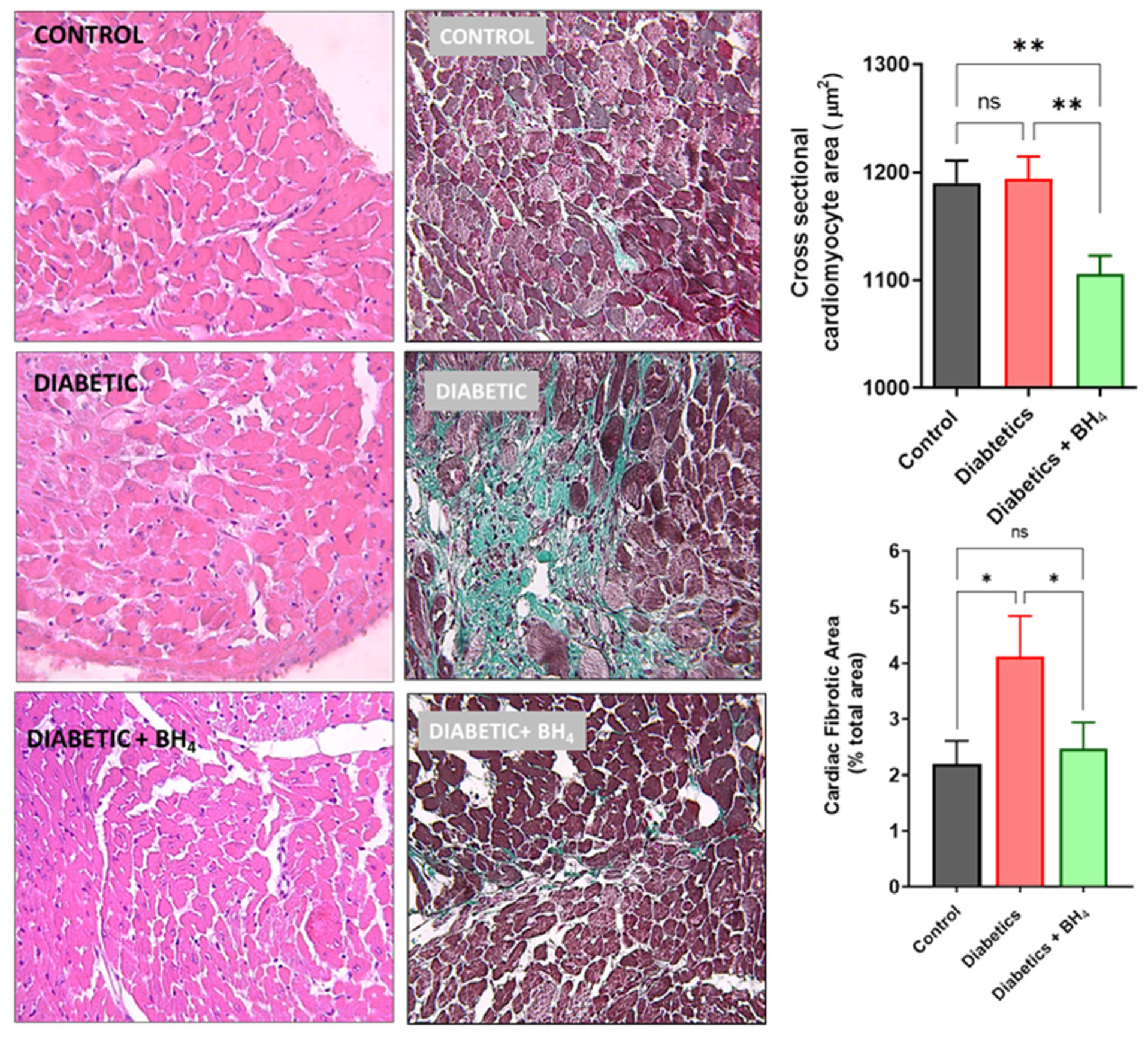
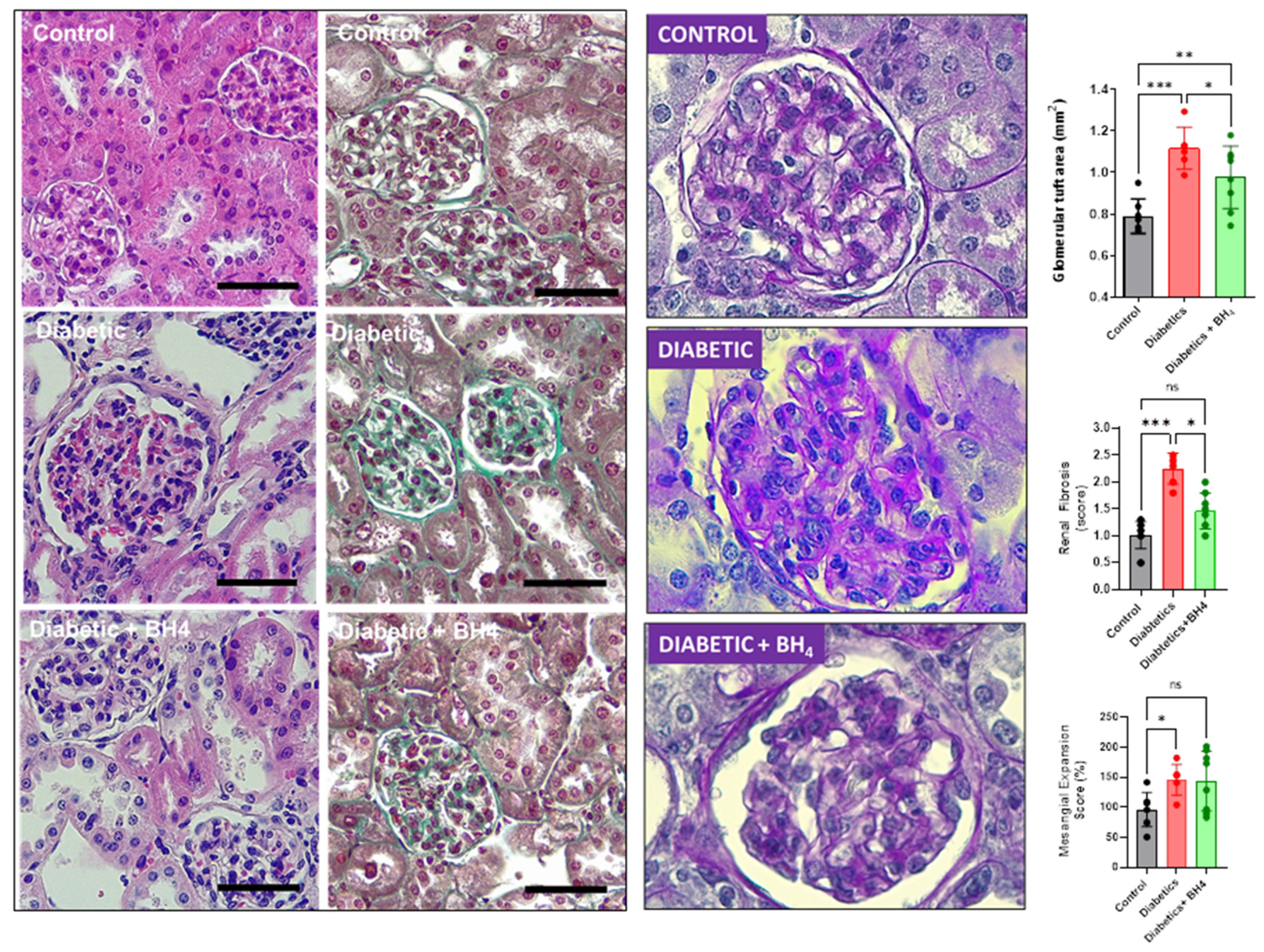
2.4. Histological Analyses
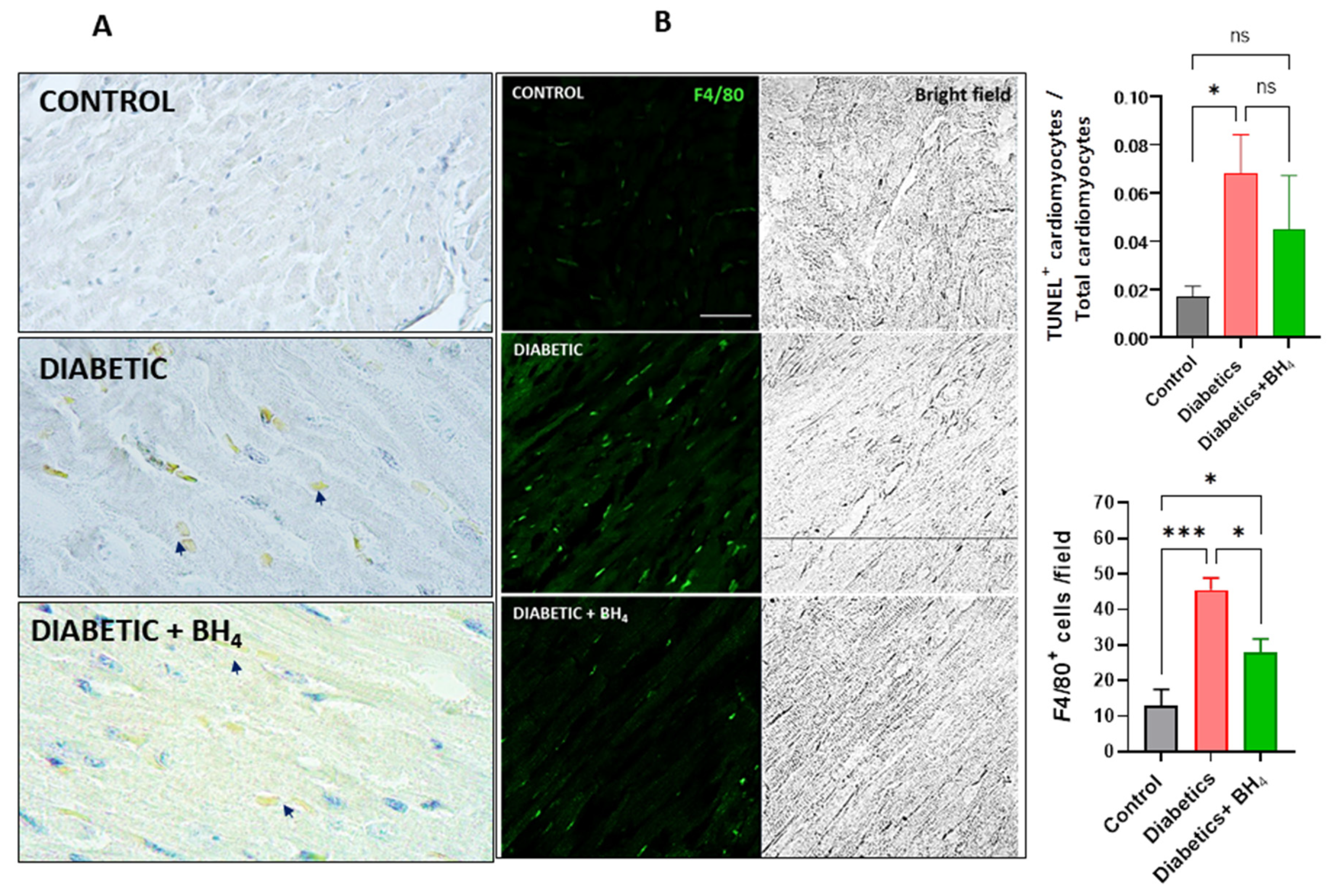
2.5. TUNEL Assay
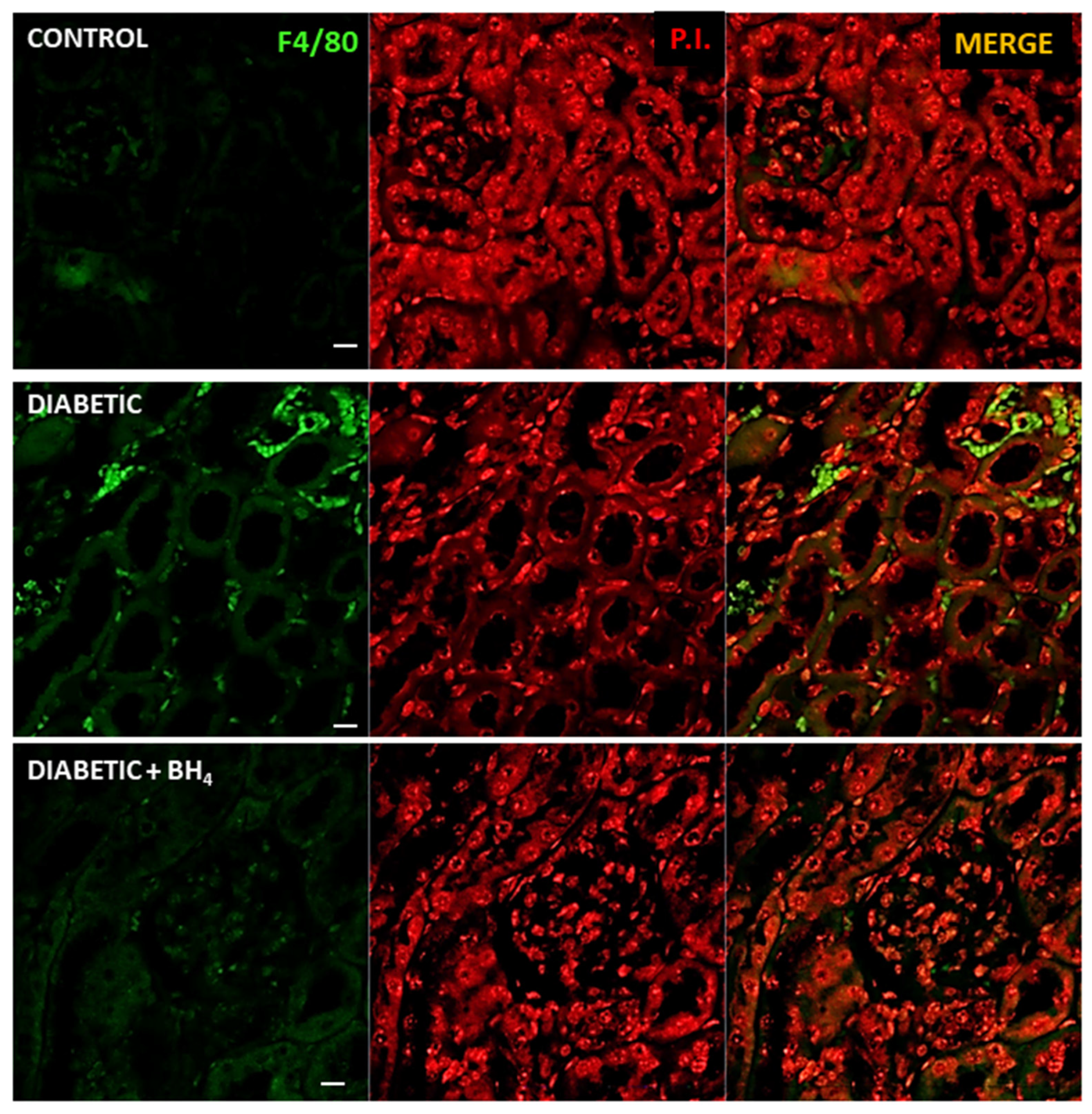
2.6. Confocal Microscopy
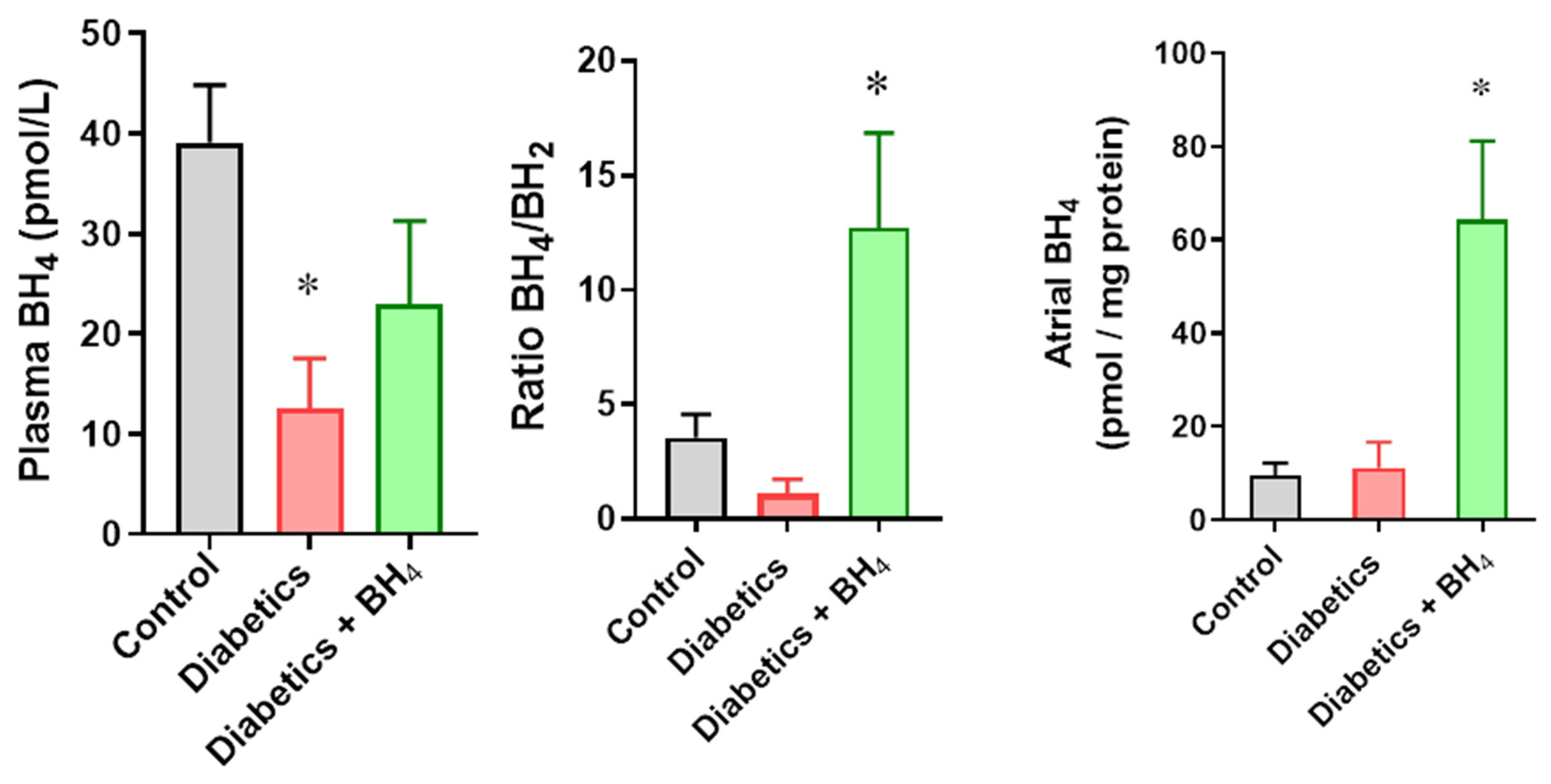
2.7. Tetrahydrobiopterin (BH4) Quantification
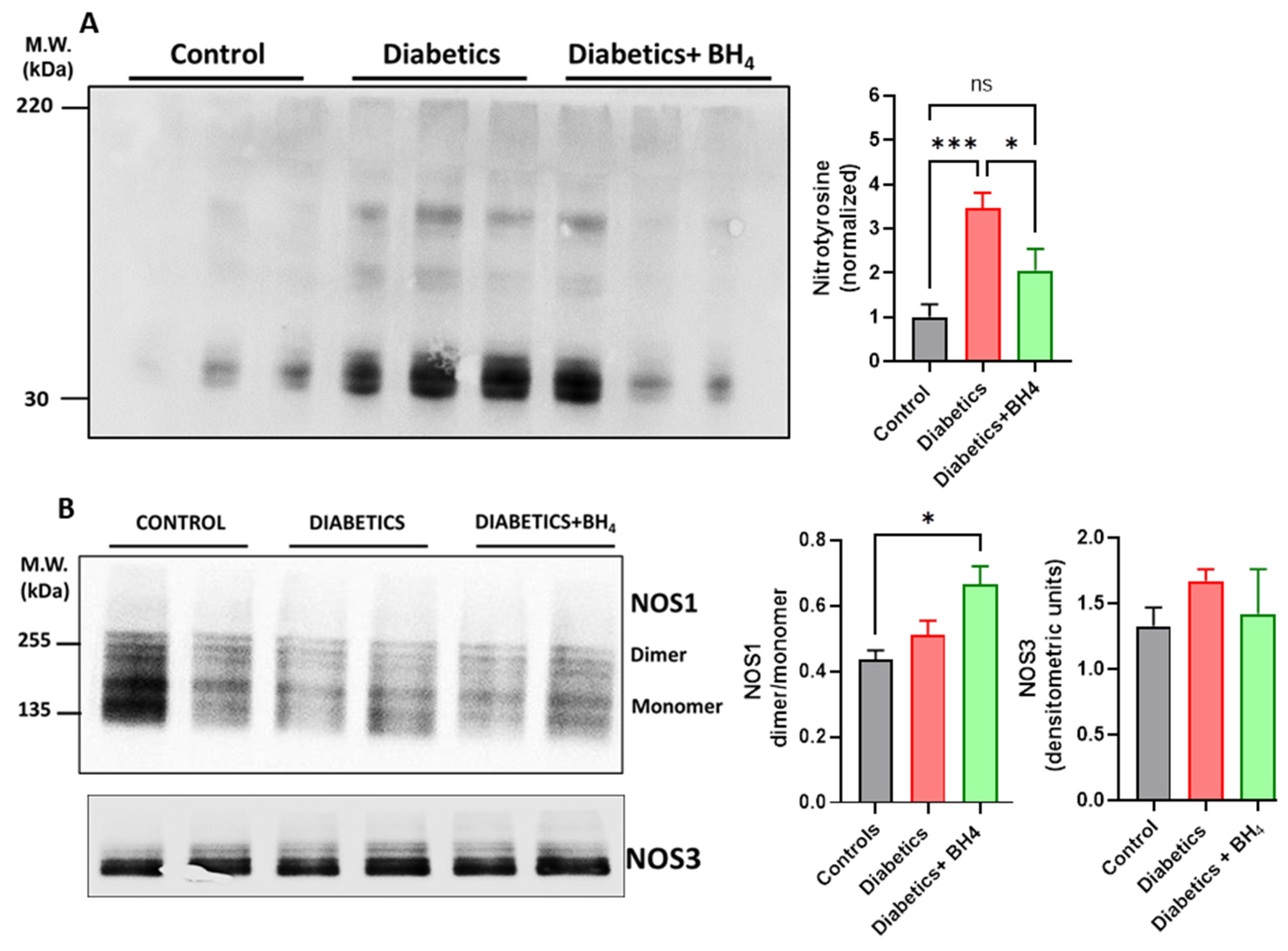
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Cardiac Remodeling
3.2. Apoptosis and Inflammatory Cells
3.3. Renal Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laditka, S.B.; Laditka, J.N. Active life expectancy of Americans with diabetes: Risks of heart disease, obesity, and inactivity. Diabetes Res. Clin. Pract. 2015, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Yuda, S.; Kouzu, H.; Miura, T. Diabetic cardiomyopathy: Pathophysiology and clinical features. Heart Fail. Rev. 2013, 18, 149–166. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. (Lausanne) 2021, 8, 695792. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Guan, Y.; Raja, R.; Ruiz-Velasco, A.; Liu, W. Mechanisms and Therapeutic Prospects of Diabetic Cardiomyopathy Through the Inflammatory Response. Front. Physiol. 2021, 12, 694864. [Google Scholar] [CrossRef]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef]
- Fu, J.; Lee, K.; Chuang, P.Y.; Liu, Z.; He, J.C. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Renal. Physiol. 2015, 308, F287–F297. [Google Scholar] [CrossRef]
- Hung, P.H.; Hsu, Y.C.; Chen, T.H.; Lin, C.L. Recent Advances in Diabetic Kidney Diseases: From Kidney Injury to Kidney Fibrosis. Int. J. Mol. Sci. 2021, 22, 11857. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Diaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yuan, S.; Zhao, X.; Qiao, M.; Li, S.; He, N.; Huang, L.; Lyu, J. Metformin Protects Cardiovascular Health in People with Diabetes. Front. Cardiovasc. Med. 2022, 9, 949113. [Google Scholar] [CrossRef]
- Wu, L.; Gunton, J.E. The Changing Landscape of Pharmacotherapy for Diabetes Mellitus: A Review of Cardiovascular Outcomes. Int. J. Mol. Sci. 2019, 20, 5853. [Google Scholar] [CrossRef] [PubMed]
- Van Ruiten, C.C.; Hesp, A.C.; van Raalte, D.H. Sodium glucose cotransporter-2 inhibitors protect the cardiorenal axis: Update on recent mechanistic insights related to kidney physiology. Eur. J. Intern. Med. 2022, 100, 13–20. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Persaud, S.J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Alp, N.J. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin. Sci. 2007, 113, 47–63. [Google Scholar] [CrossRef]
- Kim, H.L.; Park, Y.S. Maintenance of cellular tetrahydrobiopterin homeostasis. BMB Rep. 2010, 43, 584–592. [Google Scholar] [CrossRef]
- Okumura, M.; Masada, M.; Yoshida, Y.; Shintaku, H.; Hosoi, M.; Okada, N.; Konishi, Y.; Morikawa, T.; Miura, K.; Imanishi, M. Decrease in tetrahydrobiopterin as a possible cause of nephropathy in type II diabetic rats. Kidney Int. 2006, 70, 471–476. [Google Scholar] [CrossRef]
- Raij, L.; Azar, S.; Keane, W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984, 26, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, B.; Maitland, K.A.; Bayat, H.; Gu, J.; Nadler, J.L.; Corda, S.; Lavielle, G.; Verbeuren, T.J.; Zuccollo, A.; et al. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes 2006, 55, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes-Souza, N.K.; Yamaleyeva, L.M.; Lu, B.; Ramos, A.C.; Bishop, C.E.; Andersson, K.E. Superoxide overproduction and kidney fibrosis: A new animal model. Einstein, 2015; 13, 79–88. [Google Scholar]
- Zhao, X.F.; Liu, Y.H.; Han, Z.M.; Xu, Y.U. Effect of erythropoietin on the expression of dynamin-related protein-1 in rat renal interstitial fibrosis. Exp. Ther. Med. 2015, 9, 2065–2071. [Google Scholar] [CrossRef]
- Novoa, U.; Arauna, D.; Moran, M.; Nunez, M.; Zagmutt, S.; Saldivia, S.; Valdes, C.; Villasenor, J.; Zambrano, C.G.; Gonzalez, D.R. High-Intensity Exercise Reduces Cardiac Fibrosis and Hypertrophy but Does Not Restore the Nitroso-Redox Imbalance in Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2017, 2017, 7921363. [Google Scholar] [CrossRef] [PubMed]
- Vielma, A.Z.; Leon, L.; Fernandez, I.C.; Gonzalez, D.R.; Boric, M.P. Nitric Oxide Synthase 1 Modulates Basal and beta-Adrenergic-Stimulated Contractility by Rapid and Reversible Redox-Dependent S-Nitrosylation of the Heart. PLoS One 2016, 11, e0160813. [Google Scholar] [CrossRef]
- Soto, G.; Rodriguez, M.J.; Fuentealba, R.; Treuer, A.V.; Castillo, I.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin 1, a Proresolving Lipid Mediator, Ameliorates Liver Ischemia-Reperfusion Injury and Stimulates Hepatocyte Proliferation in Sprague-Dawley Rats. Int. J. Mol. Sci. 2020, 22, 540. [Google Scholar] [CrossRef]
- Valdes, C.; Arauna, D.; Gonzalez, D.; Villasenor, J. Simplified HPLC methodology for quantifying biological pterins by selective oxidation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1055–1056, 113–118. [Google Scholar] [CrossRef]
- Vielma, A.Z.; Boric, M.P.; Gonzalez, D.R. Apocynin Treatment Prevents Cardiac Connexin 43 Hemichannels Hyperactivity by Reducing Nitroso-Redox Stress in Mdx Mice. Int. J. Mol. Sci. 2020, 21, 5415. [Google Scholar] [CrossRef]
- Satoh, M.; Fujimoto, S.; Arakawa, S.; Yada, T.; Namikoshi, T.; Haruna, Y.; Horike, H.; Sasaki, T.; Kashihara, N. Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol. Dial. Transplant 2008, 23, 3806–3813. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, H.; Fan, X.; Paueksakon, P.; Harris, R.C. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int. 2012, 82, 1176–1183. [Google Scholar] [CrossRef][Green Version]
- Kidokoro, K.; Satoh, M.; Channon, K.M.; Yada, T.; Sasaki, T.; Kashihara, N. Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy. J. Am. Soc. Nephrol. 2013, 24, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Fujimoto, S.; Haruna, Y.; Arakawa, S.; Horike, H.; Komai, N.; Sasaki, T.; Tsujioka, K.; Makino, H.; Kashihara, N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2005, 288, F1144–F1152. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.M.; Papadimitriou, A.; Silva, K.C.; Lopes de Faria, J.M.; Lopes de Faria, J.B. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 2012, 61, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, Y.; Song, P.; Zhang, M.; Wang, S.; Zou, M.H. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 2007, 116, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.E.; Baumgardt, S.L.; Fang, J.; Paterson, M.; Liu, Y.; Du, J.; Shi, Y.; Qiao, S.; Bosnjak, Z.J.; Warltier, D.C.; et al. Cardiomyocyte GTP Cyclohydrolase 1 Protects the Heart Against Diabetic Cardiomyopathy. Sci. Rep. 2016, 6, 27925. [Google Scholar] [CrossRef]
- Carnicer, R.; Hale, A.B.; Suffredini, S.; Liu, X.; Reilly, S.; Zhang, M.H.; Surdo, N.C.; Bendall, J.K.; Crabtree, M.J.; Lim, G.B.; et al. Cardiomyocyte GTP cyclohydrolase 1 and tetrahydrobiopterin increase NOS1 activity and accelerate myocardial relaxation. Circ. Res. 2012, 111, 718–727. [Google Scholar] [CrossRef]
- Jo, H.; Otani, H.; Jo, F.; Shimazu, T.; Okazaki, T.; Yoshioka, K.; Fujita, M.; Kosaki, A.; Iwasaka, T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin. Exp. Pharmacol. Physiol. 2011, 38, 485–493. [Google Scholar] [CrossRef]
- Carnicer, R.; Duglan, D.; Ziberna, K.; Recalde, A.; Reilly, S.; Simon, J.N.; Mafrici, S.; Arya, R.; Rosello-Lleti, E.; Chuaiphichai, S.; et al. BH4 Increases nNOS Activity and Preserves Left Ventricular Function in Diabetes. Circ. Res. 2021, 128, 585–601. [Google Scholar] [CrossRef]
- Kim, H.K.; Ko, T.H.; Song, I.S.; Jeong, Y.J.; Heo, H.J.; Jeong, S.H.; Kim, M.; Park, N.M.; Seo, D.Y.; Kha, P.T.; et al. BH4 activates CaMKK2 and rescues the cardiomyopathic phenotype in rodent models of diabetes. Life Sci. Alliance 2020, 3. [Google Scholar] [CrossRef]
- Hashimoto, T.; Sivakumaran, V.; Carnicer, R.; Zhu, G.; Hahn, V.S.; Bedja, D.; Recalde, A.; Duglan, D.; Channon, K.M.; Casadei, B.; et al. Tetrahydrobiopterin Protects Against Hypertrophic Heart Disease Independent of Myocardial Nitric Oxide Synthase Coupling. J. Am. Heart Assoc. 2016, 5, e003208. [Google Scholar] [CrossRef]
- McNeill, E.; Crabtree, M.J.; Sahgal, N.; Patel, J.; Chuaiphichai, S.; Iqbal, A.J.; Hale, A.B.; Greaves, D.R.; Channon, K.M. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 2015, 79, 206–216. [Google Scholar] [CrossRef]
- McNeill, E.; Stylianou, E.; Crabtree, M.J.; Harrington-Kandt, R.; Kolb, A.L.; Diotallevi, M.; Hale, A.B.; Bettencourt, P.; Tanner, R.; O’Shea, M.K.; et al. Regulation of mycobacterial infection by macrophage Gch1 and tetrahydrobiopterin. Nat. Commun. 2018, 9, 5409. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef] [PubMed]
- Heidt, T.; Courties, G.; Dutta, P.; Sager, H.B.; Sebas, M.; Iwamoto, Y.; Sun, Y.; Da Silva, N.; Panizzi, P.; van der Laan, A.M.; et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 2014, 115, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.L.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.M.; Weinheimer, C.; et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 124, 263–278. [Google Scholar] [CrossRef]
- Monnerat, G.; Alarcon, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepulveda, M.; et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef]
- Bailey, J.D.; Diotallevi, M.; Nicol, T.; McNeill, E.; Shaw, A.; Chuaiphichai, S.; Hale, A.; Starr, A.; Nandi, M.; Stylianou, E.; et al. Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation. Cell Rep. 2019, 28, 218–230.e7. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Nie, Y.; Guo, H.; Zhang, F.; Zhou, X.; Yin, X. Tetrahydrobiopterin contributes to the proliferation of mesangial cells and accumulation of extracellular matrix in early-stage diabetic nephropathy. J. Pharm. Pharmacol. 2017, 69, 182–190. [Google Scholar] [CrossRef]
- Deng, C.; Wang, S.; Niu, Z.; Ye, Y.; Gao, L. Newly established LC-MS/MS method for measurement of plasma BH4 as a predictive biomarker for kidney injury in diabetes. Free Radic. Biol. Med. 2022, 178, 1–6. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Delgado-Valero, B.; Cachofeiro, V.; Martinez-Martinez, E. Fibrosis, the Bad Actor in Cardiorenal Syndromes: Mechanisms Involved. Cells 2021, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bottiglieri, T.; McCullough, P.A. The Central Role of Endothelial Dysfunction in Cardiorenal Syndrome. Cardiorenal. Med. 2017, 7, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Boudoulas, K.D.; Triposkiadis, F.; Parissis, J.; Butler, J.; Boudoulas, H. The Cardio-Renal Interrelationship. Prog. Cardiovasc. Dis. 2017, 59, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Sanford, M.; Keating, G.M. Sapropterin: A review of its use in the treatment of primary hyperphenylalaninaemia. Drugs 2009, 69, 461–476. [Google Scholar] [CrossRef] [PubMed]

| Control | Diabetics | Diabetics + BH4 | p Value | |
|---|---|---|---|---|
| n | 9 | 9 | 10 | |
| Body weight (g) | 40.7 ± 1.3 | 36.8 ± 1.0 | 34.0 ± 1.3 * | 0.0026 |
| Heart weight (g) | 0.157 ± 0.010 | 0.161 ± 0.007 | 0.139 ± 0.003 | 0.0616 |
| Heart weight/tibia length (g/mm) | 7.57 ± 0.39 | 8.59 ± 0.34 * | 7.39 ± 0.16 | 0.0495 |
| Insulin (ng/mL) | 1.09 ± 0.30 * | 0.29 ± 0.12 | 0.21 ± 0.08 | 0.0037 |
| BNP (pg/mL) | 227.9 ± 25.3 ** | 96.1 ±13.5 | 66.6 ± 12.6 | 0.0495 |
| Glucose (mg/dL) | 137 ± 7.7 * | 316 ± 69.7 | 247.3 ± 29.7 | 0.0132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novoa, U.; Soto, K.; Valdés, C.; Villaseñor, J.; Treuer, A.V.; González, D.R. Tetrahydrobiopterin (BH4) Supplementation Prevents the Cardiorenal Effects of Diabetes in Mice by Reducing Oxidative Stress, Inflammation and Fibrosis. Biomedicines 2022, 10, 2479. https://doi.org/10.3390/biomedicines10102479
Novoa U, Soto K, Valdés C, Villaseñor J, Treuer AV, González DR. Tetrahydrobiopterin (BH4) Supplementation Prevents the Cardiorenal Effects of Diabetes in Mice by Reducing Oxidative Stress, Inflammation and Fibrosis. Biomedicines. 2022; 10(10):2479. https://doi.org/10.3390/biomedicines10102479
Chicago/Turabian StyleNovoa, Ulises, Karen Soto, Cristian Valdés, Jorge Villaseñor, Adriana V. Treuer, and Daniel R. González. 2022. "Tetrahydrobiopterin (BH4) Supplementation Prevents the Cardiorenal Effects of Diabetes in Mice by Reducing Oxidative Stress, Inflammation and Fibrosis" Biomedicines 10, no. 10: 2479. https://doi.org/10.3390/biomedicines10102479
APA StyleNovoa, U., Soto, K., Valdés, C., Villaseñor, J., Treuer, A. V., & González, D. R. (2022). Tetrahydrobiopterin (BH4) Supplementation Prevents the Cardiorenal Effects of Diabetes in Mice by Reducing Oxidative Stress, Inflammation and Fibrosis. Biomedicines, 10(10), 2479. https://doi.org/10.3390/biomedicines10102479