A Review of Lean Methodology Application and Its Integration in Medical Device New Product Introduction Processes
Abstract
1. Introduction
- Assess and review Lean tools and NPI methodologies in medical device NPI processes;
- Benchmark and review the development of Lean NPI processes and methodologies in other industries;
- Put forward the best practices, challenges and synergies between Lean NPI methodologies in the medical device sector and other industries as a model for integration.
2. Literature Review
2.1. The Regulatory Landscape and Its Effect on NPI
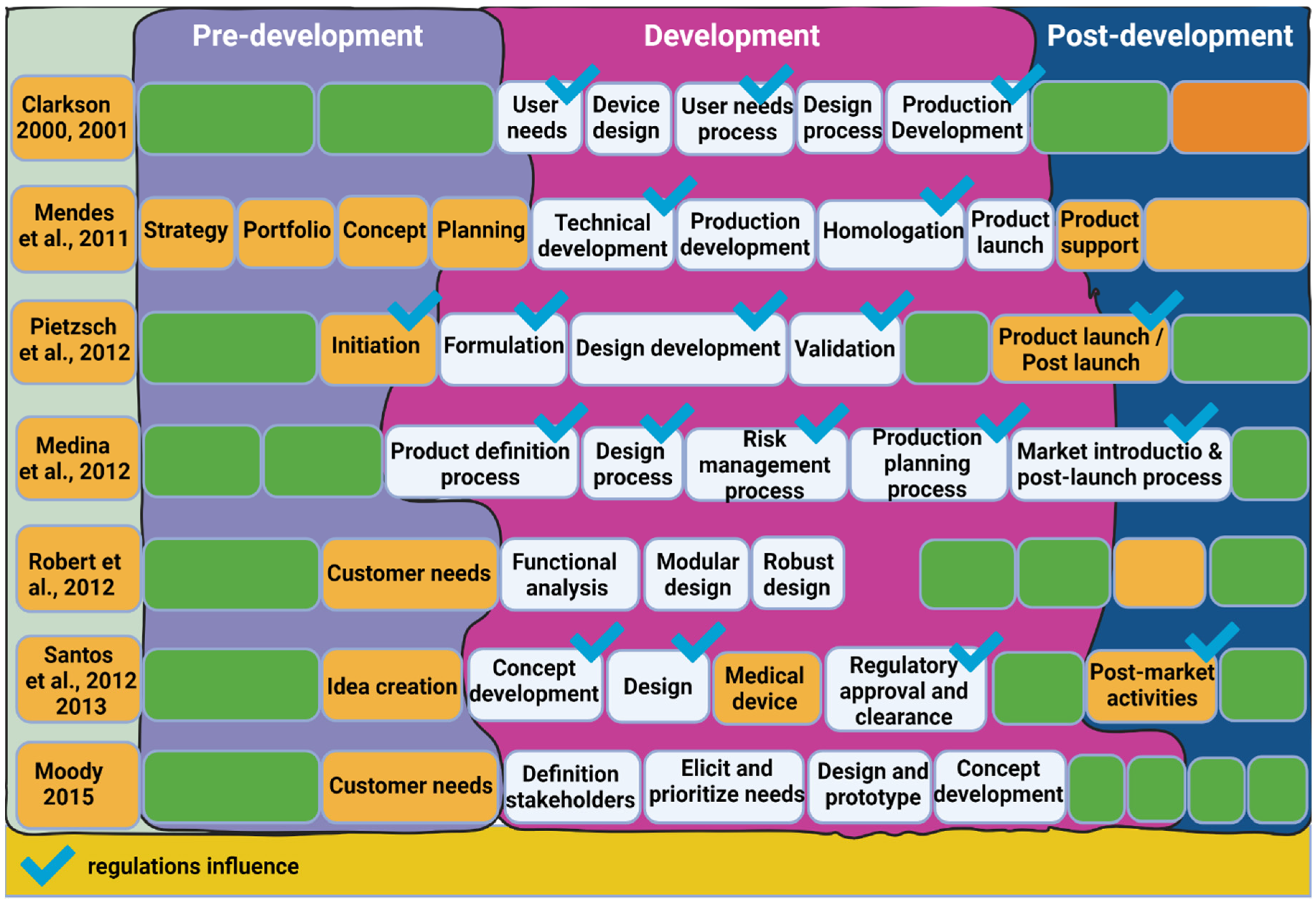
2.2. Current Medical Device NPI Approaches
2.2.1. Stage-Gate
2.2.2. Concurrent Engineering
2.2.3. Agile
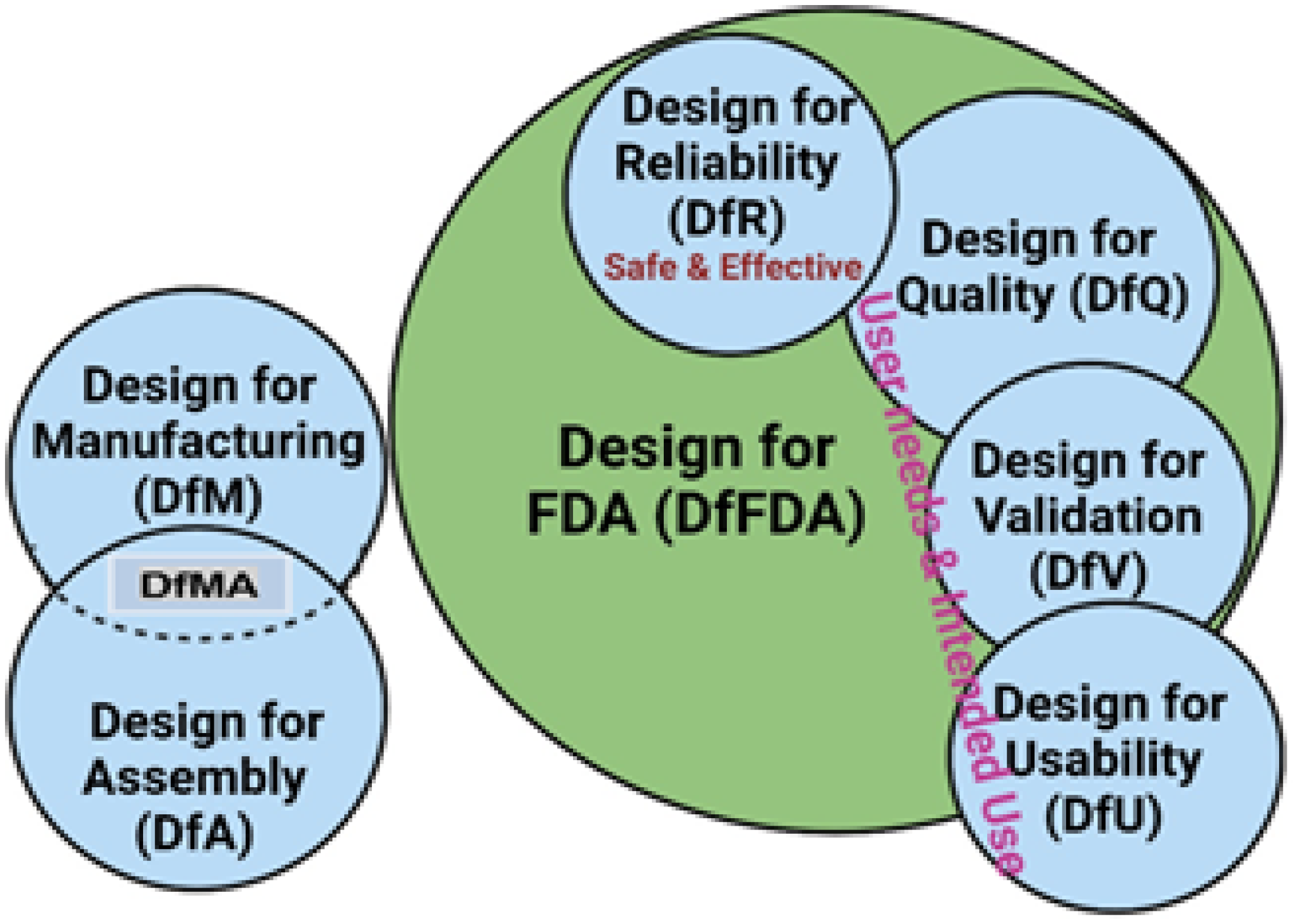
2.2.4. Design Approaches and Methodologies
2.3. Emergence of Lean NPD and NPI
2.4. Lean NPI Best Practices
2.4.1. Lean NPI Deployment Framework
2.4.2. Lean Principles
2.4.3. Metrics
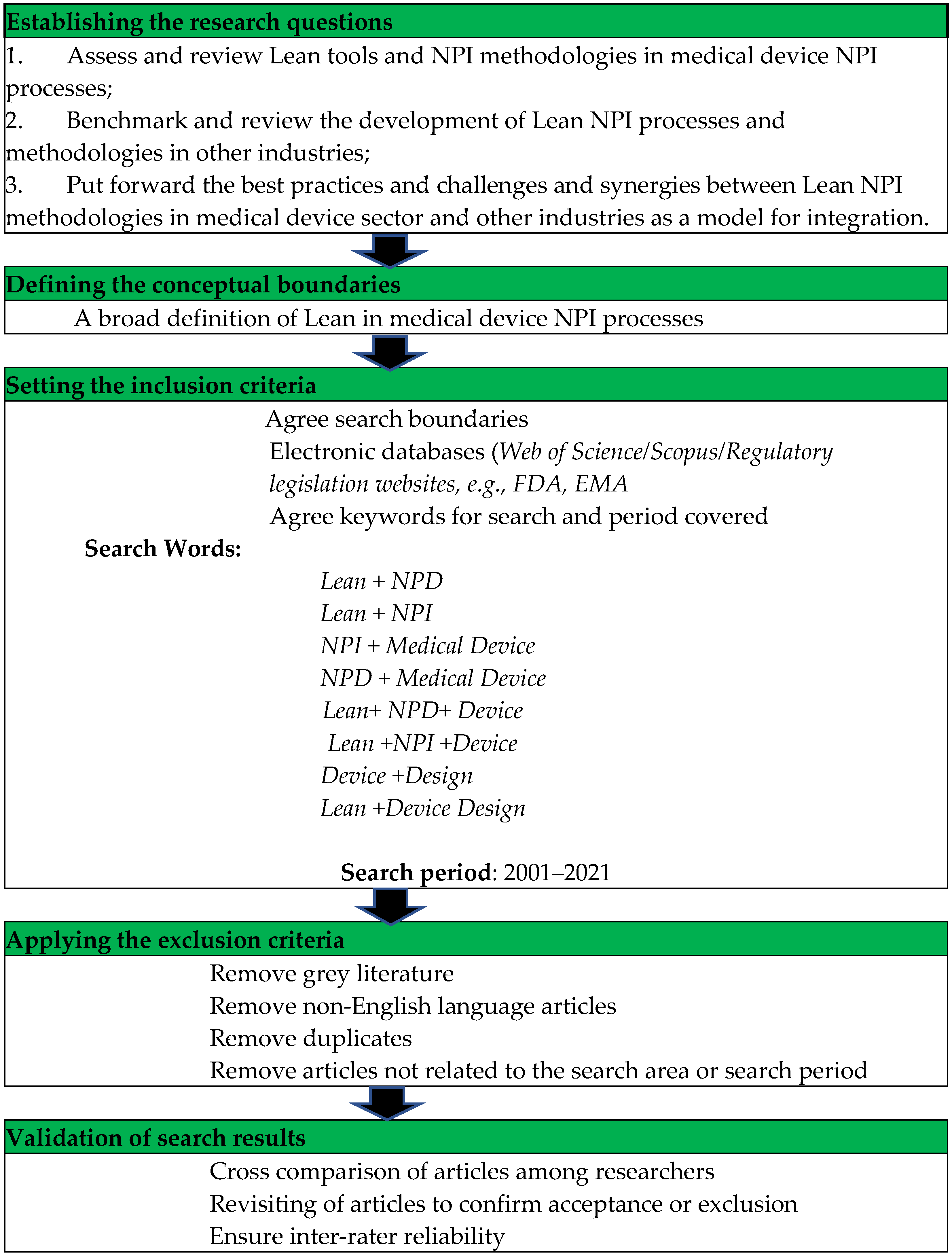
3. Methodology
4. Results from the Literature Research
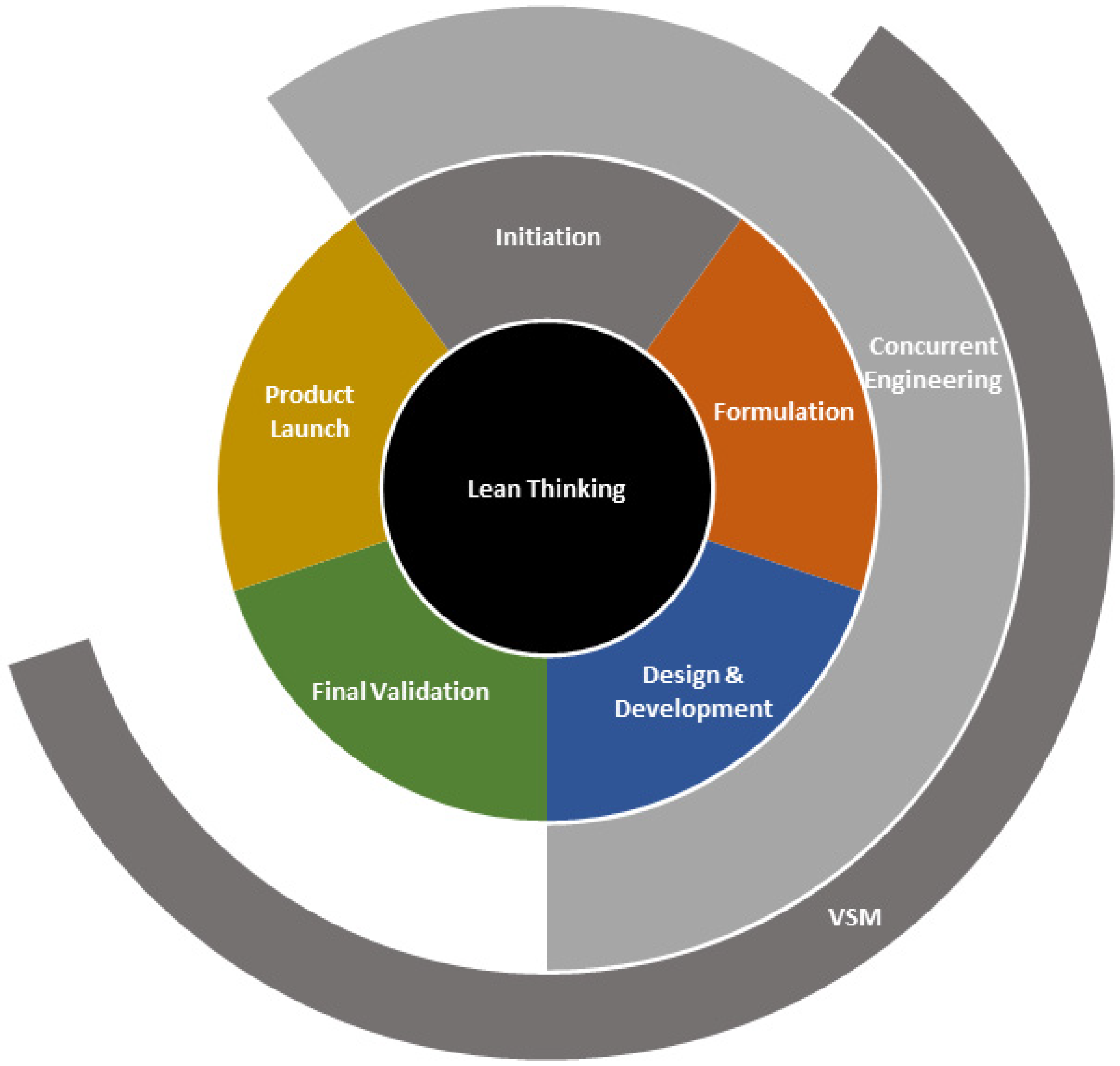
4.1. Integrating a Lean NPI Approach in the Medical Device Industry
4.2. Regulatory Considerations
4.3. Implications, Limitations and Outlook
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medtech Europe. “MedTech Europe’s Facts and Figures 2021”, MedTech Europe. 2021. Available online: https://www.medtecheurope.org/resource-library/medtech-europes-facts-and-figures-2021/ (accessed on 30 June 2021).
- Santos, I.C.; Gazelle, G.S.; Rocha, L.A.; Tavares, J.M.R. Medical device specificities: Opportunities for a dedicated product development methodology. Expert Rev. Med. Devices 2012, 9, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Krucoff, M.; Brindis, R.; Hodgson, P.; Mack, M.; Holmes, D. Medical device Innovation: Prospective Solutions for an Ecosystem in Crisis. JACC Cardiovasc. Interv. 2012, 5, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Moultrie, J.; Sutcliffe, L.; Maier, A. Exploratory study of the state of environmentally conscious design in the medical device industry. J. Clean. Prod. 2015, 108, 363–376. [Google Scholar] [CrossRef]
- Das, S.; Almonor, J. A concurrent engineering approach for the development of medical devices. Int. J. Comput. Integr. Manuf. 2000, 13, 139–147. [Google Scholar] [CrossRef]
- Medina, L.A.; Kremer, G.E.O.; Wysk, R.A. Supporting medical device development: A standard product design process model. J. Eng. Des. 2013, 24, 83–119. [Google Scholar] [CrossRef]
- Ocampo, J.; Kaminski, P. Medical device development, from technical design to integrated product development. J. Med. Eng. Technol. 2019, 43, 287–304. [Google Scholar] [CrossRef]
- Marešová, P.; Klímová, B.; Honegr, J.; Kuča, K.; Ibrahim, W.; Selamat, A. Medical device Development Process, and Associated Risks and Legislative Aspects-Systematic Review. Front. Public Health 2020, 8, 308. [Google Scholar] [CrossRef]
- Haque, B.; James-moore, M. Applying lean thinking to new product introduction. J. Eng. Des. 2004, 15, 1–31. [Google Scholar] [CrossRef]
- Pietzsch, J.B.; Shluzas, L.A.; Paté-Cornell, M.E.; Yock, P.G.; Linehan, J.H. Stage-Gate Process for the Development of Medical Devices. J. Med. Devices 2009, 3, 021004. [Google Scholar] [CrossRef]
- Lucke, L.; Mickelson, A.; Anderson, D. Proving experience speeds medical device time to market. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009. [Google Scholar]
- Bergsland, J.; Elle, O.; Fosse, E. Barriers to medical device innovation. Med. Devices Evid. Res. 2014, 7, 205–209. [Google Scholar] [CrossRef]
- Byrne, B.; McDermott, O.; Noonan, J. Applying Lean Six Sigma Methodology to a Pharmaceutical Manufacturing Facility: A Case Study. Processes 2021, 9, 550. [Google Scholar] [CrossRef]
- McDermott, O.; Antony, J.; Sony, M.; Daly, S. Barriers, and Enablers for Continuous Improvement Methodologies within the Irish Pharmaceutical Industry. Processes 2022, 10, 73. [Google Scholar] [CrossRef]
- Brown, A.; Dixon, D.; Eatock, J.; Meenan, B.; Young, T. A survey of success factors in New Product Development in the medical devices industry. In Proceedings of the IEEE International Engineering Management Conference, Estoril, Portugal, 28–30 June 2008. [Google Scholar]
- Brown, A.; Meenan, B.; Young, T. Marketing Innovation: Medical device prices follow the experience curve. J. Med. Mark. 2007, 7, 203–212. [Google Scholar] [CrossRef]
- McDermott, O.; Antony, J.; Sony, M.; Healy, T. Critical Failure Factors for Continuous Improvement Methodologies in the Irish MedTech Industry. TQM J. 2022, 34, 18–38. [Google Scholar] [CrossRef]
- Nave, D. How to compare Six Sigma, Lean and the theory of constraints. Qual. Prog. 2022, 35, 73–78. [Google Scholar]
- Pacheco, D.; Pergher, I.; Vaccaro, G.; Jung, C.; ten Caten, C. 18 comparative aspects between Lean and Six Sigma. Int. J. Lean Six Sigma 2015, 6, 161–175. [Google Scholar] [CrossRef]
- Brown, A.; Eatock, J.; Dixon, D.; Meenan, B.; Anderson, J. Quality and continuous improvement in medical device manufacturing. TQM J. 2018, 20, 541–555. [Google Scholar] [CrossRef]
- Lean Business Ireland. 2018. Shingo Prize Winners in Ireland. Available online: https://www.Leanbusinessireland.ie/shingo-prize-winners-ireland/ (accessed on 4 March 2021).
- Santos, I.C.; Gazelle, G.S.; Rocha, L.A.; Tavares, J.M.R. An ontology model for the medical device development process in Europe. In Proceedings of the1st International Conference on Design and Processes for Medical devices-PROMED, Brescia, Italy, 2–4 May 2012. [Google Scholar]
- Winter, D.; Jones, C.; Ward, C.; Gibbons, P.; McMahon, C.; Potter, K. The Application of a Lean Philosophy During Manufacture of Advanced Airframe Structures in a New Product Introduction (NPI) Environment. In Advances in Sustainable and Competitive Manufacturing Systems; Lecture Notes in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cawley, O.; Richardson, I.; Wang, X. Medical Device Software Development-A Perspective from a Lean Manufacturing Plant. SPICE Conf. 2011, 155, 84–96. [Google Scholar]
- Cawley, O.; Wang, X.; Richardson, I. Lean/Agile Software Development Methodologies in Regulated Environments-State of the Art. LESS Conf. 2010, 65, 31–36. [Google Scholar]
- Garcia, P.; Younie, D.; Fornos, J. Implementation of Lean Transactional at Tenneco Europe, Applications in Finance; SAE Technical Paper; SAE: Warrendale, PA, USA, 2012. [Google Scholar]
- Ikatrinasari, Z.; Haryanto, E. Implementation of Lean Service with Value Stream Mapping at Directorate Airworthiness and Aircraft Operation, Ministry of Transportation Republic of Indonesia. J. Serv. Sci. Manag. 2014, 7, 291–301. [Google Scholar] [CrossRef][Green Version]
- Glazkova, N.; Fortin, C.; Podladchikova, T. Application of Lean-Agile Approach for Medical Wearable Device Development. In Proceedings of the 2019 14th Annual Conference System of Systems Engineering, Anchorage, AK, USA, 19–22 May 2019; pp. 75–80. [Google Scholar]
- Martin, J.L.; Norris, B.J.; Murphy, E.; Crowe, J.A. Medical device development: The challenge for ergonomics. Appl. Ergon. 2008, 39, 271–283. [Google Scholar] [CrossRef] [PubMed]
- CDRH. “Classify Your Medical Device”, FDA, FDA, 22 October 2020. Available online: https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device (accessed on 15 August 2021).
- European Medicines Agency. “Medical devices”, European Medicines Agency, Text. 26 November 2018. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices (accessed on 15 August 2021).
- FDA CDRH. Design Control Guidance For Medical Device Manufacturers, U.S. Food and Drug Administration, FDA. 18 March 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-control-guidance-medical-device-manufacturers (accessed on 16 April 2021).
- Boylan, B.; McDermott, O.; Kinahan, N. Manufacturing Control System Development for an In Vitro Diagnostic Product Platform. Processes 2021, 9, 975. [Google Scholar] [CrossRef]
- Petersen, K.; Wohlin, C.; Baca, D. The Waterfall Model in Large-Scale Development; Bomarius, F., Oivo, M., Jaring, P., Abrahamsson, P., Eds.; Product-Focused Software Process Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 386–400. [Google Scholar]
- Sommerville, I. Software Engineering; Pearson Education: London UK, 2004. [Google Scholar]
- Lin, W.; Fan, X. Software Development Practice for FDA-Compliant Medical Devices. In Proceedings of the International Joint Conference on Computational Sciences and Optimization, Sanya, China, 24–26 April 2009; Volume 2, pp. 388–390. [Google Scholar]
- McDermott, O.; Antony, J.; Sony, M.; Looby, E. A critical evaluation and measurement of organisational readiness and adoption for continuous improvement within a medical device manufacturer. Int. J. Manag. Sci. Eng. Manag. 2022. [Google Scholar] [CrossRef]
- Bridgelal Ram, M.; Grocott, P.R.; Weir, H.C.M. Issues and challenges of involving users in medical device development. Health Expect. 2008, 11, 63–71. [Google Scholar] [CrossRef]
- Bernstein, J.I.; Joshua, I. Design Methods in the Aerospace Industry: Looking for Evidence of Set-Based Practices, Thesis, Massachusetts Institute of Technology. 1998. Available online: https://dspace.mit.edu/handle/1721.1/82675 (accessed on 16 April 2021).
- Cooper, R. Winning at New Products, 1st ed.; Addison-Wesley: Reading, MA, USA, 1986. [Google Scholar]
- Denney, D. Stage-Gate Project-Management Process in the Oil and Gas Industry. J. Pet. Technol. 2006, 58, 68–71. [Google Scholar] [CrossRef]
- Cooper, R.; Sommer, A. The Agile-Stage-Gate Hybrid Model: A Promising New Approach and a New Research Opportunity. J. Prod. Innov. Manag. 2016, 33, 513–526. [Google Scholar] [CrossRef]
- Van de Burgwal, L.; Ribeiro, C.; Van der Waal, M.; Claassen, E. Towards improved process efficiency in vaccine innovation: The Vaccine Innovation Cycle as a validated, conceptual stage-gate model. Vaccine 2018, 36, 7496–7508. [Google Scholar] [CrossRef]
- Howieson, J.; Lawley, M.; Selen, W. New Product Development in Small Food Enterprises. J. New Bus. Ideas Trends 2014, 12, 11–26. [Google Scholar]
- Wuest, T.; Liu, A.; Lu, S.; Thoben, K. Application of the Stage Gate Model in Production Supporting Quality Management. Procedia CIRP 2014, 17, 32–37. [Google Scholar] [CrossRef][Green Version]
- Högman, U.; Johannesson, H. Applying stage-gate processes to technology development—Experience from six hardware-oriented companies. J. Eng. Technol. Manag. 2013, 30, 264–287. [Google Scholar] [CrossRef]
- Blüher, T.; Amaral, D.C.; Lindow, K.; Costa, J.M.H.; Stark, R. Research opportunities in PSS design focusing on the potentials of agile approaches. Procedia CIRP. 2019, 84, 832–837. [Google Scholar] [CrossRef]
- Sommer, A.; Hedegaard, C.; Dukovska-Popovska, I.; Steger-Jensen, K. Improved Product Development Performance through Agile/Stage-Gate Hybrids: The Next-Generation Stage-Gate Process? Res. Technol. Manag. 2015, 58, 34–45. [Google Scholar] [CrossRef]
- Goldenberg, S.; Gravagna, J. A real-world perspective: Building and executing an integrated customer engagement roadmap that bridges the gaps in traditional medical device development processes. J. Med. Mark. Device Diagn. Pharm. Mark. 2018, 16. [Google Scholar] [CrossRef]
- Pahl, G.; Feldhusen, J.; Grote, K. Engineering Design, 3rd ed.; Springer: London, UK, 2007; p. 139. [Google Scholar]
- Raudberget, D. Practical Applications of Set-Based Concurrent Engineering in Industry. J. Mech. Eng. 2010, 56, 685–695. [Google Scholar]
- Salgado, E.; Dekkers, R. Lean Product Development: Nothing New Under the Sun? Int. J. Manag. Rev. 2017, 20, 903–933. [Google Scholar] [CrossRef]
- Stare, A. Agile Project Management in Product Development Projects. Procedia-Soc. Behav. Sci. 2014, 119, 295–304. [Google Scholar] [CrossRef]
- Varl, M.; Duhovnik, J.; Tavčar, J. Agile product development process transformation to support advanced one-of-a-kind manufacturing. Int. J. Comput. Integr. Manuf. 2020, 33, 590–608. [Google Scholar] [CrossRef]
- Albers, A.; Heimicke, J.; Trost, S.; Spadinger, M. Alignment of the change to agile through method-supported evaluation of agile principles in physical product development. Procedia CIRP 2020, 91, 600–614. [Google Scholar] [CrossRef]
- Bhamra, R.; Nand, A.; Yang, L.; Albregard, P.; Azevedo, G.; Corraini, D.; Emiliasiq, M. Is leagile still relevant? A review and research opportunities. Total Qual. Manag. Bus. Excell. 2020, 32, 1569–1593. [Google Scholar] [CrossRef]
- Vogel, D. Most Are not Ready for Prime Time in Medical Device Software Design and Development. DesignFax Online. 2006. Available online: https://www.semanticscholar.org/paper/Agile-Methods-%3A-Most-are-not-ready-for-prime-time-Vogel/d394e18ea4492d2fdbb86ecfa9a8bd790aee1af8 (accessed on 19 September 2022).
- McHugh, M.; Cawley, O.; McCaffcry, F.; Richardson, I.; Wang, X. An agile V-model for medical device software development to overcome the challenges with plan-driven software development lifecycles. In Proceedings of the 2013 5th International Workshop on Software Engineering in Health Care (SEHC), San Francisco, CA, USA, 20–21 May 2013. [Google Scholar]
- Gerber, C.; Goevert, K.; Schweigert-Recksiek, S.; Lindemann, U. Agile Development of Physical Products—A Case Study of Medical device Product Development. Smart Innov. Syst. Technol. 2019, 135, 823–834. [Google Scholar]
- Project Management Institute. A Guide to the Project Management Body of Knowledge, 6th ed.; Project Management Institute: Newtown Square, PA, USA, 2017. [Google Scholar]
- Rosenberger, P.; Tick, J. Relevance of PMBOK v6 Processes for Tailored Agile Project Categories. In Proceedings of the 2019 IEEE 13th International Symposium on Applied Computational Intelligence and Informatics (SACI), Timisoara, Romania, 29–31 May 2019. [Google Scholar]
- ISO 21500; Project, Programme and Portfolio Management—Context and Concepts. International Organization for Standardization: London, UK, 2021.
- Ciurana, J. Designing, prototyping and manufacturing medical devices: An overview. Int. J. Comput. Integr. Manuf. 2014, 27, 901–918. [Google Scholar] [CrossRef]
- Curran, R.; Kundu, A.; Raghunathan, S.; Eakin, D. Costing Tools for Decision Making within Integrated Aerospace Design. Concurr. Eng. 2001, 9, 327–338. [Google Scholar] [CrossRef]
- Prasad, S.; Zacharia, R.; Babu, J. Design for manufacturing (DFM) approach for Productivity Improvement in Medical Equipment Manufacturing. Int. J. Emerg. Technol. Adv. Eng. 2008, 4, 79–85. [Google Scholar]
- Dixon, D.; Eatock, J.; Meenan, B.J.; Morgan, M. Application of design of experiment (DOE) techniques to process validation in medical device manufacture. J. Valid. Technol. 2006, 12, 92–100. [Google Scholar]
- Alexander, K.; Clarkson, P. A validation model for the medical devices industry. J. Eng. Des. 2002, 13, 13197–13204. [Google Scholar] [CrossRef]
- Womack, J.; Jones, D.; Roos, D. Machine That Changed the World, 1st ed.; Simon and Schuster: New York, NY, USA, 1991. [Google Scholar]
- Womack, J.; Jones, D. Lean Thinking—Banish Waste and Create Wealth in your Corporation. J. Oper. Res. Soc. 1997, 48, 1148. [Google Scholar] [CrossRef]
- Womack, J.; Jones, D. Lean Thinking, 1st ed.; Simon and Schuster: London, UK, 2003. [Google Scholar]
- Anderson, K.M.; Grasman, S.E.; Ayoub, K.; Introne, S.; Smithwick, K. Using Lean product development to speed time to market for medical devices. In Proceedings of the Industrial Engineering Research Conference (IERC), Reno, NV, USA, 21–25 May 2011; pp. 1–7. [Google Scholar]
- Haque, B.; James-Moore, M. Measures of performance for Lean product introduction in the aerospace industry. Proceedings of the Institution of Mechanical Engineers. Part B J. Eng. Manuf. 2004, 218, 1387–1398. [Google Scholar] [CrossRef]
- Sobek, D.; Ward, A.; Liker, J. Toyota’s Principles of Set-Based Concurrent Engineering. MIT Sloan Manag. Rev. 1999, 40, 67–83. [Google Scholar]
- Khan, M.; Al-Ashaab, A.; Shehab, E.; Haque, B.; Ewers, P.; Sorli, M.; Sopelana, A. Towards Lean product and process development. Int. J. Comput. Integr. Manuf. 2013, 26, 1105–1116. [Google Scholar] [CrossRef]
- McManus, H. Product Development Value Stream Mapping (PDVSM) Manual, 1st ed.; Massachusetts Institute of Technology: Cambridge, UK, 2005. [Google Scholar]
- Tyagi, S.; Choudhary, A.; Cai, X.; Yang, K. Value stream mapping to reduce the lead-time of a product development process. Int. J. Prod. Econ. 2015, 160, 202–212. [Google Scholar] [CrossRef]
- Rother, M.; Shook, J. Learning to See, 1st ed.; Mass: The Lean Enterprise Institute: Cambridge, UK, 1998. [Google Scholar]
- Meybodi, M. The links between Lean manufacturing practices and concurrent engineering method of new product development. Benchmarking: Int. J. 2013, 20, 362–376. [Google Scholar] [CrossRef]
- Ward, A.; Sobek, D. Lean Product and Process Development, 2nd ed.; Lean Enterprise Institute: Cambridge, MA, USA, 2014. [Google Scholar]
- Freire, J.; Alarcón, L. Achieving Lean Design Process: Improvement Methodology. J. Constr. Eng. Manag. 2002, 128, 248–256. [Google Scholar] [CrossRef]
- Cooper, R.; Edgett, S. Maximizing Productivity in Product Innovation. Res. Technol. Manag. 2008, 51, 47–58. [Google Scholar] [CrossRef]
- Welo, T.; Lycke, A.; Ringen, G. Investigating the Use of Set-Based Concurrent Engineering in Product Manufacturing Companies. Procedia CIRP 2019, 84, 43–48. [Google Scholar] [CrossRef]
- Forno, A.; Pereira, F.; Forcellini, F.; Kipper, L. Value Stream Mapping: A study about the problems and challenges found in the literature from the past 15 years about application of Lean tools. Int. J. Adv. Manuf. Technol. 2014, 72, 779–790. [Google Scholar] [CrossRef]
- Al-Ashaab, A.; Golob, M.; Attia, U.; Khan, M.; Parsons, J.; Andino, A.; Perez, A.; Guzman, P.; Onecha, A.; Kesavamoorthy, S.; et al. The transformation of product development process into Lean environment using set-based concurrent engineering: A case study from an aerospace industry. Concurr. Eng. 2013, 21, 268–285. [Google Scholar] [CrossRef]
- Costa, J.; Rossi, M.; Rebentisch, E.; Terzi, S.; Taisch, M.; Nightingale, D. What to Measure for Success in Lean System Engineering Programs? Procedia Comput. Sci. 2014, 28, 789–798. [Google Scholar] [CrossRef]
- Hejazi, A.; Bhuiyan, N.; Othman, M. Performance measurement of a Lean product development process. Concurr. Eng. 2020, 28, 198–209. [Google Scholar] [CrossRef]
- Baines, T.; Williams, G.; Lightfoot, H.; Evans, S. Beyond theory: An examination of Lean new product introduction practices in the UK. Proceedings of the Institution of Mechanical Engineers. Part B J. Eng. Manuf. 2007, 221, 1593–1600. [Google Scholar]
- Al-Ashaab, A.; Golob, M.; Urrutia, U.; Gourdin, M.; Petritsch, C.; Summers, M.; El-Nounu, A. Development and application of Lean product development performance measurement tool. Int. J. Comput. Integr. Manuf. 2015, 29, 342–354. [Google Scholar] [CrossRef]
- Driva, H.; Pawar, K.; Menon, U. Performance evaluation of new product development from a company perspective. Integr. Manuf. Syst. 2001, 12, 368–378. [Google Scholar] [CrossRef]
- Browning, T. On customer value and improvement in product development processes. Syst. Eng. 2003, 6, 49–61. [Google Scholar] [CrossRef]
- Darwish, M.; Shehab, E.; Al-Ashaab, A.; Haque, B. Value Stream Mapping And Analysis Of Product Development (Engineering) Processes. In Proceedings of the 8th International Conference on Manufacturing Research ICMR 2010, Durham, UK, 14–16 September 2010. [Google Scholar]
- Kearney, A.T. The Line on Design–How to Reduce Material Cost by Eliminating Design Waste. 2003. Available online: www.atkearney.com/main (accessed on 20 June 2022).
- Anand, G.; Kodali, R. Development of a Conceptual Framework for Lean New Product Development Process. Int. J. Prod. Dev. 2008, 6, 190. [Google Scholar] [CrossRef]
- Schulze, A.; Störmer, T. Lean product development-enabling management factors for waste elimination. Int. J. Technol. Manag. 2012, 57, 71. [Google Scholar] [CrossRef]
- Slack, R. The Lean Value Principle in Military Aerospace Product Development; The Lean Aerospace Initiative (LAI)-MIT: Cambridge, MA, USA, 1999; pp. 1–17. [Google Scholar]
- Gapp, R.; Fisher, R.; Kobayashi, K. Implementing 5S within a Japanese context: An integrated management system. Manag. Decis. 2008, 46, 565–579. [Google Scholar] [CrossRef]
- Al-Aomar, R. Applying 5S Lean Technology: An Infrastructure for Continuous Process Improvement. World Acad. Sci. Eng. Technol. 2011, 60, 1606–1611. [Google Scholar]
- Bicheno, J. The Lean Toolbox for Service Systems, 1st ed.; Picsie Books: Buckingham, UK, 2008. [Google Scholar]
- Locher, D. Lean Office and Service Simplified, 1st ed.; Productivity Press: New York, NY, USA, 2011. [Google Scholar]
- Endris, K.; Khan, M.; Arias, A. Advanced process planning in Lean product and process development. In Proceedings of the 2012 18th International ICE Conference on Engineering, Technology and Innovation, Munich, Germany, 18–20 June 2012. [Google Scholar]
- Burgess, K.; Singh, P.J.; Koroglu, R. Supply chain management: A structured literature review and implications for future research. Int. J. Oper. Prod. Manag. 2006, 26, 703–729. [Google Scholar] [CrossRef]
- Petticrew, M. Systematic reviews from astronomy to zoology: Myths and misconceptions. BMJ 2001, 322, 98–101. [Google Scholar] [CrossRef]
- Parameswaran, U.D.; Ozawa-Kirk, J.L.; Latendresse, G. To Live (Code) or to Not: A New Method for Coding in Qualitative Research. Qualitative Social Work; SAGE Publications: Thousand Oaks, CA, USA, 2020; Volume 19, pp. 630–644. [Google Scholar]
- Karlsson, C.; Ahlstrom, P. The Difficult Path to Lean Product Development. J. Prod. Innov. Manag. 1996, 13, 283–295. [Google Scholar] [CrossRef]
- León, H.; Farris, J. Lean Product Development Research: Current State and Future Directions. Eng. Manag. J. 2011, 23, 29–51. [Google Scholar] [CrossRef]
- Siiskonen, M. Integrated Product and Production Platforms for Pharmaceutical Products: Design Thinking for the Development of Personalized Medicines; Chalmers University of Technology: Gothenburg, Sweden, 2019. [Google Scholar]
- Sony, M. Pros and cons of implementing Industry 4.0 for the organizations: A review and synthesis of evidence. Prod. Manuf. Res. Taylor Fr. 2020, 8, 244–272. [Google Scholar] [CrossRef]
- Synnes, E.; Welo, T. Enhancing Integrative Capabilities through Lean Product and Process Development. Procedia CIRP 2016, 54, 221–226. [Google Scholar] [CrossRef][Green Version]
- Moore, S. Next Generation Medical Device Manufacture; A strategic assessment. J. Enterp. Excell. 2016, 1. [Google Scholar]

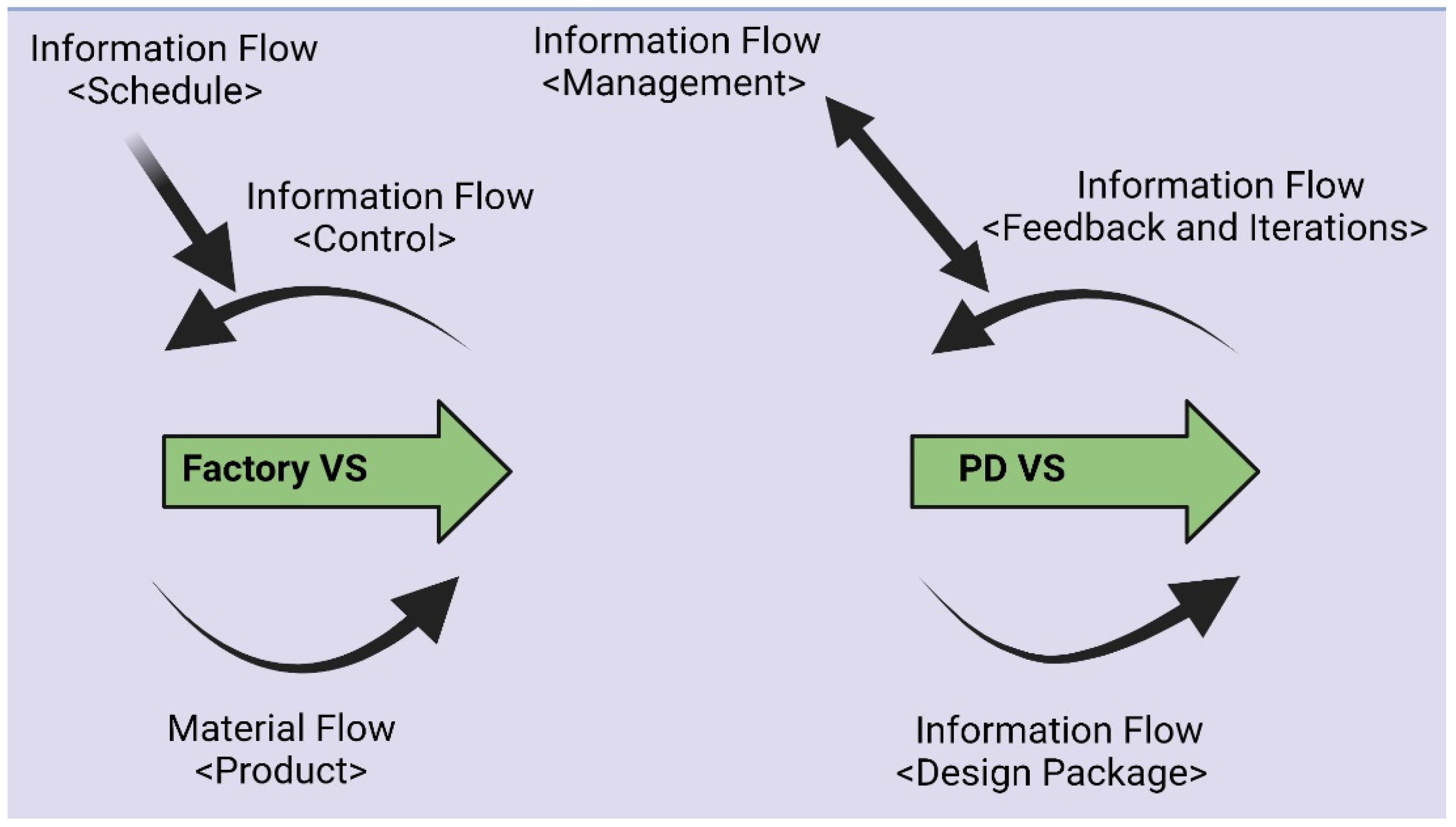

| Manufacturing | Engineering | |
|---|---|---|
| Value | Visible at each step, defined goal | Harder to see, emergent goals |
| Value Stream | Parts and material | Information and knowledge |
| Flow | Iterations are waste | Planned iterations must be efficient |
| Pull | Driven by takt time | Driven by needs of enterprise |
| Perfection | Process repeatable without errors | Process enables enterprise improvement |
| Seven Wastes | Applied to NPI |
|---|---|
| Transportation | • Complex structure for decision making and approval • Information incompatibility. File transfer • Information handled by multiple people before arrival • Poor data interface and management |
| Unnecessary motion | • Retrieving printed materials • Poor physical layout and 5S • Information forwarded to wrong people • Data acquired then not used |
| Unnecessary inventory | • Unnecessary detail or ”just-in-case” information • Poor configuration/revision management • Obsolete and outdated documents/information |
| Waiting | • Information waiting—information is delivered too early—maybe absolute by the time it is used • People waiting—information is delivered too late—process cannot continue without this information |
| Inappropriate/overprocessing | • Too many meetings yielding no results or consensus • Failure to identify and manage design risk requirements • Avoiding use of technological advances/aides • Excessive approvals • Excessive formatting and customization of documents • Unnecessary serial processing • Too many iterations or re-work, out of sequence working • Excessive verification and testing • Underutilization of knowledge • Overspecification/overdesign |
| Overproduction or early production | • Too many products, designs, or projects • Over design, over-specification, over tolerancing • Reductant development—i.e., Reuse not practiced |
| Concurrent Engineering Philosophy | Lean Philosophy |
|---|---|
| • Lacks an enterprise-wide common strategic direction or statement for implementation. It is naturally directed towards improving NPD process and thus promotes specialized tools such as DfX tools, etc. • It lacks a life-cycle approach, and the focus is not on ‘how to’, but on ‘what to do’ • Liable to different interpretations and definitions • It does not classify and contextualize waste and waste elimination occurs as a by-product of CE activities • It promotes customer focus and improves the information flow without a clearly defined systematic approach • It provides overlapping activities and the flow of partial information based on downstream process needs. It greatly improves the information flow and is seen as the engineering version of a manufacturing-type pull system. | • Lean is by definition an enterprise initiative with a common format for various business processes with the single strategic goal of eliminating waste and improving the flow of value. Its basic/original definition does not, however, address adequately the needs of NPI processes • Life cycle approach is used to drive continuous waste elimination to provide an implementation route map for Lean • ‘Value’ and ‘Waste’ are considered the main factors • Waste is initially identified, and then classified and contextualized within given value streams to be eliminated in the last step • It promotes (a) creation of value stream maps based on customer demands and (b) flow is only possible after waste is eliminated, and (c) the value-creating process can be eliminated at a customer-defined rate • The concepts of takt time, single piece flow and the pacemaker process are promoted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slattery, O.; Trubetskaya, A.; Moore, S.; McDermott, O. A Review of Lean Methodology Application and Its Integration in Medical Device New Product Introduction Processes. Processes 2022, 10, 2005. https://doi.org/10.3390/pr10102005
Slattery O, Trubetskaya A, Moore S, McDermott O. A Review of Lean Methodology Application and Its Integration in Medical Device New Product Introduction Processes. Processes. 2022; 10(10):2005. https://doi.org/10.3390/pr10102005
Chicago/Turabian StyleSlattery, Owen, Anna Trubetskaya, Sean Moore, and Olivia McDermott. 2022. "A Review of Lean Methodology Application and Its Integration in Medical Device New Product Introduction Processes" Processes 10, no. 10: 2005. https://doi.org/10.3390/pr10102005
APA StyleSlattery, O., Trubetskaya, A., Moore, S., & McDermott, O. (2022). A Review of Lean Methodology Application and Its Integration in Medical Device New Product Introduction Processes. Processes, 10(10), 2005. https://doi.org/10.3390/pr10102005








