Selective Destruction of Soluble Polyurethaneimide as Novel Approach for Fabrication of Insoluble Polyimide Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
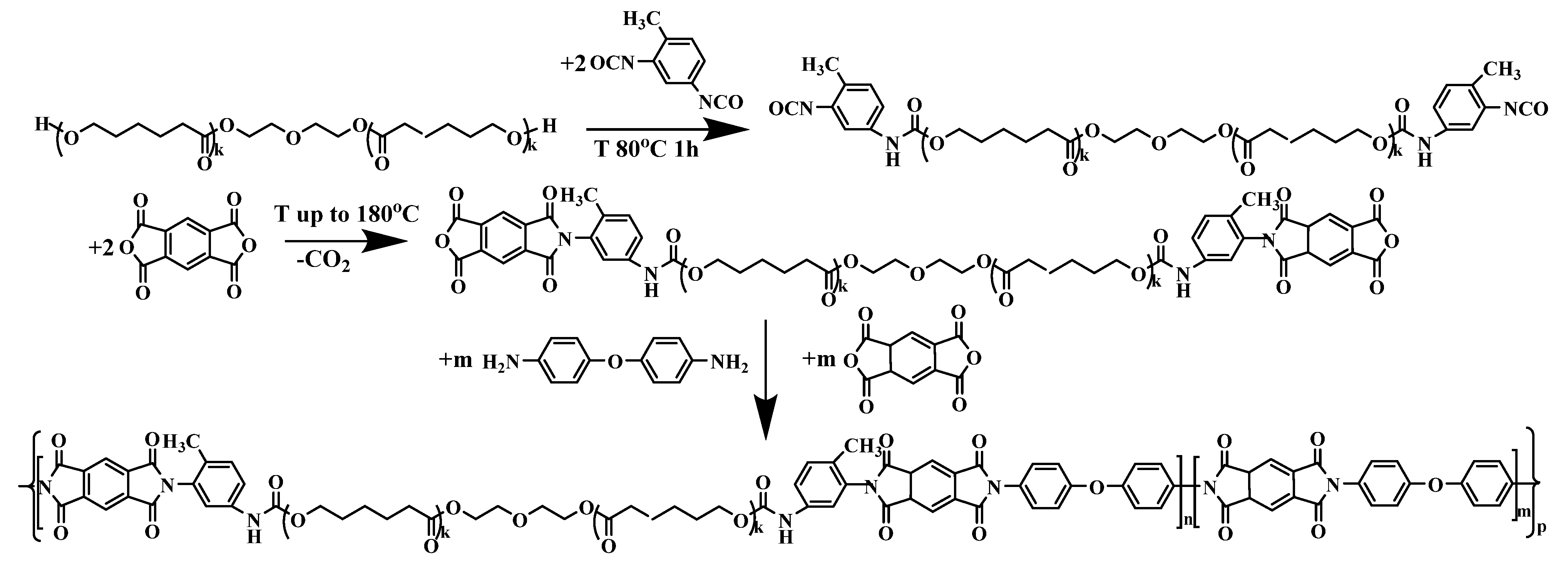
2.2. Synthesis Method
2.3. Synthesis of an Initial Macromonomer with Urethane Groups in the Chain
2.4. Synthesis of Copoly(Urethane-Imide) Prepolymer
2.5. Obtaining Film Samples of Copoly(Urethane-Imide)
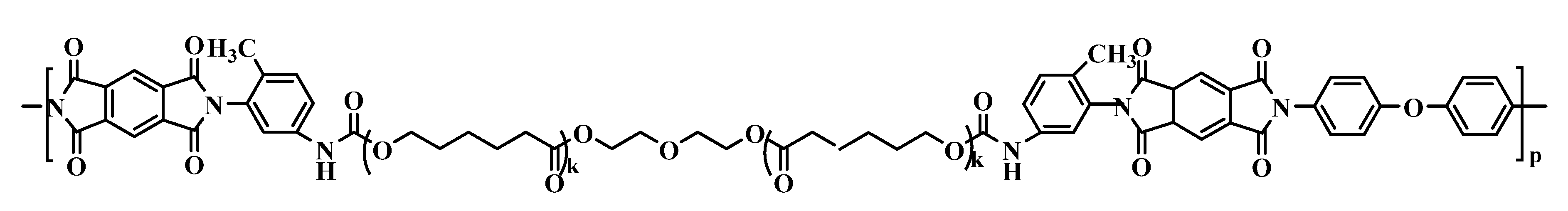
2.6. The Synthesized Polymer Corresponds to the Structural Formula
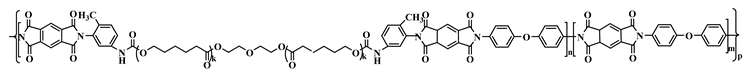 where n = 1, m = 10.
where n = 1, m = 10.2.7. Thermolysis of Copoly(Urethane-Imide)
2.8. Hydrolysis of Thermalized Samples of Copoly(Urethane-Imide)
2.9. Methods of Research
3. Results
3.1. TGA Studies of Synthesized Copolymers
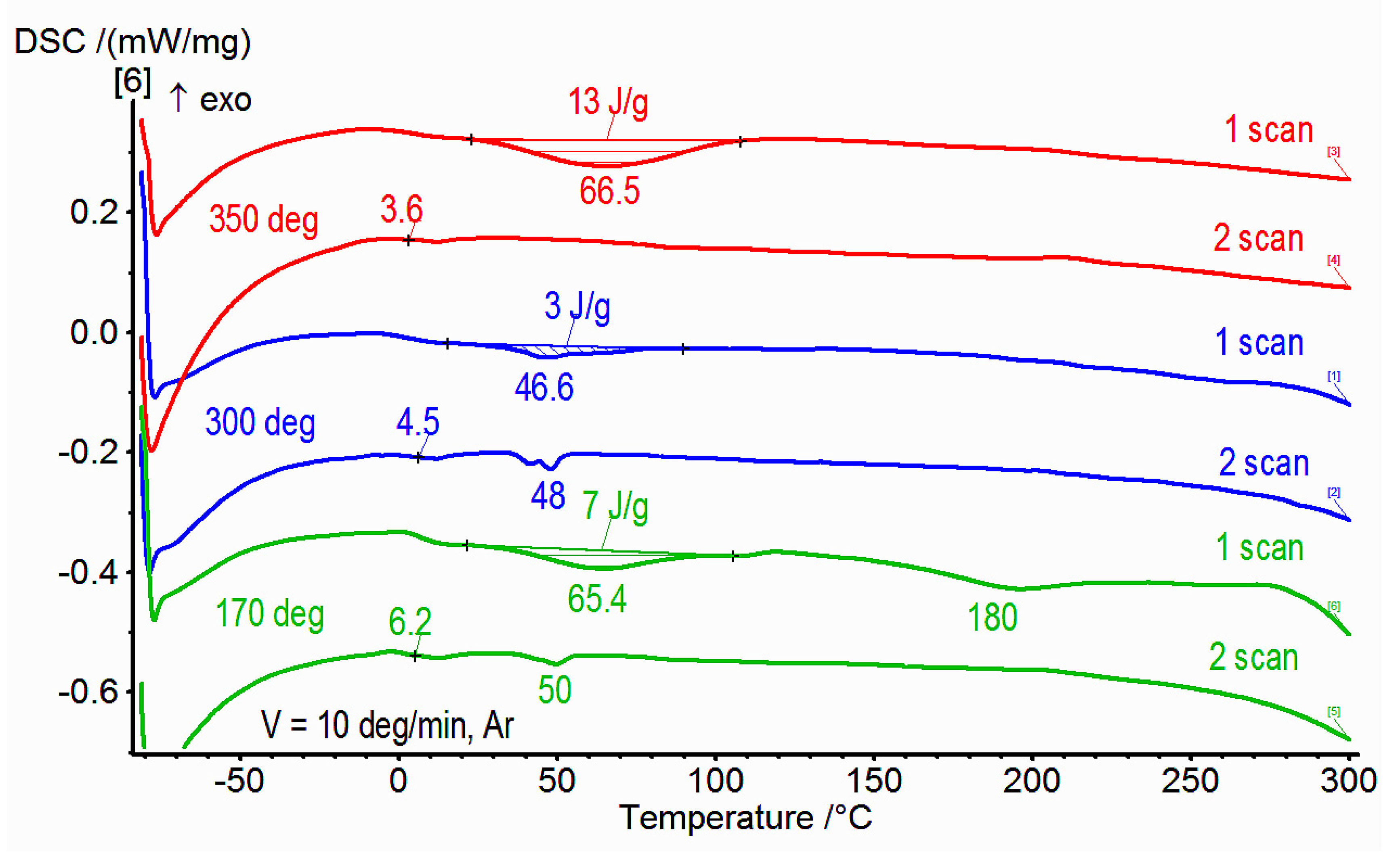
3.2. DSC Studies of Synthesized Copolymers
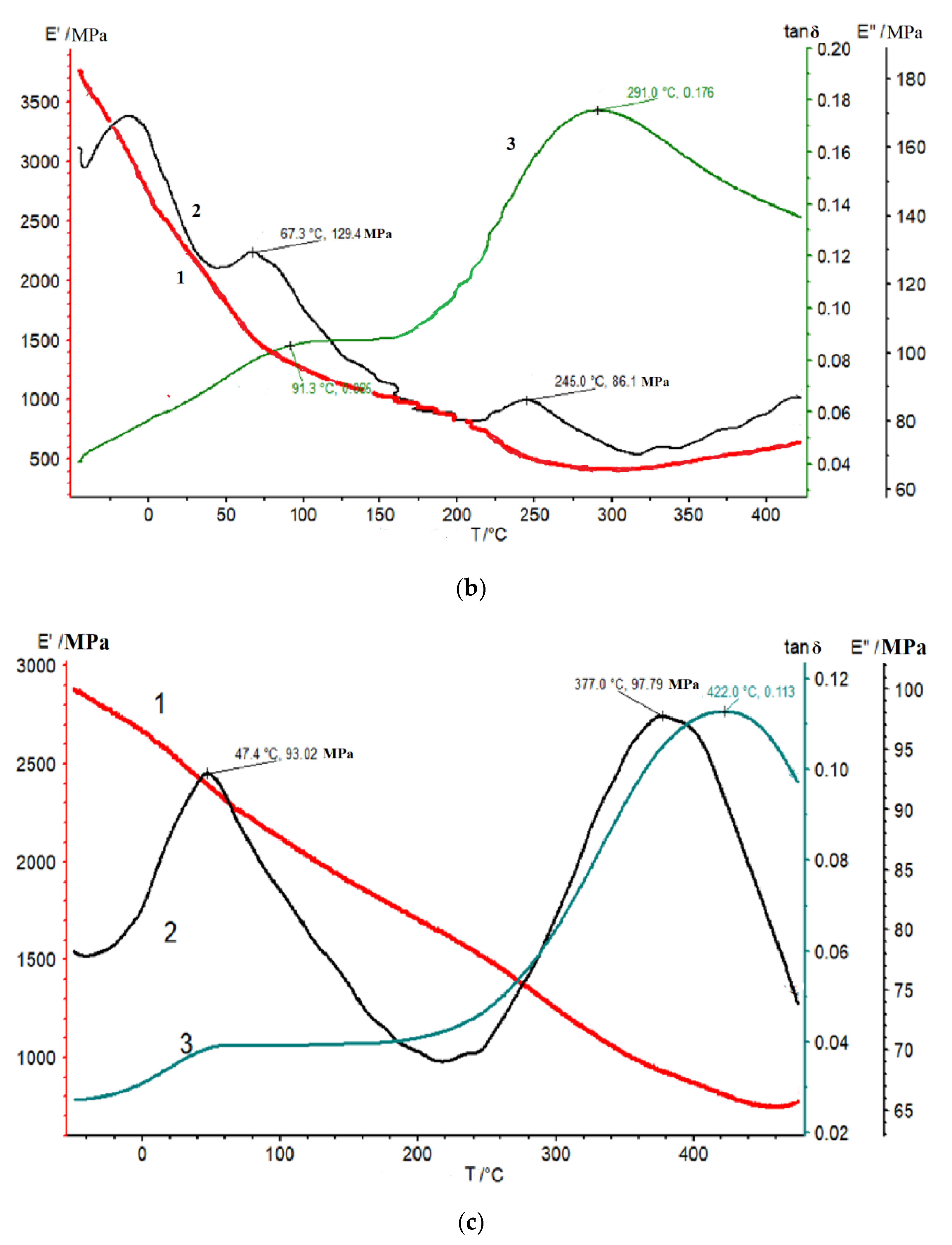
3.3. DMA Studies and Mechanical Properties of Thermalized Samples
3.4. Comparative Study by IR Spectroscopy of the Obtained Copolymers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Symbols and Acronyms
| TGA | thermogravimetric analysis; |
| DTG | differential thermogravimetric analysis; |
| DSC | differential scanning calorimetry; |
| DMA | dynamic mechanical analysis; |
| NMR 1H | nuclear magnetic resonance spectra; |
| DMSO-d6 | deuterated dimethyl sulfoxide; |
| IR | infrared spectra; |
| η | viscosity, cm3 g−1.; |
| τ5 | 5% mass loss of the sample; |
| τ10 | 5% mass loss of the sample; |
| ΔH | enthalpy of melting; |
| E′ | storage modulus |
| E″ | loss modulus; |
| E, MPa | young’s module; |
| σt, MPa | tensile strength; |
| εb, % | elongation at break; |
| Tg | glass transition temperature; |
| Tm | melting point; |
| tan δ | the tangent of the mechanical loss angle. |
References
- Mittal, K.L. (Ed.) Polyimides: Synthesis, Characterization and Applications; Plenum Press: New York, NY, USA, 1984; Volume 1. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides Thermally Stable Polymers; Consultants Bureau: New York, NY, USA, 1987; p. 318. [Google Scholar]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Megusar, J. Low temperature fast-neutron and gamma irradiation of Kapton® polyimide films. J. Nucl. Mater. 1997, 245, 185–190. [Google Scholar] [CrossRef]
- Abadie, M.J.M.; Rusanov, A.L. Practical Guide to Polyimides Shawbury; Rapra: Karnataka, UK, 2007; 84p. [Google Scholar]
- Pulyalina, A.; Tataurov, M.; Faykov, I.; Rostovtseva, V.; Polotskaya, G. Polyimide Asymmetric Membrane vs. Dense Film for Purification of MTBE Oxygenate by Pervaporation. Symmetry 2020, 12, 436. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Synthesis, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Schumann, W.; Strathmann, H. Polyimide Membrane and Process for Making Same. U.S. Patent 3,925,211, 30 January 1975. [Google Scholar]
- Chang, J.; Niu, H.; Wu, D. High performance polyimide fibers. Struct. Prop. High-Perform. Fibers 2017, 301–323. [Google Scholar] [CrossRef]
- Pan, Z.; Han, S.; Wang, J.; Qi, S.; Tian, G.; Wu, D. Polyimide fabric-reinforced polyimide matrix composites with excellent thermal, mechanical, and dielectric properties. High Perform. Polym. 2020, 32, 1085–1093. [Google Scholar] [CrossRef]
- Liu, J.; Min, Y.; Chen, J.; Zhou, H.; Wang, C. Preparation of the Ultra-Low Dielectric Constant Polyimide Fiber Membranes Enabled by Electrospinning. Macromol. Rapid Commun. 2007, 28, 215–219. [Google Scholar] [CrossRef]
- Xie, J.F. An Electrospun Polyimide Ultrathin Fiber Membrane for Particle Filtration of Hot Gas. Adv. Mater. Res. 2014, 1048, 489–492. [Google Scholar] [CrossRef]
- Pei, X.; Chen, G.; Liu, J.; Fang, X. Influence of crystalline polyimide hard block on the properties of poly(imide siloxane) copolymers. Polymer 2015, 56, 229–236. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Chen, G.; Zhang, A.; Fang, X. Melt-Processable Semicrystalline Polyimides Based on 1,4-Bis(3,4-Dicarboxyphenoxy)benzene Dianhydride (HQDPA): Synthesis, Crystallization, and Melting Behavior. Polymers 2017, 9, 420. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Li, P. Fabrication of PMDA-ODA Hollow Fibers with Regular Cross-Section Morphologies and Study on the Formation Mechanism. J. Membr. Sci. 2017, 544, 1–11. [Google Scholar] [CrossRef]
- Ueda, T.; Inoue, S.-I. Thermoplasticity in Polyurethane-Imide Elastomers. Org. Polym. Mater. 2018, 8, 11. [Google Scholar] [CrossRef]
- Jonquieres, A.; Clement, R.; Lochon, P. Permeability of block copolymers to vapors and liquid. Prog. Polym. Sci. 2002, 27, 1803–1877. [Google Scholar] [CrossRef]
- Solimando, X.; Babin, J.; Arnal-Herault, C.; Wang, M.; Barth, D.; Roizard, D.; Doillon-Halmenschlager, J.-R.; Poncot, M.; Royaud, I.; Alcouffe, P.D.; et al. Highly selective multi-block poly (ether-urea-imide) s for CO2/N2 separation: Structure-morphology-properties relationships. Polymer 2017, 131, 56. [Google Scholar] [CrossRef]
- Sang, X.; Wang, R.; Chen, X.; Zhang, L.; An, M.; Shen, Y. A Review on Synthesis and Property of Polyurethane-imide. Adv. Mater. Res. 2011, 284—286, 1746. [Google Scholar] [CrossRef]
- Ueda, T.; Nishio, T.; Inoue, S. Influences of Diamines on the Morphologies and the Chemical, Thermal, and Mechanical Properties of Polyurethane-Imide Elastomers. Open J. Org. Polym. Mater. 2017, 7, 47. [Google Scholar] [CrossRef]
- Masiulanis, B.; Hrouz, J.; Baldrian, J.; Ilavský, M.; Dušek, K. Dynamic Mechanical Behavior and Structure of Polyurethaneimides. J. Appl. Polym. Sci. 1987, 34, 1941. [Google Scholar] [CrossRef]
- Holden, G.; Kricheldorf, H.; Quirk, R. Thermoplastic Elastomers, 3rd ed.; Hanser Publication: Cincinnati, OH, USA, 2004; p. 540. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons International Right. Inc.: Hoboken, NJ, USA, 2014; p. 439. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Volgin, I.V.; Andreeva, M.V.; Larin, S.V.; Didenko, A.L.; Vaganov, G.V.; Borisov, I.L.; Volkov, A.V.; Klushin, L.I.; Lyulin, S.V. Transport Properties of Thermoplastic R-BAPB Polyimide: Molecular Dynamics Simulations and Experiment. Polymers 2019, 11, 1775. [Google Scholar] [CrossRef]
- Feng, W.; Li, J.; Fang, C.; Zhang, L.; Zhu, L. Controllable thermal annealing of polyimide membranes for highly-precise organic solvent nanofiltration. J. Membr. Sci. 2022, 643, 120013. [Google Scholar] [CrossRef]
- Chen, K.; Li, P.; Zhang, H.; Sun, H.; Yang, X.; Yao, D.; Pang, X.; Han, X.; Niu, Q.J. Organic solvent nanofiltration membrane with improved permeability by in-situ growth of metal-organic frameworks interlayer on the surface of polyimide substrate. Sep. Purif. Technol. 2020, 251, 117387. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Zhang, X.; Cao, B.; Li, P. Formation of Macrovoid-Free PMDA-MDA Polyimide Membranes Using a Gelation/Non-Solvent-Induced Phase Separation Method for Organic Solvent Nanofiltration. Ind. Eng. Chem. Res. 2019, 58, 6712–6720. [Google Scholar] [CrossRef]
- Anokhina, T.; Borisov, I.; Yushkin, A.; Vaganov, G.; Didenko, A.; Volkov, A. Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers 2020, 12, 2785. [Google Scholar] [CrossRef] [PubMed]
- Ohya, H.; Kudryavtsev, V.V.; Semenova, S.I. Polyimide Membranes: Applications, Fabrications, and Properties; Kodansha Inc.: New York, NY, USA, 1996; p. 314. [Google Scholar]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Farahani, D.A.; Hossein, M.; Dangchen, M.; Pegah, N.A. Nanocomposite membranes for organic solvent nanofiltration. Mater. Sci. Chem. Sep. Purif. Rev. 2018, 1–30. [Google Scholar] [CrossRef]
- Galizia, M.; Bye, K.P. Advances in Organic Solvent Nanofiltration Rely on Physical Chemistry and Polymer Chemistry. Front. Chem. Sec. Polym. Chem. 2018, 6, 47. [Google Scholar] [CrossRef]
- Koenitzer, B.A. Polyurehane-Imide Membranes and Ther Use for the Separation of Aromatics from Non-Aromatics. U.S. Patent #4,929,358, 29 May 1990. [Google Scholar]
- Gallyamov, A.A. Structure, Properties and Application of Polyurethane Destruction by Amines—De-Amines and Polyamines. 2017. Available online: https://elar.usfeu.ru/handle/123456789/5334 (accessed on 26 August 2022).
- Bykova, E.N.; Gofman, I.V.; Ivankova, E.M.; Nikolaeva, A.L.; Yakimansky, A.V.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Influence of Nanoparticles of Various Types As Fillers on Resistance to Hydrolysis of Films of Heat-Resistant Polyimide. Nanosyst. Phys. Chem. Math. 2019, 10, 666–673. [Google Scholar] [CrossRef]
- Kotanen, S.; Poikelispaa, M.; Efimov, A.; Harjunalanen, T.; Mills, C.; Laaksonen, T.; Sarlin, E. Hydrolytic stsbility of polyurethane/polyhydroxyuretane hybrid adhesives. Int. J. Adhes. Adhes. 2021, 110, 102950. [Google Scholar] [CrossRef]
- Czeiszperger, R. Polyurethane Elastomer Hydrolytic Stability: A Comprehensive Review; Anderson Development Company: 2012. Available online: https://www.andersondevelopment.com/pdf/PU%20Technical%20Papers-added%2003162021/PU%20Hydrolytic%20Stability.pdf (accessed on 26 August 2022).
- Didenko, A.L.; Kuznetcov, D.A.; Smirnova, V.E.; Bogdanov, E.A.; Vaganov, G.V.; Ivanov, A.G.; Kobykhno, I.A.; Vasil’eva, E.S.; Tolochko, O.V.; Svetlichnyi, V.M.; et al. Synthesis, Heat Resistance, and Mechanical Properties of Cross-Linked Urethane–Imide Copolymers Containing Blocks of Two Structurally Diff erent Aliphatic Fragments (Polyether and Polyester) in the Backbone. Russ. J. Appl. Chem. 2021, 94, 1240–1259. [Google Scholar] [CrossRef]
- Didenko, A.L.; Kuznetcov, D.A.; Smirnova, V.E.; Popova, E.N.; Vaganov, G.V.; Ivanov, A.G.; Svetlichnyi, V.M.; Yudin, V.E.; Kudryavtsev, V.V. Co-poly (urethane-imide) s based on poly[di(ethylene glycol) adipate] and their compositions with thermoplastic polyimide: Synthesis and properties. Russ. Chem. Bull. 2020, 69, 369–377. [Google Scholar] [CrossRef]
- Didenko, A.L.; Kuznetsov, D.A.; Vaganov, G.V.; Smirnova, V.E.; Popova, E.N.; Ivanov, V.M. Svetlichnyi, V.E. Yudin, V.V. Kudryavtsev, Multiblock Copoly (urethane–imide) s with the Properties of Thermoplastic Elastomers. Polym. Sci. Ser. C 2020, 62, 90–110. [Google Scholar] [CrossRef]
- de Visser, A.C.; Driessen, A.A.; Wolke, J.G.C. Segmented copolyether-imides, 2. Die Makromol. Chem. Rapid Commun. 1980, 1, 177. [Google Scholar] [CrossRef]
- Yeganeh, H.; Shamekhi, M.A. Poly (urethane-imide), a New Generation of Thermoplastic Elastomers with Enhanced Thermal Stability. Polymer 2004, 45, 359. [Google Scholar] [CrossRef]
- Gerkin, R.M.; Hilker, B.L. Block Copolymers: Segmented: Encyclopedia of Materials: Science and Technology; Elsevier Ltd: Amsterdam, The Netherlands, 2001; p. 10388. [Google Scholar] [CrossRef]
- Vasnev, V.A.; Kuchanov, S.I. Non-equilibrium Copolycondensation in Homogeneous Systems. Russ. Chem. Rev. 1973, 42, 1020–1033. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Chichester, UK, 2000. [Google Scholar] [CrossRef]
- Infrared Spectroscopy Thompson, J.M. Infrared Spectroscopy; Pan Stanford Publishing Pt. Ltd.: Singapore, 2018. [Google Scholar]
- Bistričić, L.; Baranović, G.; Leskovac, M.; Govorčin Bajsić, E. Hydrogen bonding and mechanical properties of thin films of polyether-based polyurethane–silica nanocomposites. Eur. Polym. J. 2010, 46, 1975–1987. [Google Scholar] [CrossRef]
- Zimmer, B.; Nies, C.; Schmitt Possart, W. Chemistry, polymer dynamics and mechanical properties of a two-part polyurethane elastomer during and after crosslinking. Part I: Dry conditions. Polymer 2017, 115, 77. [Google Scholar] [CrossRef]





| No. | Polymer Type and Processing | Polymer Properties | ||
|---|---|---|---|---|
| E, MPa | σt, MPa | εb, % | ||
| 1 | multiblock (segmental) copoly(urethane-imide) (from Scheme 2) | 4 ± 1 | 33 ± 5 | 889 ± 176 |
| 2 | copoly(urethane-imide) (from Scheme 1) 170 °C Thermolysis–2 h | 1796 ± 229 | 113 ± 8 | 172 ± 15 |
| 3 | copoly(urethane-imide) (from Scheme 1) 300 °C Thermolysis–0.5 h | 1494 ± 96 | 103 ± 11 | 106 ± 12 |
| 4 | copoly(urethane-imide) (from Scheme 1) 350 °C Thermolysis–0.5 h | 2452 ± 331 | 131 ± 16 | 27 ± 5 |
| 5 | poly-(4,4′-oxydiphenylene)pyromellitimide | 2115 ± 226 | 122 ± 10 | 34 ± 4 |
| No. | Polymer Type and Processing | Polymer Properties | ||
|---|---|---|---|---|
| E, MPa | σt, MPa | εb, % | ||
| 2 | copoly(urethane-imide) (from Scheme 1) 170 °C Thermolysis–2 h–hydrolysis 24 h | 1038 ± 98 | 117 ± 10 | 137 ± 12 |
| 3 | copoly(urethane-imide) (from Scheme 1) 300 °C Thermolysis–0,5 h–hydrolysis 48 h | 1870 ± 150 | 119 ± 11 | 72 ± 9 |
| 5 | poly-(4,4′-oxydiphenylene)pyromellitimide | 2115 ± 226 | 121 ± 10 | 34 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didenko, A.L.; Ivanov, A.G.; Smirnova, V.E.; Vaganov, G.V.; Anokhina, T.S.; Borisov, I.L.; Volkov, V.V.; Volkov, A.V.; Kudryavtsev, V.V. Selective Destruction of Soluble Polyurethaneimide as Novel Approach for Fabrication of Insoluble Polyimide Films. Polymers 2022, 14, 4130. https://doi.org/10.3390/polym14194130
Didenko AL, Ivanov AG, Smirnova VE, Vaganov GV, Anokhina TS, Borisov IL, Volkov VV, Volkov AV, Kudryavtsev VV. Selective Destruction of Soluble Polyurethaneimide as Novel Approach for Fabrication of Insoluble Polyimide Films. Polymers. 2022; 14(19):4130. https://doi.org/10.3390/polym14194130
Chicago/Turabian StyleDidenko, Andrey L., Aleksey Gennad’evich Ivanov, Valentina E. Smirnova, Gleb V. Vaganov, Tatyana S. Anokhina, Ilya L. Borisov, Vladimir V. Volkov, Alexey V. Volkov, and Vladislav V. Kudryavtsev. 2022. "Selective Destruction of Soluble Polyurethaneimide as Novel Approach for Fabrication of Insoluble Polyimide Films" Polymers 14, no. 19: 4130. https://doi.org/10.3390/polym14194130
APA StyleDidenko, A. L., Ivanov, A. G., Smirnova, V. E., Vaganov, G. V., Anokhina, T. S., Borisov, I. L., Volkov, V. V., Volkov, A. V., & Kudryavtsev, V. V. (2022). Selective Destruction of Soluble Polyurethaneimide as Novel Approach for Fabrication of Insoluble Polyimide Films. Polymers, 14(19), 4130. https://doi.org/10.3390/polym14194130












