Important Roles and Potential Uses of Natural and Synthetic Antimicrobial Peptides (AMPs) in Oral Diseases: Cavity, Periodontal Disease, and Thrush
Abstract
1. Introduction
2. Treatment by Antibiotics
3. The Efficacy of Natural Antimicrobial Peptides (AMPs)
3.1. Histatins
3.2. Defensins
3.3. Human Cathelicidin (LL-37)
4. Synthetic Antimicrobial Peptides (sAMPs)
5. Perspectives and Concluding Remarks
5.1. Natural AMPs
5.2. Antimicrobial Mechanisms of Action
5.3. Nanostructured AMPs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira Zandona, A.; Santiago, E.; Eckert, G.J.; Katz, B.P.; Pereira de Oliveira, S.; Capin, O.R.; Mau, M.; Zero, D.T. A natural history of dental caries lesions: A 4-year observational study. J. Dent. Res. 2012, 91, 841–846. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral. Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Beighton, D. Can the ecology of the dental biofilm be beneficially altered? Adv. Dent. Res. 2009, 21, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Chandrabhan, D.; Hemlata, R.; Renu, B.; Pradeep, V. Isolation of dental caries bacteria from dental plaque and effect of toothpastes on acidogenic bacteria. Open J. Med. Microbiol. 2012, 2, 65–69. [Google Scholar] [CrossRef][Green Version]
- Lippert, F. The effects of lesion baseline characteristics and different Sr: Ca ratios in plaque fluid-like solutions on caries lesion de- and remineralization. Arch. Oral Biol. 2012, 57, 1299–1306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, A.; Thakur, S.R.; Shetty, S.B. Anti-microbial efficacy of green tea and chlorhexidine mouth rinses against Streptococcus mutans, Lactobacilli spp. and Candida albicans in children with severe early childhood caries: A randomized clinical study. J. Ind. Soc. Pedo. Prev. Dent. 2016, 34, 65–70. [Google Scholar]
- Lemos, J.A.; Burne, R.A. A model of efficiency: Stress tolerance by Streptococcus mutans. Microbiology 2008, 154, 3247–3255. [Google Scholar] [CrossRef]
- Nakano, K.; Nomura, R.; Nakagawa, I.; Hamada, S.; Ooshima, T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J. Clin. Microbiol. 2004, 42, 198–202. [Google Scholar] [CrossRef]
- Nakano, K.; Ooshima, T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 2009, 4, 891–902. [Google Scholar] [CrossRef]
- Siderov, J.; Duggan, J. Arsenic trioxide associated toothache. J. Onc. Pharm. Pract. 2010, 16, 127–128. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins-a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Miller, B.F. Inhibition of experimental dental caries in the rat by fluoride and iodoacetic acid. Exp. Biol. Med. 1938, 39, 389–393. [Google Scholar] [CrossRef]
- Clarke, J.K. On the bacterial factor in the etiology of dental caries. Brit. J. Exp. Pathol. 1924, 5, 612–620. [Google Scholar]
- Keyes, P.H. Present and future measures for dental caries control. J. Ame. Dent. Ass. 1969, 79, 1395–1404. [Google Scholar] [CrossRef]
- Newbrun, E.; Sharma, M. Further studies on extracellular glucans synthesized by glucosyltransferases of oral streptococci. Caries Res. 1976, 10, 255–272. [Google Scholar] [CrossRef]
- Simon-Soro, A.; Mira, A. Solving the etiology of dental caries. Trends Microbiol. 2015, 23, 76–82. [Google Scholar] [CrossRef]
- Sodata, P.; Juntavee, A.; Juntavee, N.; Peerapattana, J. Optimization of adhesive pastes for dental caries prevention. AAPS PharmSciTech. 2017, 18, 3087–3096. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Period. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioactive Mater. 2018, 4, 43–55. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Zaura, E. The numerous microbial species in oral biofilms: How could antibacterial therapy be effective? Adv. Dent. Res. 2012, 24, 108–111. [Google Scholar] [CrossRef]
- Kalesinskas, P.; Kacergius, T.; Ambrozaitis, A.; Peciuliene, V.; Ericson, D. Reducing dental plaque formation and caries development. A review of current methods and implications for novel pharmaceuticals. Stomatologija 2014, 16, 44–52. [Google Scholar]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38, S11–S15. [Google Scholar] [CrossRef]
- Pemberton, M.N.; Gibson, J. Chlorhexidine and hypersensitivity reactions in dentistry. Br. Dent. J. 2012, 213, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.M.; Wyche, C.J.; Boyd, L.D.; Mallonee, L.F. Clinical Practice of the Dental Hygienist, 13th ed.; Jones Bartlett Learning: Burlington, MA, USA, 2021; pp. 1042–1043. [Google Scholar]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic antimicrobial peptides from eukaryotic organisms. Current Protein Peptide Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhou, L.; Jiang, W.; Kuang, R.; Li, J.; Tao, R.; Ni, L. An in vitro synergetic evaluation of the use of nisin and sodium fluoride or chlorhexidine against Streptococcus mutans. Peptides 2011, 32, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Pietkiewicz, J.; Bronowicka-Szydełko, A.; Dzierzba, K.; Danielewicz, R.; Gamian, A. Glycation of the muscle specific enolase by reactive carbonyls: Effect of temperature and the protection role of carnosine; pirydoxamine and phosphatidylserine. Protein J. 2011, 30, 149–158. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, J.; Brandt, B.W.; Zhu, Y.; Li, J.; van Loveren, C.; Deng, D.M. Identification and functional analysis of genome mutations in a fluoride-resistant Streptococcus mutans strain. PloS ONE 2015, 10, e0122630. [Google Scholar] [CrossRef]
- Balagopal, S.; Arjunkumar, R. Chlorhexidine: The gold standard antiplaque agent. J. Pharma. Sci. Res. 2013, 5, 270–274. [Google Scholar]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward chlorhexidine in oral bacteria-is there cause for concern. Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Rema, T.; Medihala, P.; Lawrence, J.R.; Vidovic, S.; Leppard, G.G.; Reid, M.; Korber, D.R. Proteomic analyses of chlorhexidine tolerance mechanisms in Delftia acidovorans biofilms. mSphere 2016, 1, e00017-15. [Google Scholar] [CrossRef]
- Karpinski, T.M.; Szkaradkiewicz, A.K. Chlorhexidine-pharmaco-biological activity and application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar]
- Ruiz-Linares, M.; Ferrer-Luque, C.M.; Arias-Moliz, T.; de Castro, P.; Aguado, B.; Baca, P. Antimicrobial activity of alexidine; chlorhexidine and cetrimide against Streptococcus mutans biofilm. Ann. Clin. Microbiol. Antimicro. 2014, 13, 41. [Google Scholar] [CrossRef]
- Goto, S.; Yoshitomi, H.; Fujii, S. The effect of quaternary ammonium compounds on stomach emptying. Yakugaku Zasshi. 1977, 97, 801–804. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.-X.; Xu, H.H. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef]
- Schweizer, H.P. Triclosan: A widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 2001, 202, 1–7. [Google Scholar] [CrossRef]
- Escalada, M.G.; Harwood, J.L.; Maillard, J.-Y.; Ochs, D. Triclosan inhibition of fatty acid synthesis and its effect on growth of Escherichia coli and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2005, 55, 879–882. [Google Scholar] [CrossRef]
- Westfall, C.; Flores-Mireles, A.L.; Robinson, J.I.; Lynch, A.J.L.; Hultgren, S.; Henderson, J.P.; Levin, P.A. The widely used antimicrobial triclosan induces high levels of antibiotic tolerance in vitro and reduces antibiotic efficacy up to 100-fold in vivo. Antimicrob. Agents Chemother. 2019, 63, e02312-18. [Google Scholar] [CrossRef]
- Available online: https://beyondpesticides.org/dailynewsblog/2012/08/johnson-and-johnson-to-phase-out-triclosan-regulators-remain-unresponsive/ (accessed on 1 October 2022).
- Adler, I.; Muino, A.; Aguas, S.; Harada, L.; Diaz, M.; Lence, A.; Labbrozzi, M.; Muino, J.M.; Elsner, B.; Avagnina, A.; et al. Helicobacter pylori and oral pathology: Relationship with the gastric infection. World J. Gastroenterol. 2014, 20, 9922–9935. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Muddana, K. Viral infections of oral cavity. J. Family Med. Prim. Care 2020, 9, 36–42. [Google Scholar] [CrossRef]
- Al-Azzam, S.; Ding, Y.; Liu, J.; Pandya, P.; Ting, J.P.; Afshar, S. Peptides to combat viral infectious diseases. Peptides 2020, 134, 170402. [Google Scholar] [CrossRef]
- Angebault, C.; Andremont, A. Antimicrobial agent exposure and the emergence and spread of resistant microorganisms: Issues associated with study design. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 32, 581–595. [Google Scholar] [CrossRef]
- Chon, S.Y.; Doan, H.Q.; Mays, R.M.; Singh, S.M.; Gordon, R.A.; Tyring, S.K. Antibiotic overuse and resistance in dermatology. Dermatol. Ther. 2012, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a microbial biofilm. Car Res. 2004, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic therapy in dentistry. Int. J. Dent. 2021, 28, 6667624. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 2014, 159, 1300–1311. [Google Scholar] [CrossRef]
- Haghgoo, R.; Mehran, M.; Afshari, E.; Zadeh, H.F.; Ahmadvand, M. Antibacterial effects of different concentrations of Althaea officinalis root extract versus 0.2% chlorhexidine and penicillin on Streptococcus mutans and Lactobacillus (in vitro). J. Int. Soc. Prevent. Comm. Dent. 2017, 7, 180–185. [Google Scholar]
- de Matos, B.M.; Ribeiro, Z.E.A.; Balducci, I.; Figueiredo, M.S.; Back-Brito, G.N.; Mota, A.J.; Braga, J.A.; Koga-Ito, C.Y. Oral microbial colonization in children with sickle cell anaemia under long-term prophylaxis with penicillin. Arch. Oral Biol. 2014, 59, 1042–1047. [Google Scholar] [CrossRef]
- Jousselin, A.; Manzano, C.; Biette, A.; Reed, P.; Pinho, M.G.; Rosato, A.E.; Kelley, W.L.; Renzoni, A. The Staphylococcus aureus chaperone PrsA is a new auxiliary factor of oxacillin resistance affecting penicillin-binding protein 2A. Antimicrob. Agents Chemother. 2015, 60, 1656–1666. [Google Scholar] [CrossRef]
- Jeong, S.; Paeng, I.R. Sensitivity and selectivity on aptamer-based assay: The determination of tetracycline residue in bovine milk. Sci. World J. 2012, 2012, 159456. [Google Scholar] [CrossRef]
- Patil, S.D.; Sharma, R.; Srivastava, S.; Navani, N.K.; Pathania, R. Downregulation of yidC in Escherichia coli by antisense RNA expression results in sensitization to antibacterial essential oils eugenol and carvacrol. PloS ONE 2013, 8, e57370. [Google Scholar] [CrossRef]
- Lew, H.T.; Frenc, S.W. Tetracycline nephrotoxicity and nonoliguric acute renal failure. Arch. Intern. Med. 1966, 118, 123–128. [Google Scholar] [CrossRef]
- Kennedy, R.; Alibhai, M.; Shakib, K. Tetracycline: A cure all? Brit. J. Oral Maxillo. Surg. 2014, 52, 382–383. [Google Scholar] [CrossRef]
- Bottino, M.C.; Munchow, E.A.; Albuquerque, M.T.P.; Kamocki, K.; Shahi, R.; Gregory, R.L.; Chu, T.G.; Pankajakshan, D. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2085–2092. [Google Scholar] [CrossRef]
- Sigeti, J.S.; Guiney, D.G., Jr.; Davis, C.E. Mechanism of action of metronidazole on Bacteroides fragilis. J. Infect. Dis. 1983, 148, 1083–1089. [Google Scholar] [CrossRef]
- Available online: https://www.mayoclinic.org/drugs-supplements/metronidazole-oral-route/side-effects/drg-20064745 (accessed on 1 October 2022).
- Sefton, A.M. Macrolides and changes in the oral flora. Clin. Trial Int. J. Antimicrob. Agents 1999, 11 (Suppl. 1), S23–S29, discussion S31–S32. [Google Scholar] [CrossRef]
- Alvarez-Elcoro, S.; Enzler, M.J. The macrolides: Erythromycin; clarithromycin; and azithromycin. Mayo Clin. Proc. 1999, 74, 613–634. [Google Scholar] [CrossRef]
- Williams, J.D.; Maskell, J.P.; Shain, H.; Chrysos, G.; Sefton, A.M.; Fraser, H.Y.; Hardie, J.M. Comparative in-vitro activity of azithromycin; macrolides (erythromycin; clarithromycin and spiramycin) and streptogramin RP 59500 against oral organisms. J. Antimicrob. Chemother. 1992, 30, 27–37. [Google Scholar] [CrossRef]
- Poehlsgaard, J.; Douthwaite, S. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 2005, 3, 870–881. [Google Scholar] [CrossRef]
- Arenz, S.; Wilson, D.N. Bacterial protein synthesis as a target for antibiotic inhibition. Cold Spring Harb. Perspect. Med. 2016, 6, a025361. [Google Scholar] [CrossRef]
- Available online: https://www.healthline.com/health/clindamycin-for-tooth-infection (accessed on 1 October 2022).
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity; mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification; design; application and research progress in multiple fields. Front. Microbiol. 2020, 11, e582779. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Antibiofilm peptides against oral biofilms. J. Oral Microbiol. 2017, 9, 1327308. [Google Scholar] [CrossRef]
- Bulet, P.; Urge, L.; Ohresser, S.; Hetru, C.; Otvos, L., Jr. Enlarged scale chemical synthesis and range of activity of drosocin; an O-glycosylated antibacterial peptide of Drosophila. Eur. J. Biochem. 1996, 238, 64–69. [Google Scholar] [CrossRef]
- Hale, J.D.; Hancock, R.E. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti-Infective Ther. 2007, 5, 951–959. [Google Scholar] [CrossRef]
- Matthyssen, T.; Li, W.; Holden, J.A.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. The potential of modified and multimeric antimicrobial peptide materials as superbug killers. Front. Chem. 2022, 9, 795433. [Google Scholar] [CrossRef]
- Sang, Y.; Blecha, F. Antimicrobial peptides and bacteriocins: Alternatives to traditional antibiotics. Anim. Health Res. Rev. 2008, 9, 227–235. [Google Scholar] [CrossRef]
- Supanchart, C.; Thawanaphong, S.; Makeudom, A.; Bolscher, J.G.; Nazmi, K.; Kornak, U.; Krisanaprakornkit, S. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J. Dent. Res. 2012, 91, 1071–1077. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure; function; and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef]
- Devine, D.A. Antimicrobial peptides in defence of the oral and respiratory tracts. Mol. Immunol. 2003, 40, 431–443. [Google Scholar] [CrossRef]
- De Sousa-Pereira, P.; Amado, F.; Abrantes, J.; Ferreira, R.; Esteves, P.J.; Vitorino, R. An evolutionary perspective of mammal salivary peptide families: Cystatins; histatins; statherin and PRPs. Arch. Oral Biol. 2013, 58, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation; characterization; primary structure; and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; van den Keijbus, P.A.; Kroeze, K.L.; Nazmi, K.; Gibbs, S.; Bolscher, J.G.; Veerman, E.C. Histatins enhance wound closure with oral and non-oral cells. J. Dent. Res. 2009, 88, 846–850. [Google Scholar] [CrossRef]
- Richardson, C.; Johnsson, M.; Raj, P.; Levine, M.; Nancollas, G. The influence of histatin-5 fragments on the mineralization of hydroxyapatite. Arch. Oral Biol. 1993, 38, 997–1002. [Google Scholar] [CrossRef]
- Tay, W.M.; Hanafy, A.I.; Angerhofer, A.; Ming, L. A plausible role of salivary copper in antimicrobial activity of histatin-5-metal binding and oxidative activity of its copper complex. Bioorg. Med. Chem. Lett. 2009, 19, 6709–6712. [Google Scholar] [CrossRef]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.; Zhang, M.Z.; Zhang, S. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol. 2018, 18, 54. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Selsted, M.E.; Harwig, S.; Ganz, T.; Schilling, J.W.; Lehrer, R. Primary structures of three human neutrophil defensins. J. Clin. Investig. 1985, 76, 1436. [Google Scholar] [CrossRef]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins, natural peptide antibiotics of human neutrophils. J. Clin. Investig. 1985, 76, 1427. [Google Scholar] [CrossRef]
- Wilde, C.; Griffith, J.; Marra, M.; Snable, J.; Scott, R. Purification and characterization of human neutrophil peptide 4; A novel member of the defensin family. J. Biol. Chem. 1989, 264, 11200–11203. [Google Scholar] [CrossRef]
- Gomes-Pde, S.; Fernandes, M.H. Defensins in the oral cavity: Distribution and biological role. J. Oral Pathol. Med. 2010, 39, 1–9. [Google Scholar] [CrossRef]
- Gorr, S.-U. Antimicrobial peptides in periodontal innate defense. Front. Oral Biol. 2012, 15, 84–98. [Google Scholar]
- Dale, B.A.; Krisanaprakornkit, S. Defensin antimicrobial peptides in the oral cavity. J. Oral Pathol. Med. 2001, 30, 321–327. [Google Scholar] [CrossRef]
- Selsted, M.E.; Tang, Y.-Q.; Morris, W.L.; McGuire, P.A.; Novotny, M.J.; Smith, W.; Henschen, A.H.; Cullor, J.S. Purification; primary structures; and antibacterial activities of β-defensins; a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 1993, 268, 6641–6648. [Google Scholar] [CrossRef]
- Gardner, M.S.; Rowland, M.D.; Siu, A.Y.; Bundy, J.L.; Wagener, D.K.; Stephenson, J.L., Jr. Comprehensive defensin assay for saliva. Anal. Chem. 2009, 81, 557–566. [Google Scholar] [CrossRef]
- Tao, R.; Jurevic, R.J.; Coulton, K.K.; Tsutsui, M.T.; Roberts, M.C.; Kimball, J.R.; Wells, N.; Berndt, J.; Dale, B.A. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 2005, 49, 3883–3888. [Google Scholar] [CrossRef]
- Krisanaprakornkit, S.; Weinberg, A.; Perez, C.N.; Dale, B.A. Expression of the peptide antibiotic human b-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect. Immun. 1998, 66, 4222–4228. [Google Scholar] [CrossRef]
- Abiko, Y.; Jinbu, Y.; Noguchi, T.; Nishimura, M.; Kusano, K.; Amaratunga, P.; Shibata, T.; Kaku, T. Upregulation of human beta-defensin 2 peptide expression in oral lichen planus, leukoplakia and candidiasis. An immunohistochemical study. Pathol. Res. Pract. 2002, 198, 537–542. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, D.J.; Han, S.H.; Kang, M.J.; Lee, J.Y.; Jeong, Y.J.; Lee, S.J.; Kim, T.H.; Ahn, S.G.; Yoon, J.H.; et al. Diverse toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect. Immun. 2014, 82, 1914–1920. [Google Scholar] [CrossRef]
- Ouhara, K.; Komatsuzawa, H.; Yamada, S.; Shiba, H.; Fujiwara, T.; Ohara, M.; Sayama, K.; Hashimoto, K.; Kurihara, H.; Sugai, M. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides; β-defensins and LL37; produced by human epithelial cells. J. Antimicrob. Chemother. 2005, 55, 888–896. [Google Scholar] [CrossRef]
- Joly, S.; Maze, C.; McCray, P.B., Jr.; Guthmiller, J.M. Human β-defensins 2 and demonstrate strain selective activity against oral microorganisms. J. Clin. Microbiol. 2004, 42, 1024–1029. [Google Scholar] [CrossRef]
- Shin, J.E.; Choi, Y. Treponema denticola suppresses expression of human β-defensin-2 in gingival epithelial cells through inhibition of TNFα production and TLR2 activation. Mol. Cells 2010, 29, 407–412. [Google Scholar] [CrossRef]
- Agawa, Y.; Lee, S.; Ono, S.; Taniguchi, T.; Anzai, K.; Kirino, Y. Interaction with phospholipid bilayers; ion channel formation; and antimicrobial activity of basic amphipathic α-helical model peptides of various chain lengths. J. Biol. Chem. 1991, 266, 20218–20222. [Google Scholar] [CrossRef]
- Diamond, G.; Ryan, L. Beta-defensins: What are they really doing in the oral cavity? Oral Dis. 2011, 17, 628–635. [Google Scholar] [CrossRef]
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002, 23, 291–296. [Google Scholar] [CrossRef]
- Perez-Rodriguez, A.; Eraso, E.; Quindós, G.; Mateo, E. Antimicrobial peptides with anti-Candida activity. Int. J. Mol. Sci. 2022, 23, 9264. [Google Scholar] [CrossRef]
- Ji, S.; Hyun, J.; Park, E.; Lee, B.-L.; Kim, K.-K.; Choi, Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J. Periodo. Res. 2007, 42, 410–419. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect. Immu. 1995, 63, 1291–1297. [Google Scholar] [CrossRef]
- Oren, Z.; Shai, Y. Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: Structure-function study. Biochemistry 1997, 36, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Jain, H. Cationic antimicrobial peptide: LL-37 and its role in periodontitis. Front. Biol. 2017, 12, 116–123. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Kamali Kakhki, R.; Safdari, H.; Khaledi, A.; Ghazvini, K. LL-37: Review of antimicrobial profile against sensitive and antibiotic-resistant human bacterial pathogens. Gene Rep. 2019, 17, 100519. [Google Scholar] [CrossRef]
- Greer, A.; Zenobia, C.; Darveau, R.P. Defensins and LL-37: A review of function in the gingival epithelium. Periodontol 2000 2013, 63, 67–79. [Google Scholar] [CrossRef]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Chapple, D.S. Peptide antibiotics: Minireview. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef]
- Boto, A.; Perez de la Lastra, J.; Gonzalez, C. The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Calixto, G.M.F.; Chorilli, M.; Spolidorio, D.M.; Santos-Filho, N.A.; Cilli, E.M.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081–3091. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Souza, P.F.N.; Vasconcelos, I.M.; Dias, L.P.; Martins, T.F.; Van Tilburg, M.F.; Guedes, M.I.F.; Sousa, D.O.B. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo- CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability. Biochimie 2019, 157, 10–21. [Google Scholar] [CrossRef]
- Dias, L.P.; Souza, P.F.N.; Oliveira, J.T.A.; Vasconcelos, I.M.; Araújo, N.M.S.; Tilburg, M.F.V.; Guedes, M.I.F.; Carneiro, R.F.; Lopes, J.L.S.; Sousa, D.O.B. RcAlb-PepII, a synthetic small peptide bioinspired in the 2S albumin from the seed cake of Ricinus communis is a potent antimicrobial agent against Klebsiella pneumoniae and Candida parapsilosis. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183092. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, Y.; Lyu, X.; Dong, N.; Shan, A. Antimicrobial activity; improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Sci. Rep. 2016, 6, 27258. [Google Scholar] [CrossRef]
- Wieprecht, T.; Dathe, M.; Beyermann, M.; Krause, E.; Maloy, W.L.; MacDonald, D.L.; Bienert, M. Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry 1997, 36, 6124–6132. [Google Scholar] [CrossRef]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.L.; MacDonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef]
- Avrahami, D.; Shai, Y. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 2002, 41, 2254–2263. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Marques, L.S.M.; Oliveira, J.T.A.; Lima, P.G.; Dias, L.P.; Neto, N.A.S.; Lopes, F.E.S.; Sousa, J.S.; Silva, A.F.B.; Caneiro, R.F.; et al. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020, 175, 132–145. [Google Scholar] [CrossRef]
- Kim, M.K.; Kang, H.K.; Ko, S.J.; Hong, M.J.; Bang, J.K.; Seo, C.H.; Park, Y. Mechanisms driving the antibacterial and antibiofilm properties of Hp1404 and its analogue peptides against multidrug-resistant Pseudomonas aeruginosa. Sci. Rep. 2018, 8, 1763. [Google Scholar] [CrossRef]
- Fernandez-Vidal, M.; Jayasinghe, S.; Ladokhin, A.S.; White, S.H. Folding amphipathic helices into membranes: Amphiphilicity trumps hydrophobicity. J. Mol. Biol. 2007, 370, 459–470. [Google Scholar] [CrossRef]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Benincasa, M.; Skerlavaj, B.; Gennaro, R.; Pellegrini, A.; Zanetti, M. In vitro and in vivo antimicrobial activity of two α-helical cathelicidin peptides and of their synthetic analogs. Peptides 2003, 24, 1723–1731. [Google Scholar] [CrossRef]
- Portelinha, J. Examining the Role of Histidine in the Antimicrobial Peptide Gaduscidin-1. Master’s Thesis, University of Connecticut, Mansfield, CT, USA, 2018. Available online: https://opencommons.uconn.edu/gs_theses/1284 (accessed on 1 October 2022).
- Hristova, K.; Wimley, W.C. A look at arginine in membranes. J. Membr. Biol. 2011, 239, 49–56. [Google Scholar] [CrossRef]
- Arias, M.; Piga, K.; Hyndman, M.; Vogel, H. Improving the activity of Trp-rich antimicrobial peptides by Arg/Lys substitutions and changing the length of cationic residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Wu, Z.; Nuding, S.; Groscurth, S.; Marcinowski, M.; Beisner, J.; Buchner, J.; Schaller, M.; Stange, E.F.; Wehkamp, J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β 2-defensin 1. Nature 2011, 469, 419–423. [Google Scholar] [CrossRef]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Wang, Y.; Zhang, J.; Zhao, S.; Yang, G. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci. Rep. 2015, 5, 16336. [Google Scholar] [CrossRef]
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shia, W.; et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef]
- Guo, L.; Edlund, A. Targeted antimicrobial peptides: A novel technology to eradicate harmful Streptococcus mutans. J. Calif. Dent. Assoc. 2017, 45, 557–564. [Google Scholar]
- Kreling, P.F.; Aida, K.L.; Massunari, L.; Caiaffa, K.S.; Percinoto, C.; Bedran, T.B.L.; Spolidorio, D.M.P.; Abuna, G.F.; Cilli, E.M.; Duque, C. Cytotoxicity and the effect of cationic peptide fragments against cariogenic bacteria under planktonic and biofilm conditions. Biofouling 2016, 32, 995–1006. [Google Scholar] [CrossRef]
- Hirt, H.; Hall, J.W.; Larson, E.; Gorr, S.U. A D-enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii. PLoS ONE 2018, 13, e0194900. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.; Schneider, V.A.; Agustiandari, H.; van Dijk, A.; Tjeerdsma-van Bokhoven, J.L.; Bikker, F.J.; Haagsman, H.P. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS ONE 2014, 9, e95939. [Google Scholar] [CrossRef] [PubMed]
- Winfred, S.B.; Meiyazagan, G.; Panda, J.J.; Nagendrababu, V.; Deivanayagam, K.; Chauhan, V.S.; Venkatraman, G. Antimicrobial activity of cationic peptides in endodontic procedures. Eur. J. Dent. 2014, 8, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Chauhan, V.S. Rationale-based; de novo design of dehydrophenylalanine-containing antibiotic peptides and systematic modification in sequence for enhanced potency. Antimicrob. Agents Chemother. 2011, 55, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Gao, T.; Zhang, N.; He, J.; Wu, F. Covalent immobilization of DJK-5 peptide on porous titanium for enhanced antibacterial effects and restrained inflammatory osteoclastogenesis. Colloids Surf. B Biointerfaces 2021, 202, 111697. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Zhou, Z.; Tu, H.; Ren, Q.; Wang, X.; Ding, L.; Zhou, X.; Zhang, L. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch. Oral Biol. 2017, 80, 41–50. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Huth, K.C.; Hickel, R.; Kollmuss, M. Inhibitory effect of LL-37 and human lactoferricin on growth and biofilm formation of anaerobes associated with oral diseases. Anaerobe 2021, 67, 102301. [Google Scholar] [CrossRef]
- Ahn, K.B.; Kim, A.R.; Kum, K.Y.; Yun, C.H.; Han, S.H. The synthetic human beta-defensin-3 C15 peptide exhibits antimicrobial activity against Streptococcus mutans, both alone and in combination with dental disinfectants. J. Microbiol. 2017, 55, 830–836. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Blaauboer, M.E.; Nazmi, K.; Scheres, N.; Bolscher, J.G.; Veerman, E.C. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010, 391, 541–548. [Google Scholar] [CrossRef]
- Hong, S.Y.; Oh, J.E.; Kwon, M.; Choi, M.J.; Lee, J.H.; Lee, B.L.; Moon, H.M.; Lee, K.H. Identification and characterization of novel antimicrobial decapeptides generated by combinational chemistry. Antimicrob. Agents Chemother. 1998, 42, 2534–2541. [Google Scholar] [CrossRef]
- Concannon, S.P.; Crowe, T.D.; Abercrombie, J.J.; Molina, C.M.; Hou, P.; Sukumaran, D.K.; Raj, P.A.; Leung, K.P. Susceptibility of oral bacteria to an antimicrobial decapeptide. J. Med. Microbiol. 2003, 52, 1083–1093. [Google Scholar] [CrossRef]
- Hee Na, D.H.; Faraj, J.; Capan, Y.; Leung, K.P.; DeLuca, P.P. Stability of antimicrobial decapeptide (KSL) and its analogues for delivery in the oral cavity. Pharmaceu. Res. 2007, 24, 1544–1550. [Google Scholar] [CrossRef]
- Sousa, M.G.C.; Xavier, P.D.; Cantuaria, A.P.C.; Porcino, R.A.; Almeida, J.A.; Franco, O.L.; Rezende, T.M.B. Host defense peptide IDR-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microb. Pathogen. 2020, 152, 104634. [Google Scholar] [CrossRef]
- Wang, H.; Ai, L.; Zhang, Y.; Cheng, J.; Yu, H.; Li, C.; Zhang, D.; Pan, Y.; Lin, L. The Effects of antimicrobial peptide Nal-P-113 on inhibiting periodontal pathogens and improving periodontal status. Biomed. Res. Int. 2018, 2018, 1805793. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lin, L.; Fu, W.; Yu, H.Y.; Yu, N.; Tan, L.S.; Cheng, J.-W.; Pan, Y.P. Preventive effects of the novel antimicrobial peptide Nal-P-113 in a rat Periodontitis model by limiting the growth of Porphyromonas gingivalis and modulating IL-1beta and TNF-alpha production. BMC Compl. Alter. Med. 2017, 17, 426. [Google Scholar] [CrossRef]
- do Nascimento Dias, J.; de Souza Silva, C.; de Araújo, A.R.; Souza, J.M.T.; de Holanda Veloso Júnior, P.H.; Cabral, W.F.; da Glória da Silva, M.; Eaton, P.; de Souza de Almeida Leite, J.R.; Nicola, A.M.; et al. Mechanisms of action of antimicrobial peptides ToAP2 and NDBP-5.7 against Candida albicans planktonic and biofilm cells. Sci. Rep. 2020, 10, 10327. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Song, J.-w.; Gong, F.; Li, S.-B.; Chang, H.-Y.; Xie, H.-M.; Gao, H.-W.; Tan, Y.-X.; Ji, S.-P. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Saeedi, N.; Mesbahfar, E.; Farrokh, P.; Salimi, F.; Rezaei, A. Design and characterization of new antimicrobial peptides derived from Aurein 1.2 with enhanced antibacterial activity. Biochimie 2021, 181, 42–51. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Biopolymers 2008, 90, 369–383. [Google Scholar] [CrossRef]
- Tan, J.; Huang, J.; Huang, Y.; Chen, Y. Effects of single amino acid substitution on the biophysical properties and biological activities of an amphipathic α-helical antibacterial peptide against gram-negative bacteria. Molecules 2014, 19, 10803–10817. [Google Scholar] [CrossRef]
- Saint Jean, K.D.; Henderson, K.D.; Chrom, C.L.; Abiuso, L.E.; Renn, L.M.; Caputo, G.A. Effects of hydrophobic amino acid substitutions on antimicrobial peptide behavior. Probiotics Antimicro. Prot. 2018, 10, 408–419. [Google Scholar] [CrossRef]
- Torcato, I.M.; Huang, Y.H.; Franquelim, H.G.; Gaspar, D.; Craik, D.J.; Castanho, M.A.; Henriques, S.T. Design and characterization of novel antimicrobial peptides; R-Bp100 and RW-Bp100; with activity against gram-negative and gram-positive bacteria. Biochim. Biophys. Acta (Bba) Biomembr. 2013, 1828, 944–955. [Google Scholar] [CrossRef]
- Rothbard, J.; Jessop, T.; Wender, P. Adaptive translocation: The role of hydrogen bonding and membrane potential in the uptake of guanidinium-rich transporters into cells. Adv. Drug Deliv. Rev. 2005, 57, 495–504. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Strom, M.B.; Rekdal, O.; Svendsen, J.S. Antimicrobial activity of short arginine- and tryptophan-rich peptides. J. Pept. Sci. 2002, 8, 431–437. [Google Scholar] [CrossRef]
- Manabe, T.; Kawasaki, K. D-form KLKLLLLLKLK-NH2 peptide exerts higher antimicrobial properties than its L-form counterpart via an association with bacterial cell wall components. Sci. Rep. 2017, 7, 43384. [Google Scholar] [CrossRef]
- Shahmiri, M.; Cornell, B.; Mechler, A. Phenylalanine residues act as membrane anchors in the antimicrobial action of Aurein 1.2. Biointerphases 2017, 12, 05G605. [Google Scholar] [CrossRef]
- Mattiuzzo, M.; Bandiera, A.; Gennaro, R.; Benincasa, M.; Pacor, S.; Antcheva, N.; Scocchi, M. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 2007, 66, 151–163. [Google Scholar] [CrossRef]
- Bacalum, M.; Janosi, L.; Zorila, F.; Tepes, A.-M.; Ionescu, C.; Bogdan, E.; Hadade, N.; Craciun, L.; Grosu, I.; Turcu, I.; et al. Modulating short tryptophan- and arginine-rich peptides activity by substitution with histidine. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1844–1854. [Google Scholar] [CrossRef]
- Lointier, M.; Aisenbrey, C.; Marquette, A.; Tan, J.H.; Kichler, A.; Bechinger, B. Membrane pore-formation correlates with the hydrophilic angle of histidine-rich amphipathic peptides with multiple biological activities. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183212. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, K.S.; Lee, J.; Yoo, J.; Lee, J.; Chung, J. Diptericine-like protein: An immune response gene regulated by the anti-bacterial gene induction pathway in Drosophila. Gene 2001, 271, 233–238. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Kim, H.J.; Kim, Y.I.; Kang, Y.J.; Lee, I.H.; Jin, B.R.; Han, Y.S.; Cheon, H.M.; Ha, N.G.; Seo, S.J. Comparative analysis of two attacin genes from Hyphantria cunea. Compar. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 213–220. [Google Scholar] [CrossRef] [PubMed]
- D’Este, F.; Benincasa, M.; Cannone, G.; Furlan, M.; Scarsini, M.; Volpatti, D.; Gennaro, R.; Tossi, A.; Skerlavaj, B.; Scocchi, M. Antimicrobial and host cell-directed activities of Gly/Ser-rich peptides from Salmonid cathelicidins. Fish Shell. Immunol. 2016, 59, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chou, S.; Xu, L.; Zhu, X.; Dong, N.; Shan, A.; Chen, Z. High specific selectivity and membrane-active mechanism of the synthetic centrosymmetric a-helical peptides with Gly-Gly pairs. Sci. Rep. 2015, 5, 15963. [Google Scholar] [CrossRef] [PubMed]
- Zweytick, D.; Deutsch, G.; Andrä, J.; Blondelle, S.E.; Vollmer, E.; Jerala, R.; Lohner, K. Studies on lactoferricin-derived Escherichia coli membrane-active peptides reveal differences in the mechanism of N-acylated versus nonacylated peptides. J. Biol. Chem. 2011, 286, 21266–21276. [Google Scholar] [CrossRef] [PubMed]
- Kamysz, E.; Sikorska, E.; Jaśkiewicz, M.; Bauer, M.; Neubauer, D.; Bartoszewska, S.; Baranska-Rybak, W.; Kamysz, W. Lipidated analogs of the LL-37-derived peptide fragment KR12- structural analysis; surface-active properties and antimicrobial activity. Int. J. Mol. Sci. 2020, 21, 887. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, T.; Gou, S.; He, Y.; Zhu, N.; Zhu, Y.; Wang, L.; Liu, H.; Zhang, Y.; Yao, J.; et al. Design and synthesis of new N-terminal fatty acid modified-antimicrobial peptide analogues with potent in vitro biological activity. Eur. J. Med. Chem. 2019, 182, 111636. [Google Scholar] [CrossRef]
- Rounds, T.; Straus, S.K. Lipidation of antimicrobial peptides as a design strategy for future alternatives to antibiotics. Int. J. Mol. Sci. 2020, 21, 9692. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Brit. J. Pharmacol. 2017, 18, 2967–2983. [Google Scholar] [CrossRef]
- Mainjot, A.; D’Hoore, W.; Vanheusden, A.; Van Nieuwenhuysen, J.-P. Antibiotic prescribing in dental practice in Belgium. Int. Endodontic J. 2009, 42, 1112–1117. [Google Scholar] [CrossRef]
- Kloos, W.; Bannerman, T.; Murray, P.; Baron, E.; Pfaller, M. Manual of Clinical Microbiology; American Society for Microbiology (ASM): Washington, DC, USA, 1991. [Google Scholar]
- Gonzalez-Estrada, A.; Radojicic, C. Penicillin allergy: A practical guide for clinicians. Cleveland Clinic J. Med. 2015, 82(5), 295–300. [Google Scholar] [CrossRef]
- Peedikayil, F. Antibiotics in odontogenic infections–An update. J. Antimicrobial. 2016, 2, 1000107. [Google Scholar]
- Dancer, S.J. The problem with cephalosporins. J. Antimicrob. Chemotherapy. 2001, 48, 463–478. [Google Scholar] [CrossRef]
- Li, W.; Tailhades, J.; O’Brien-Simpson, N.M.; Separovic, F.; Otvos, L.; Hossain, M.A.; Wade, J.D. Proline-rich antimicrobial peptides: Potential therapeutics against antibiotic-resistant bacteria. Amino Acids 2014, 46, 2287–2294. [Google Scholar] [CrossRef]
- Gagnon, M.-C.; Strandberg, E.; Grau-Campistany, A.; Wadhwani, P.; Reichert, J.; Bürck, J.; Rabanal, F.; Auger, M.; Paquin, J.-F.; Ulrich, A.S. Influence of the length and charge on the activity of α-helical amphipathic antimicrobial peptides. Biochemistry 2017, 56, 1680–1695. [Google Scholar] [CrossRef]
- Hoffknecht, B.C.; Worm, D.J.; Bobersky, S.; Prochnow, P.; Bandow, J.E.; Metzler-Nolte, N. Influence of the multivalency of ultrashort Arg-Trp-based antimicrobial peptides (AMP) on their antibacterial activity. ChemMedChem 2015, 10, 1564–1569. [Google Scholar] [CrossRef]
- Manzo, G.; Scorciapino, M.A.; Wadhwani, P.; Bürck, J.; Montaldo, N.P.; Pintus, M.; Sanna, R.; Casu, M.; Giuliani, A.; Pirri, G.; et al. Enhanced amphiphilic profile of a short β-stranded peptide improves its antimicrobial activity. PLoS ONE 2015, 10, e0116379. [Google Scholar] [CrossRef]
- Zhou, C.; Qi, X.; Li, P.; Chen, W.N.; Mouad, L.; Chang, M.W.; Leong, S.S.; Chan-Park, M.B. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of α-amino acid-N-carboxyanhydrides. Biomacromolecules 2010, 11, 60–67. [Google Scholar] [CrossRef]
- Su, X.; Zhou, X.; Tan, Z.; Zhou, C. Highly efficient antibacterial diblock copolypeptides based on lysine and phenylalanine. Biopolymers 2017, 107, e23041-8. [Google Scholar] [CrossRef]
- Stone, T.A.; Cole, G.B.; Ravamehr-Lake, D.; Nguyen, H.Q.; Khan, F.; Sharpe, S.; Deber, C.M. Positive charge patterning and hydrophobicity of membrane-active antimicrobial peptides as determinants of activity; toxicity; and pharmacokinetic stability. J. Med. Chem. 2019, 62, 6276–6286. [Google Scholar] [CrossRef]
- Baehni, P.C.; Takeuchi, Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003, 9 (Suppl. 1), 23–29. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Krishnappa, S. Various approaches for prevention of dental caries with emphasis on probiotics: A review. IOSR J. Dent. Med. Sci. 2014, 1, 62–67. [Google Scholar] [CrossRef]
- Yadav, K.; Prakash, S.; Yadav, N.P.; Sah, R.S. Multi-drug resistance of bacterial isolates among dental caries patients. Janaki Med. Coll. J. Med. Sci. 2016, 3, 37. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Han, D.; Cao, W.; Zheng, L.; Xie, Z.; Liu, H. Identification of Streptococcus mutans genes involved in fluoride resistance by screening of a transposon mutant library. Mol. Oral Microbiol. 2020, 35, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.D.S.M.; Azevedo, J.; Leal, H.F.; Queiroz, A.T.L.D.; da Silva Filho, H.P.; Reis, J.N. Bacterial diversity and prevalence of antibiotic resistance genes in the oral microbiome. PLoS ONE 2020, 15, e0239664. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.-L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M. Host-defense peptides with therapeutic potential from skin secretions of frogs from the family pipidae. Pharmaceuticals 2014, 7, 58–77. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Wang, X.; Liang, J.; Zhang, C.; Zhang, K.; Lin, G.; Lai, R. The first antimicrobial peptide from sea amphibian. Mol. Immunol. 2008, 45, 678–681. [Google Scholar] [CrossRef]
- Cao, J.; de la Fuente-Nunez, C.; Ou, R.W.; Torres, M.D.T.; Pande, S.G.; Sinskey, A.J.; Lu, T.K. Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synthet. Biol. 2018, 7, 896–902. [Google Scholar] [CrossRef]
- Tang, S.-S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.-F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation; characterisation; and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Gamal, M.I.; Abdin, S.; Alkhazraji, H.; Kamal, L.; Hammad, S.; El-Awady, F.; Waleed, D.; Kourbaj, L. Recent updates of marine antimicrobial peptides. Saudi Pharm. J. 2018, 26, 396–409. [Google Scholar] [CrossRef]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W.; Park, J.Y.; Seo, J.K.; Kim, D.G. Myticusin-beta; antimicrobial peptide from the marine bivalve, Mytilus coruscus. Fish Shellf. Immunol. 2020, 99, 342–352. [Google Scholar] [CrossRef]
- Huang, H.-N.; Rajanbabu, V.; Pan, C.-Y.; Chan, Y.-L.; Wu, C.-J.; Chen, J.-Y. A cancer vaccine based on the marine antimicrobial peptide pardaxin (GE33) for control of bladder-associated tumors. Biomaterials 2013, 34, 10151–10159. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, Y.; Qiu, Z.; Liu, Z.; Qiao, J.; Li, Y.; Caiyin, Q. Nisin variants generated by protein engineering and their properties. Bioengineering 2022, 9, 251. [Google Scholar] [CrossRef]
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek J. Microbiol. 1996, 69, 193–202. [Google Scholar] [CrossRef]
- Sears, P.M.; Smith, B.S.; Stewart, W.K.; Gonzalez, R.N.; Rubino, S.D.; Gusik, S.A.; Kulisek, E.S.; Projan, S.J.; Blackburn, P. Evaluation of a nisin-based germicidal formulation on teat skin of live cows. J. Dairy Sci. 1992, 75, 3185–3190. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, D.K.; Jani, R.H.; Blumbach, J.; Ganguli, B.N.; Klesel, N.; Limbert, M.; Seibert, G. Mersacidin; a new antibiotic from Bacillus. In vitro and in vivo antibacterial activity. J. Antibiot. 1992, 45, 839–845. [Google Scholar] [CrossRef]
- Kruszewska, D.; Sahl, H.G.; Bierbaum, G.; Pag, U.; Hynes, S.O.; Ljungh, A. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J. Antimicrob. Chemother. 2004, 54, 648–653. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin; apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Irazazabal, L.N.; Porto, W.F.; Ribeiro, S.M.; Casale, S.; Humblot, V.; Ladram, A.; Franco, O.L. Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim. Biophys. Acta 2016, 1858, 2699–2708. [Google Scholar] [CrossRef]
- Castle, N.A.; Haylett, D.G.; Jenkinson, D.H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989, 12, 59–65. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cheng, J.W.; Yu, H.Y.; Lin, L.; Chih, Y.H.; Pan, Y.P. Efficacy of a novel antimicrobial peptide against periodontal pathogens in both planktonic and polymicrobial biofilm states. Acta Biomater. 2015, 25, 150–161. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, K.; Ling, J.; Peng, Z.; Huang, X.; Liu, H.; Gu, L. Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch. Oral Biol. 2011, 56, 869–876. [Google Scholar] [CrossRef]
- Hilpert, K.; Volkmer-Engert, R.; Walter, T.; Hancock, R.E.W. High throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 2005, 23, 1008–1012. [Google Scholar] [CrossRef]
- Deslouches, B.; Phadke, S.M.; Lazarevic, V.; Cascio, M.; Islam, K.; Montelaro, R.C.; Mietzner, T.A. De novo generation of cationic antimicrobial peptides: Influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 2005, 49, 316–322. [Google Scholar] [CrossRef]
- Han, X.; Kou, Z.; Jiang, F.; Sun, X.; Shang, D. Interactions of designed Trp-containing antimicrobial peptides with DNA of multidrug-resistant Pseudomonas aeruginosa. DNA Cell Biol. 2020, 40, 414–424. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, Q.; Dong, W.; Liang, H.; Bi, X. The Effects of LPS on the activity of Trp-containing antimicrobial peptides against gram-negative bacteria and endotoxin neutralization. Acta Biomater. 2016, 33, 153–165. [Google Scholar] [CrossRef]
- Bi, X.; Wang, C.; Ma, L.; Sun, Y.; Shang, D. Investigation of the role of tryptophan residues in cationic antimicrobial peptides to determine the mechanism of antimicrobial action. J. Appl. Microbiol. 2013, 115, 663–672. [Google Scholar] [CrossRef]
- Borgwardt, D.S.; Martin, A.D.; Van Hemert, J.R.; Yang, J.; Fischer, C.L.; Recker, E.N.; Nair, P.R.; Vidva, R.; Chandrashekaraiah, S.; Progulske-Fox, A.; et al. Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses. Sci. Rep. 2015, 4, 3904. [Google Scholar] [CrossRef]
- van Dijk, I.A.; Veerman, E.C.I.; Reits, E.A.J.; Bolscher, J.G.M.; Stap, J. Salivary peptide histatin 1 mediated cell adhesion: A possible role in mesenchymal-epithelial transition and in pathologies. Biol. Chem. 2018, 399, 1409–1419. [Google Scholar] [CrossRef]
- Curvelo, J.A.R.; Moraes, D.C.; Anjos, C.A.D.; Portela, M.B.; Soares, R.M.A. Histatin 5 and human lactoferrin inhibit biofilm formation of a fluconazole resistant Candida albicans clinical isolate. An. Acad. Bras. Cienc. 2019, 91, e20180045. [Google Scholar] [CrossRef] [PubMed]
- Armata Pharmaceuticals, Inc. A Phase 2 Study to Evaluate the Microbiology; Safety and Tolerability of C16G2 Varnish and Strip in Adolescent and Adult Subjects (C3J17−206−00). Available online: https://clinicaltrials.gov/ct2/show/NCT03196219 (accessed on 24 August 2022).
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.G.; Liu, S.W.; Walsh, K.A.; Adams, G.G.; Stanton, D.P.; Ward, B.R.; Shen, P.; O’Brien-Simpson, N.M.; Reynolds, E.C. Streptococcus mutans biofilm disruption by κ-casein glycopeptide. J. Dent. 2013, 41, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Harris, F.; Mura, M.; Phoenix, D.A. An Atlas of anionic antimicrobial peptides from amphibians. Curr. Protein Pept. Sci. 2018, 19, 823–838. [Google Scholar] [CrossRef]
- Cabras, T.; Patamia, M.; Melino, S.; Inzitari, R.; Messana, I.; Castagnola, M.; Petruzzelli, R. Pro-oxidant activity of histatin 5 related Cu (II)-model peptide probed by mass spectrometry. Biochem. Biophys. Res. Commun. 2007, 358, 277–284. [Google Scholar] [CrossRef]
- Zawisza, I.; Mital, M.; Polkowska-Nowakowska, A.; Bonna, A.; Bal, W. The impact of synthetic analogs of histidine on copper (II) and nickel (II) coordination properties to an albumin-like peptide. Possible leads towards new metallodrugs. J. Inorg. Biochem. 2014, 139, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides, origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Lin, B.; Li, R.; Handley, T.N.G.; Wade, J.D.; Li, W.; O’Brien-Simpson, N.M. Cationic antimicrobial peptides are leading the way to combat oropathogenic infections. ACS Infect. Dis. 2021, 7, 2959–2970. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. WIREs Nanomed. Nanobiotechnol. 2013, 5, 49–66. [Google Scholar] [CrossRef]
- Deshayes, C.; Arafath, M.N.; Apaire-Marchais, V.; Roger, E. Drug delivery systems for the oral administration of antimicrobial peptides, promising tools to treat infectious diseases. Front. Med. Technol. 2022, 3, 778645. [Google Scholar] [CrossRef]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Lo, E.C.; Samaranayake, L.P. Antibacterial effects of silver diamine fluoride on multispecies cariogenic biofilm on caries. An. Clin. Microbiol. Antimicrob. 2013, 12, 4. [Google Scholar] [CrossRef]
- Eckert, R.; He, J.; Yarbrough, D.K.; Qi, F.; Anderson, M.H.; Shi, W. Targeted killing of Streptococcus mutans by a pheromone-guided “smart” antimicrobial peptide. Antimicrob. Agents Chemother. 2006, 50, 3651–3657. [Google Scholar] [CrossRef]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-assisted self-assembled polypeptide nanogels to selectively combat bacterial infection. ACS Appl. Mater. Interf. 2019, 11, 33599–33611. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe1, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Engler, A.C.; Shukla, A.; Puranam, S.; Buss, H.G.; Jreige, N.; Hammond, P.T. Effects of side group functionality and molecular weight on the activity of synthetic antimicrobial polypeptides. Biomacromolecules 2011, 12, 1666–1674. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chang, H.Y.; Lu, J.K.; Huang, Y.C.; Harroun, S.G.; Tseng, Y.T.; Li, Y.J.; Huang, C.C.; Chang, H.T. Self-assembly of antimicrobial peptides on gold nanodots: Against multidrug-resistant bacteria and wound-healing application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Rai, A.; Pinto, S.; Velho, T.R.; Ferreira, A.F.; Moita, C.; Trivedi, U.; Evangelista, M.; Comune, M.; Rumbaugh, K.P.; Simões, P.N. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 2016, 85, 99–110. [Google Scholar] [CrossRef]
- Akrami, M.; Balalaie, S.; Hosseinkhani, S.; Alipour, M.; Salehi, F.; Bahador, A.; Haririan, I. Tuning the anticancer activity of a novel pro-apoptotic peptide using gold nanoparticle platforms. Sci. Rep. 2016, 6, 31030. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef]
- Zhong, Z.; Male, K.B.; Luong, J.H.T. More recent progress in the preparation of Au nanostructures, properties, and applications. Anal. Lett. 2003, 36, 3097–3118. [Google Scholar] [CrossRef]
- Tang, L.; Azzi, J.; Kwon, M.; Mounayar, M.; Tong, R.; Yin, Q.; Moore, R.; Skartsis, N.; Fan, T.M.; Abdi, R.; et al. Immunosuppressive activity of size-controlled PEG-PLGA nanoparticles containing encapsulated cyclosporine A. J. Transplant. 2012, 2012, 896141. [Google Scholar] [CrossRef] [PubMed]
- Laridi, R.; Kheadr, E.E.; Benech, R.O.; Vuillemard, J.C.; Lacroix, C.; Fliss, I. Liposomeencapsulated nisin Z: Optimization, stability and release during milk fermentation. Int. Dairy J. 2003, 13, 325–336. [Google Scholar] [CrossRef]
- Benech, R.O.; Kheadr, E.E.; Laridi, R.; Lacroix, C.; Fliss, I. Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl. Environ. Microbiol. 2002, 68, 3683–3690. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Yang, L.; Narsimhan, G.; Bhunia, A.K.; Yao, Y. Designing carbohydrate nanoparticles for prolonged efficacy of antimicrobial peptide. J. Control. Release 2011, 150, 150–156. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, M.; de Melo Carrasco, L.D. Novel formulations for antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 18040–18083. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Sibuyi, N.R.S.; Madiehe, A.M.; Meyer, M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Haakansson, J.; Bjoern, C.; Lindgren, K.; Sjoestroem, E.; Sjoestrand, V.; Mahlapuu, M. Efficacy of the novel topical antimicrobial agent PXL150 in a mouse model of surgical site infections. Antimicrob. Agents Chemother. 2014, 58, 2982–2984. [Google Scholar] [CrossRef]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nakagami, H.; Maeda, A.; Morishita, R.; Miyazaki, N.; Ogawa, T.; Tabata, Y.; Kikuchi, Y.; Hayashi, H.; Tatsu, Y.; et al. Development of a novel antimicrobial peptide, AG-30, with angiogenic properties. J. Cell. Mol. Med. 2009, 13, 535–546. [Google Scholar] [CrossRef]
- Na, D.H.; Faraj, J.; Capan, Y.; Leung, K.P.; DeLuca, P.P. Chewing gum of antimicrobial decapeptide (KSL) as a sustained antiplaque agent: Preformulation study. J. Control. Release 2005, 107, 122–130. [Google Scholar] [CrossRef]
- Faraj, J.A.; Dorati, R.; Schoubben, A.; Worthen, D.; Selmin, F.; Capan, Y.; Leung, K.; DeLuca, P.P. Development of a peptide-containing chewing gum as a sustained release antiplaque antimicrobial delivery system. AAPS PharmSciTech. 2007, 8, E177–E185. [Google Scholar] [CrossRef]
- Sanna, V.; Roggio, A.M.; Siliani, S.; Piccinini, M.; Marceddu, S.; Mariani, A.; Sechi, M. Development of novel cationic chitosan-and anionic alginate–coated poly (D, L-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int. J. Nanomed. 2012, 7, 5501. [Google Scholar]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wu, H.; Yin, T.; Wang, L.; Shan, A. Nanostructured antimicrobial peptides: Crucial steps of overcoming the bottleneck for clinics. Front. Microbiol. 2001, 12, 710199. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Das, P.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.H.T.; Gedanken, A. Antimicrobial activities of Zn-doped CuO microparticles decorated on polydopamine against sensitive and antibiotic-resistant bacteria. ACS Appl. Polym. Mater. 2020, 2, 5878–5888. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.T.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio. Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial peptides: Interaction with model and biological membranes and synergism with chemical antibiotics. Front. Chem. 2018, 6, 204. [Google Scholar] [CrossRef]
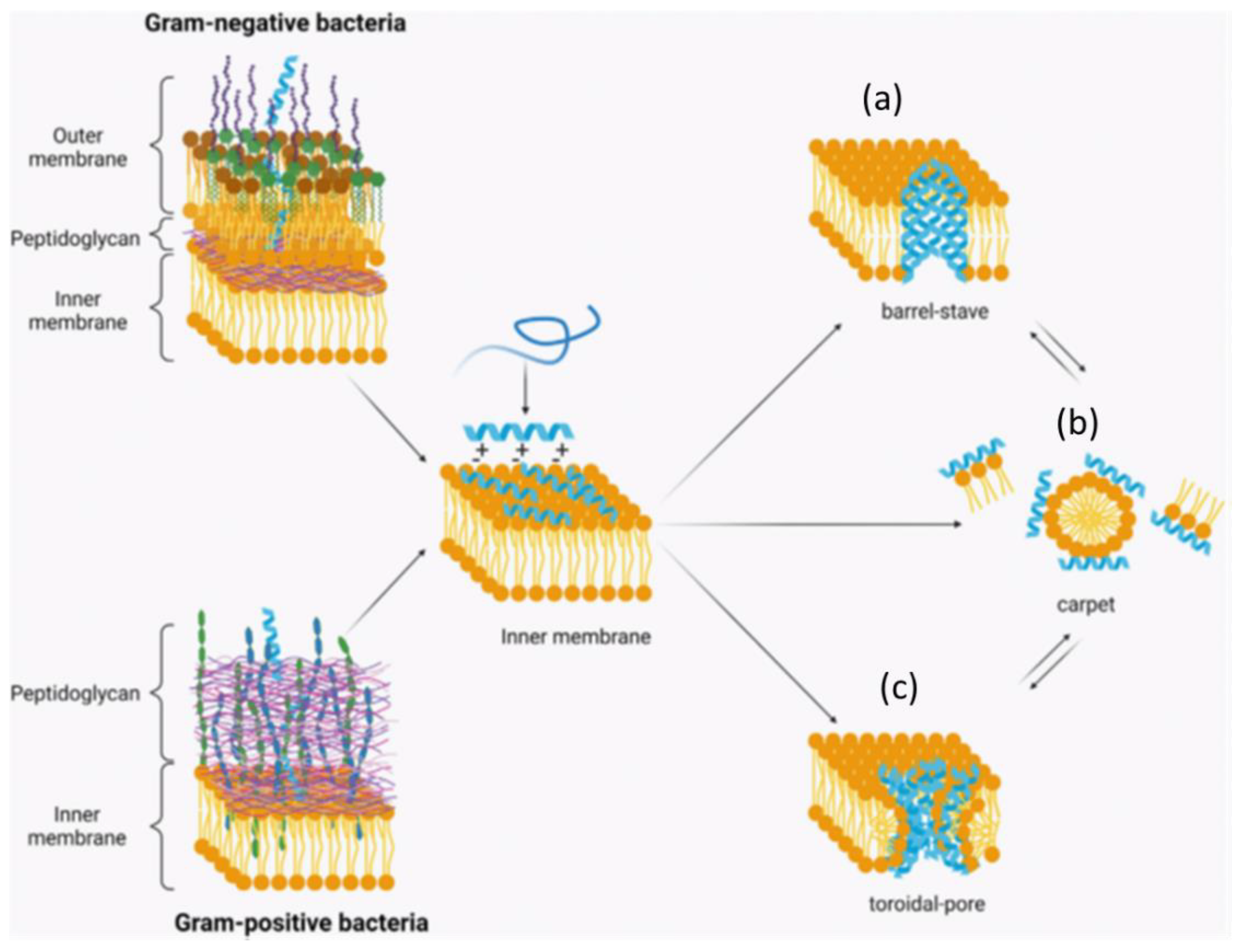
| Small Molecules | General Observations | Ref. |
|---|---|---|
| Fluoride (F−, an ionized form of the element fluorine, F) | A key component in potable water, mouthwashes, toothpaste, and oral supplements to ward off dental cavities | |
| Binding to the tooth surface to promote remineralization and balance acid-stimulated demineralization | [24] | |
| Fluoride impedes the enolase activity of S. mutans and other Streptococci. The enzyme reversibly catalyzes D-2-phosphoglycerate and phosphoenolpyruvate in glycolysis and gluconeogenesis. | [27,28] | |
| Fluoride might invoke dental and skeletal fluorosis and emerging fluoride-resistant oral bacteria. | [29] | |
| Chlorhexidine- a cationic polybiguanide-C22H30Cl2N10, Mw = 505.45 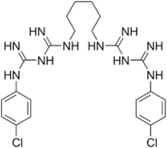 | A “gold standard” of antiplaque agents. | [30] |
| Effective against bacteria and yeasts by disrupting their inner cell membranes. | [30,31] | |
| Plaque adhesion is prevented as the acidic groups of salivary glycoproteins are blocked by chlorhexidine. | ||
| Reduces the bacterial attachment to tooth surfaces by competing with Ca agglutination. | [32] | |
| Inducing DNA damage in oral mucosal cells, white blood cells, kidney cells, and cellular apoptosis. | [33,34] | |
| Quaternary Ammonium Salts (positive charge) | Widely used in mouth rinses to inhibit oral plaque. | [35] |
| Binding to the negatively charged bacterial cells to invoke bacterial lysis. | [36] | |
| Several side effects: convulsions, hypotension, gastrointestinal symptoms, coma, and even fatality. | [37] | |
Triclosan- C12H7Cl3O2, Mw = 289.54 | Added to many consumer products including toothpaste to reduce or prevent bacterial contamination. | [38] |
| This broad-spectrum agent blocks enoyl–acyl carrier protein reductase, an enzyme required for microbial lipid synthesis | [39] | |
| Susceptible to bacterial resistance. | [40] | |
| By 2015, Johnson & Johnson had removed triclosan from all of its products. | [41] |
| Antibiotics | Mechanisms, Drug Resistance, and Side Effect | Ref. |
|---|---|---|
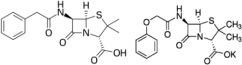 Penicillin: β-lactam antibiotic Penicillin G (benzylpenicillin) or penicillin V (phenoxymethylpenicillin) is frequently prescribed PenG- C16H18N2O4S, Pen V Mw = 334.4 C16H18N2O5S Mw = 350.39 | Inhibits the formation of peptidoglycan in the cell walls. | |
| Effective against G+ Streptococci and Staphylococci, and some G− bacteria. | [49] | |
| Bacterial resistance as they produce mecA that encodes PBP2a with low binding affinity to β-lactams. | [50,51] | |
| Diarrhea, nausea, rash, urticaria, hypersensitivity, and neurotoxicity. | [52] | |
 Tetracyclines-C22H24N2O8, Mw = 444.440 | Binds to the mRNA translation complex via its 30S ribosomal subunit to inhibit protein synthesis. | [53,54] |
| Cramps, stomach burning, diarrhea, sore mouth, nephrotoxicity, non-oliguric acute renal failure, and teeth discoloration. | [55,56,57] | |
Metronidazole- C6H9N3O3, Mw = 171.16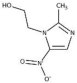 | Effective against oral obligate anaerobes as it inhibits nucleic acid synthesis by disrupting DNA. | [58] |
| Causing several side effects including nausea, headaches, and tachycardia. | [59] | |
| Potential use in treating periodontitis | ||
 Macrolides (Erythromycin: C37H67NO13, MW = 733.93) | Effective against Streptococci, Staphylococci, Pneumococci, and Enterococci. | [60] |
| Myopathy, enterohepatic recycling, and cholestasis. | [61] | |
| Erythromycin can decrease 35% of the plaque amount after one week. | [62] | |
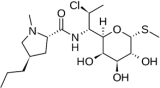 Clindamycin C18H33ClN2O5S, Mw = 424.98 | Inhibits bacterial protein synthesis by disrupting ribosomal translocation. | [63] |
| Effective against anaerobic bacteria. | [64] | |
| An alternative to treat patients with allergy to penicillin or penicillin-resistant infection. | ||
| Diarrhea, nausea, abdominal pain, vomiting pseudomembranous colitis, and contact dermatitis | [65] |
| Amino Acids | Abbreviation with 3 Letter Code or One Single Capital Letter Code |
|---|---|
| Polar (6 AAs) but not charged | Asparagine (Asn, N), Cysteine (Cys, C), Glutamine (Gln, Q), Threonine (Thr, T), Tyrosine (Tyr, Y), Serine (Ser, S) |
| Hydrophobic (9 AAs) | Alanine (Ala, A), Glycine (Gly, G), Isoleucine (Ile, I), Leucine (Leu, L), Methionine (Met, M), Phenylalanine (Phe, F), Proline (Pro, P), Tryptophan (Trp, W), Valine (Val, V). Mostly their carbon and hydrogen, have very small dipole moments and tend to be repelled from water |
| Negative charged (pH 7) (2 AAs) | Aspartate (Asn, D), Glutamate (Glu, E) |
| Positive charges (pH 7) (3, AAs) | Arginine (Arg, R), Histidine (His, H), Lysine (Lys, K), |
| Histatins | General Observations, AA Sequence of Histatins (One Letter Code) |
|---|---|
| His-1 (salivary glands-bone marrow): 38 AA, Mw ∼4929 Da | DSPHEKRHHGYR [His-2 or RKFHEKHHSHREFPFYGDYGSNYLYDN] |
| His-5 (salivary glands): 24 AA, Mw ∼3037 Da | DSHA[His-12]R[His-8] or DSHA-KRHHGYK- R-KFHEKHHSHRGY |
| His- 3: 32 AA, Mw ∼4063 Da Proteolytic fragments in saliva | [His-5]RSNYLYDN or [His-6]SNYLYDN |
| His-2 (salivary glands) (27 AA) | RKFHEKHHSHREFPFYGDYGSNYLYDN |
| His-4 (20 AA) | [His-7]RSNYLYDN or RKFHEKHHSHRGY-RSNYLYDN |
| His-6 (25 AA) | [His-5]-R |
| His-7 (13 AA) | R[His-8] or R-KFHEKHHSHRGY |
| His-8 (12 AA) | KFHEKHHSHRGY |
| His-9 (14 AA) | [His-7]R or RKFHEKHHSHRGY-R |
| His-10 (13 AA) | [His-8]R or KFHEKHHSHRGY-R |
| His-11 (8 AA) | [His-12]R or KRHHGYK-R |
| His-12 (7 AA) | KRHHGYK |
| hBD | Microorganisms and Minimum Inhibition Concentrations (MUC) | Ref. |
|---|---|---|
| hBD1,2,3 | - Activities against S. mutans, E. faecalis, and other oral pathogens. hBD-2 and -3 are inducible by the bacterial LPS, whereas hBD-1 averts normal flora from becoming opportunistic. | [93] |
| hBD-2 | - Effective against C. albicans and is also induced by lichen-planus related inflammation. | [94] |
| β-defensins | - Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum are two periodontal pathogens. | [95] |
| hBD-3 | - MIC = 12.5 mg/L for F. nucleatum, 100–200 mg/L for P. gingivalis, A. actinomycetemcomitans, and Prevotella intermedia. | [96] |
| hBD-2/hBD-3 | - MIC = 3.9–250 μg/mL and 1.4–250 μg/mL) for several oral pathogens and Candida spp. | [97] |
| β-defensins | - Pathogens including T. denticola likely interfere with the signal pathway to subdue the expression of β-defensins to develop the resistance against the peptides | [98] |
| α-Defensins | Sequence One Letter Code | |
|---|---|---|
| hNP-1 (30 AAs) | A-[hNP-2] (Disulfide bridge (DB): 2-30, 4-19, 9-29) | |
| hNP-2 (29 AAs) | CYCRIPACIAGERRYGTCIYQGRLWAFCC Begins and ends with cysteine residues. DB: 1-29, 3-18, 8-28 | |
| hNP-3 (30 AAs) | D-[hNP-2] (DB: same as hNP-1) | |
| hNP-4 | VCSC RLVFC RRTEL RVGNC LIGG VSFTY CCTRVD (DB: same as hNP-1) | |
| α-defensins regulate complement activation and enhance macrophage phagocytosis. | [101] | |
| β-defensins | Sequence One Letter Code | |
| hBD-1 | DHYNCVSSGG QCLYSACPIF TKIQGTCYRG KAKCCK (36 AA residues with six Cys forming three intramolecular disulfides) P. gingivalis, A. actinomycetemcomitans, and F. nucleatum | |
| hBD-2 | GIGDPVTCLK SGAICHPVFC PRRYKQIGTC GLPGTKCCKK P Effective against G-bacteria and C. albicans, but less effective against G+ bacteria. | [102] |
| hBD-3 | GIINTLQKYY CRVRGGRCAV LSCLPKEEQI GKCSTRGRKC CRRKK (45 AAs, Disulfide bridge: 11-40, 18-33, 23-41) Effective against several bacteria including S. mutans and P. gingivalis | |
| Charge | Ref. |
|---|---|
| A charge of +1 provided by Arg or Lys residues is needed but there is no correlation between high positive charges with high activities. | [115] |
| However, high positive charges (>+9) are related to high hemolytic activities. The Lys substitution is less hemolytic than the Arg | [116] |
| substitution. In general, analogs with net positive charges invoke high cytotoxicity. | |
| Hydrophobicity/Hydrophobic Moment (HM) | |
| The second strongest force behind the charge drives AMPs (to the bacterial membrane). A single AA replacement might alter hydrophobicity and subsequently activity. The α-helical peptide with high hydrophobicity of its nonpolar face possesses high activities. | [117,118,119] |
| However, there is limited hydrophobicity to attain maximal activity without any undesirable effects. The side chain and bulkiness of AMPs also affect the antimicrobial activity. Phe > Ile > Leu > Val for the order of side chain length and Phe > Leu > Ile > Val for bulkiness. Increasing overall hydrophobicity might increase activity and hemolysis, provided there is no change in amphipathicity, helicity, or net charge. The hydrophobic moment (HM) or an index of amphipathicity is also attributed to antimicrobial activity, indicating the peptide’s capability to switch from a polar face to a nonpolar face right after its insertion into the membrane. | [120,121] |
| The hydrophobic moment is defined as the vertical sum of individual AA hydrophobicity of a specific peptide over that of an ideal α-helix peptide. For instance, Hp1404-T1 (HM = 0.699), has 16-fold less antimicrobial activity compared to Hp1404-T1e with an HM of 0.831. | [121] |
| Compared to hydrophobicity, helical amphipathicity is a stronger force in interfacial binding. | [122] |
| Length | |
| AMPs often have <20 amino acid residues (AAR). | [123] |
| A peptide transversing the lipid bilayer requires 18 AAR, 2–4 AAR per turn for an α-helix, and 7–8 AAR (two turns) for amphipathic faces. | [124] |
| The smaller peptides are considered safer than their bigger counterparts. | [116] |
| Compared to melittin (15 AA), an analog derived from the peptide C-terminus exhibits 7-fold lower antimicrobial activity and 300-fold lower hemolytic activity. | [125] |
| AA Sequence | |
| A proper peptide sequence often results in an α-helix and histidine is not often found in AMPs, as its size impairs the AMP insertion into the membrane. | [126,127,128] |
| Arg is most critical to antimicrobial activity to display both hydrophobic and electrostatic interactions with bacterial membranes. | [127,128] |
| The cysteine function in AMPs is still unknown; however, the replacement of Cys with Ala or Leu reduces the hBD-1 activity. Peptides could not penetrate the membrane if their cysteines form disulfide bridges. | [129] |
| Self-Association | |
| To a certain extent, peptide self-association (dimerize) enhances antimicrobial activity | [130] |
| However, strong self-association prevents the penetration of AMPs into the cytoplasmic membrane. | |
| Synthetic AMPs: Design and Performances |
|---|
| AMPCol on smooth titanium surface: A synthetic antimicrobial peptide (Tet213, KRWWKWWRRC) is conjugated to free amines of collagen IV to form AMPCol. The coating of AMPCol on smooth titanium surfaces sustains its release to prevent peri-implantitis [131]. C16G2: It targets S. mutans and S. salivarius. It shows an overall potency of C16G2 against G- species. C16G2 is also effective against two G+ bacteria from human skin flora [132]. Peptide (CSP), sequence (SGSLSTFFRLFNRSFTQALGK), CSPC16, preserves pheromone activity, whereas the 8-TFFRLFNR region within CSPC16, CSP M8, is sufficient for its specific delivery to S. mutans [133]. D1–23 (FLPKTLRKFFARI RGGRAAVLNA) with low cytotoxicity outperforms chlorohexidine against S. mutans biofilm and it is also effective against S. mitis and S. salivarius [134]. It also shows D-GL13K (GKIIKLKASLKLL-NH2) [135]. PR39 (RRRPRPPYLP RPRPPPFFPP RLPPRIPPGF PPRFPPRFP-NH2 (only 7 different AAs) [136] and VSL2 [AcA∆FKA∆FWK∆FVK∆FVK-NH2] where Ac is an acetyl group [137] and ∆F = ΔPhe, α,β-dihydrophenylalanine, show activity against E. faecalis at different levels [138]. DJK-5: VQWRAIRVRVRVIR shows activities against E. faecalis, P. aeruginosa, and a mixture of S. aureus, S. epidermidis [139]. GH12: α- helical AMP with a sequence of GLLWHLLHHLLH-NH2 is effective against oral Streptococci and reduces the exopolysaccharide production by S. mutans [140,141]. HBD3–15: Derived from hBD-3, consists of 15 AAs (GKCSTRGRKCCRRKK). Effective against S. mutans, S. gordonii, and E. faecalis on biofilm formation [134,142]. IDR-1002 with a sequence of VQRWLIVWRIRK-NH2 exhibits antibacterial properties and anti-inflammatory of S. aureus and E. faecalis [143]. KSL [KKVVFKVKFK-NH2] KSL inhibits oral pathogens associated with dental caries and plaque [144]. KSL is cleaved at K6–V7 in the human saliva and F5–K6 in simulated gastric fluids [145]. An analog of KSL, where the L6 residue is replaced with W, is stable and preserves its activity against several oral pathogens [146]. P-113/Nal-P-113: Biocompatible His-5 is easily degradable in saliva, plasma, and serum [147,148]. Nal-P-113 is synthesized from His-5 (AKRHHGYKRKFH) by substituting histidine with β-naphthylalanine. This modified peptide is highly stable in physiological conditions and is effective against P. gingivalis, an asaccharolytic G- anaerobic bacterium. ToAP2 (FFGTLFKLGSKLIPGVMKLFSKKKER, 26 AA) and NDBP-5.7 (ILSAIWSGIKSLF-NH2), the two peptides are effective against C. albicans, but the former is more active and effective than the latter [149]. |
| Activity/Selectivity/Cytotoxicity |
|---|
| Charge effect AR-23 (melittin-related peptide with 23 AA, a charge of +4, and an α-helical amphipathic structure, GIGAVLKVLTTGLPALISWIKRKRQQ-NH2). If Ala is replaced by Arg or Lys, a variant with 3 substitutions acquires a net charge of +7 with reduced hemolytic activity. Hemolysis is more pronounced with the Arg substitution than the Lys substitution. Interpretation of the charge effect is complicated and nebulous as the substitutions also alter the amphipathicity, helicity, and hydrophobicity of the peptide [150]. Aurein 1,2 (53.04% α-helix) is a natural peptide with 13-AA (GLFDIIKKIAESF) and a net charge of +1. Replacement of Asp 4 and Glu 11 with Lys increases the net charge to +5. The modified peptide (78.44% α-helix) exhibits improved efficacy against E. coli and P. aeruginosa [151]. Further substitution, Ala 10 for Try, the resulting peptide with 36.36% α-helical content exhibits higher antimicrobial activity and lowest hemolysis. Such results are more relevant to the charge increase, not the increased α-helicity. The AA sequence and the Trp substitution position are also two major contributing factors. V13Kʟ with 26 AA and a net charge of +7: [Ac-AKWKSFLKTFKSAKKTVLHTALKAISS–amide] On the polar face, a variant of this peptide with a charge decrease to +4 significantly reduces both antimicrobial and hemolytic activities. A variant (+8) acquires higher antimicrobial activity, whereas the hemolytic activity remains unchanged. Any further increase in charge improves activity, however, the peptide becomes significantly more hemolytic [152]. |
| Hydrophobicity vs. hemolysis V13Kʟ: If Val 16 is substituted with Leu, the variant (V16L) becomes more hydrophobic with slightly increased activity. However, it causes 53.9% hemolysis vs. 28.3% [153]. When Val 16 is substituted with Ala, a new variant (V16A) becomes less hydrophobic and causes only 14.3% hemolysis. It has the same MIC for clinically isolated P. aeruginosa ATC2853 and E. coli, compared to V13Kʟ. C18G: A platelet factor IV-derived AMP [ALWKKLLKKLLKSAKKLG] The substitution of Leu to Phe or Ile has no effect on its MIC against 5 tested bacteria. The replacement by Val, however, increases the MIC values against all such bacteria. The effect is more pronounced with α-aminoisobutyric acid substitution [154]. |
| Amino acid sequence and composition Lys and Arg with similar charges are often used to design synthetic AMPs. Higher activity is obtained with the Arg substitution over the Lys substitution [155]. The Arg side chain interacts with two bacterial lipid head groups, compared to one for the Lys side chain [156]. Arg-rich AMPs by ion-pair–π interactions promote enhanced peptide-membrane interactions as exemplified by indolicidin [ILPWKWPWWPWRR-NH2] and tritrpticin [VRRFPWWWPFLRR], two Arg and Trp rich peptides [157]. Octa 2 with a sequence of RRWWRWWR is another Trp- and Arg-rich peptide with activities against E. coli, P. aeruginosa, and S. aureus. Short Trp- and Arg-rich AMPs can be derived from murine or bovine lactoferricin with strong activities [158]. D-amino acids might have higher activities than ʟ-amino acids. The D-form of sapecin B (88 AAs) has a significantly lower MIC than its L-isomer counterpart: 16-fold with S. aureus and only 2-fold with E. coli [159]. However, the D-forms and L-forms of Kn2–7 [FIKRIARLLRKIF], mastoparan M [INKAIAALAKKLL-NH2], and temporins (10–14 Aas) have comparable activities. Phen 3 and 13 in Aurein 1.2 bind to bacterial membranes, thus their substitution by Ala decreases activity [160]. Proline (nonpolar AA)-rich peptides (PrAMPs) enter the cytoplasm via the inner membrane transporter SbmA to target ribosomes or interfere with protein synthesis [161]. Octa 2: replacing Arg residues with His, the modified peptide shows good therapeutic potential [162]. L4H4 [NH2-KKALLAHALAHLALLALHLALHLKKA-Amide]: by inserting four His sequences in Leu and Ala, the modified amphiphilic magainin shows good properties for cell penetration and antibacterial activity [163]. Attacins and diptericins have 14% to 22% glycine residues [164,165]. Salmonid cathelicidins (glycine-rich) activate phagocyte-mediated microbicidal activity, unlike the conventional mode of AMPs [166]. The glycine-rich central–symmetrical GG3 is considered a candidate against G-bacteria [167]. Cys-rich peptides (defensins). Antimicrobial activities stem from their unique amino acid sequence against G− and G+ bacteria, fungi, and enveloped viruses. |
| Side change The N-terminal with added Acyl groups or fatty acid chains enhances the antimicrobial activity; in contrast, the use of octanoyl, or 6-methyl-octanoyl results in weak activity [168]. LL-37 [LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES] with increasing hydrophobicity, and activity is obtained when different-length fatty acid chains are added to the peptide. Increasing alkyl chain lengths beyond 8 carbons (C8), however, reduces activity as the modified AMP is more prone to self-assembly [169]. C8-KR12-NH2 shows only <10% hemolysis at very high concentrations. However, the incorporation of C10 significantly increases hemolytic activity. Adding fatty acid chains to anoplin (an amphipathic, α-helical peptide of wasp venom, [GLLKRIKTLL-NH2] results in improved activity with increased hemolysis. The latter is correlated to the chain length though hemolysis is only 10% [170]. The lipidation effect on AMP activity is further reviewed elsewhere [171,172]. |
| Natural AMPs | Characteristics and Antimicrobial Activities | Ref. |
|---|---|---|
| Amphibian-Derived | Magainin 1 [GIGKFLHSAGKFGKAFVGEIMKS] and magainin 2 [GIGKFLHSAKKFGKAFVGEIMNS], skin secretions of frogs. Both have 23 Aas each and differ by two substitutions. | [185] |
| Insect-Derived | Cancrin [GSAQPYKQLHKVVNWDPYG] (The first peptide from the sea amphibian Rana cancrivora). Cecropin-31–37 Aas (guppy silkworm, bees, Drosophila). | [186] |
| Microorganisms-Derived | Nisin and gramicidin (linear peptides with 15 amino acids) from Lactococcus lactis Bacillus brevis, and Bacillus subtilis. | [187] |
| Plant-derived | Thionins (45–48 AA, 6–8 Cys forming 3–4 disulfide bonds). The C-terminal region with 12 conserved Cys residues contributes to its stability). | [188] |
| Marine-derived | As-CATH4 (immunity-stimulating effect in vivo). Myticusin-beta is a promising antimicrobial agent. The GE33-based vaccine can enhance antitumor immunity in mice. GE33 is also known as pardaxin with the following sequence: GFFALIPKISSPLFKTLSAVGSALSSSGGQE-OH. | [189,190,191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luong, A.D.; Buzid, A.; Luong, J.H.T. Important Roles and Potential Uses of Natural and Synthetic Antimicrobial Peptides (AMPs) in Oral Diseases: Cavity, Periodontal Disease, and Thrush. J. Funct. Biomater. 2022, 13, 175. https://doi.org/10.3390/jfb13040175
Luong AD, Buzid A, Luong JHT. Important Roles and Potential Uses of Natural and Synthetic Antimicrobial Peptides (AMPs) in Oral Diseases: Cavity, Periodontal Disease, and Thrush. Journal of Functional Biomaterials. 2022; 13(4):175. https://doi.org/10.3390/jfb13040175
Chicago/Turabian StyleLuong, Albert Donald, Alyah Buzid, and John H. T. Luong. 2022. "Important Roles and Potential Uses of Natural and Synthetic Antimicrobial Peptides (AMPs) in Oral Diseases: Cavity, Periodontal Disease, and Thrush" Journal of Functional Biomaterials 13, no. 4: 175. https://doi.org/10.3390/jfb13040175
APA StyleLuong, A. D., Buzid, A., & Luong, J. H. T. (2022). Important Roles and Potential Uses of Natural and Synthetic Antimicrobial Peptides (AMPs) in Oral Diseases: Cavity, Periodontal Disease, and Thrush. Journal of Functional Biomaterials, 13(4), 175. https://doi.org/10.3390/jfb13040175








