The Identification and Characterization of the KNOX Gene Family as an Active Regulator of Leaf Development in Trifolium repens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of TrKNOX Genes in T. repens
2.2. Phylogeny and Classification of TrKNOX Genes
2.3. Gene Structure, Motif, and Chromosomal Localization Analysis of TrKNOX Genes
2.4. Evolutionary Analysis of TrKNOX Genes with Several Related Species
2.5. Protein–Protein Interaction (PPI) Network Construction of the Crucial TrKNOX Genes in T. repens Using the Interolog Approach
2.6. Plant Materials
2.7. Expression Patterns Analysis of TrKNOX Genes Using qRT-PCR
3. Results
3.1. Identification of TrKNOX Genes in T. repens
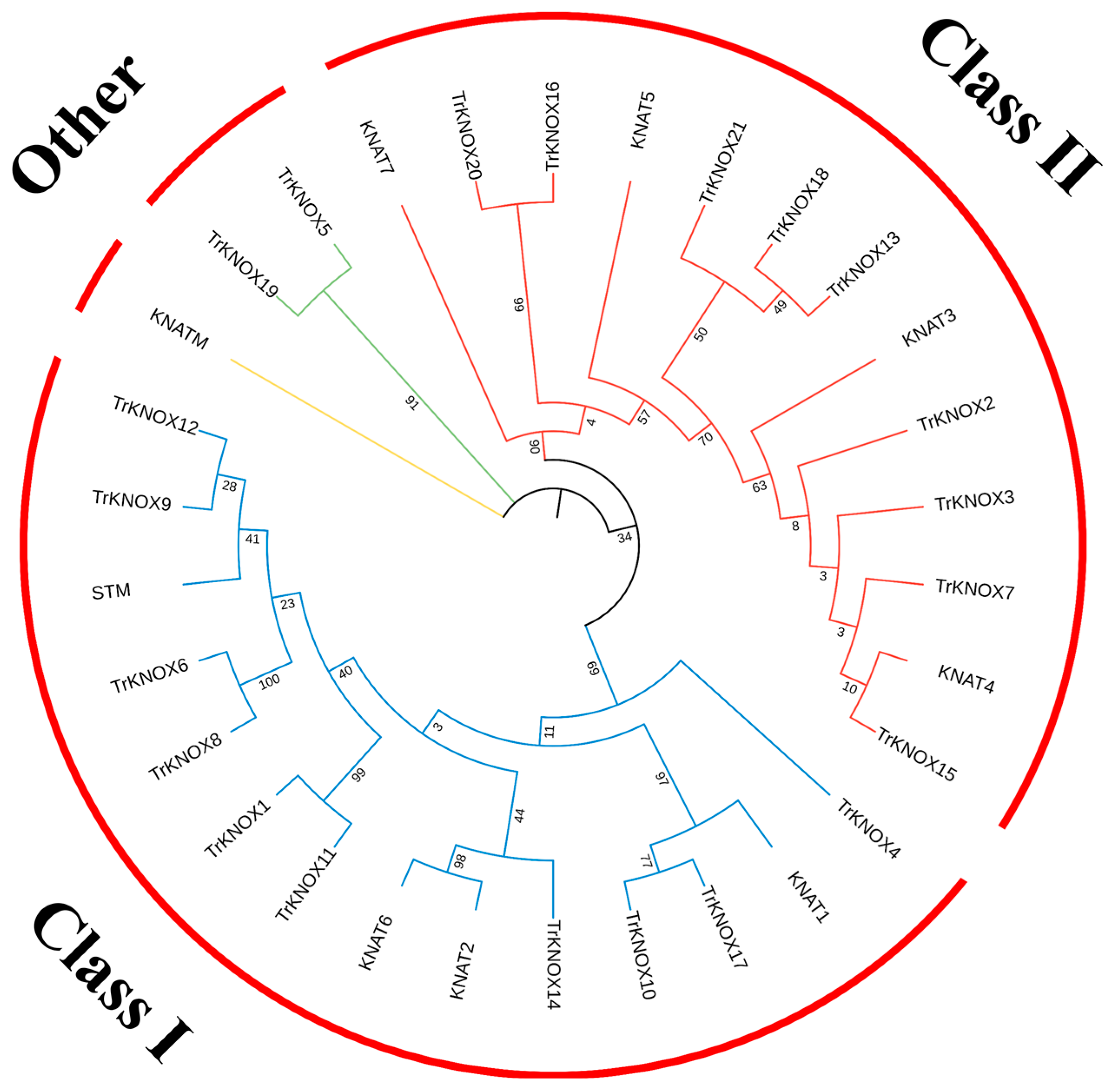
3.2. Phylogenetic Analysis and Classification of TrKNOX Genes
3.3. Gene Structure and Conserved Motifs Analysis of TrKNOX
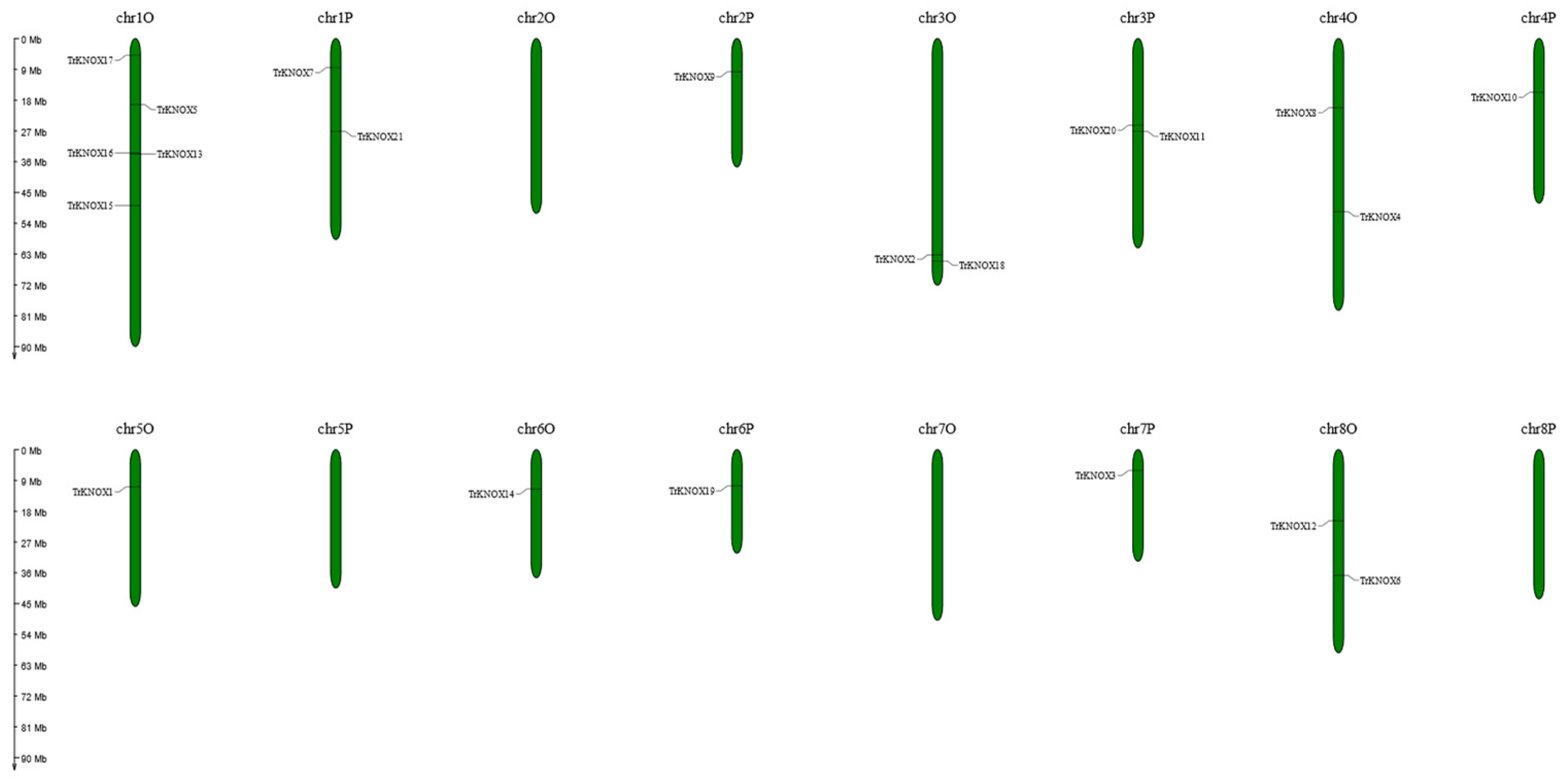
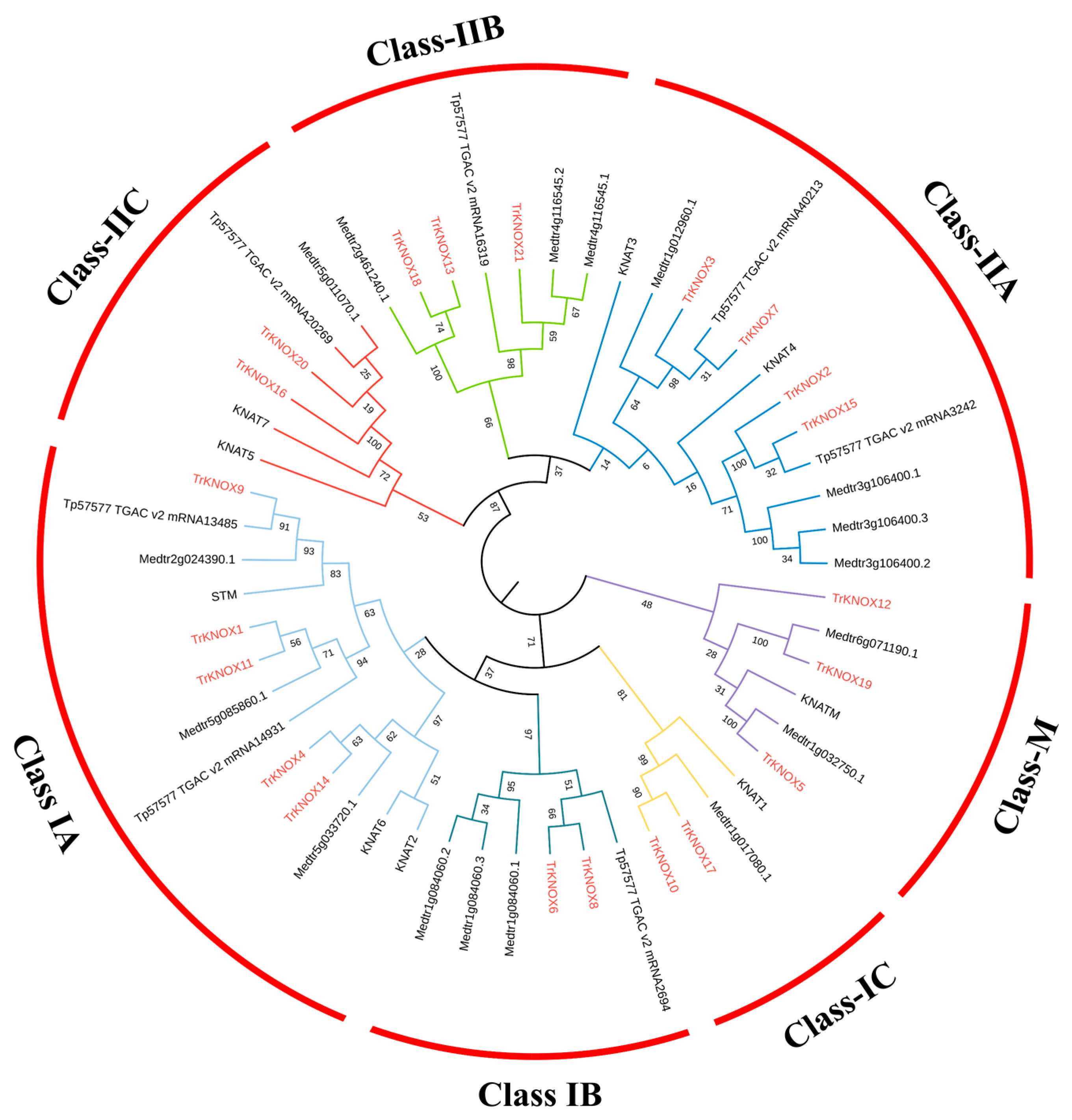
3.4. Chromosomal Locations and Evolutionary Analysis of TrKNOX Genes with Several Related Species
3.5. Expression Analysis of TrKNOX Genes during Leaf Development
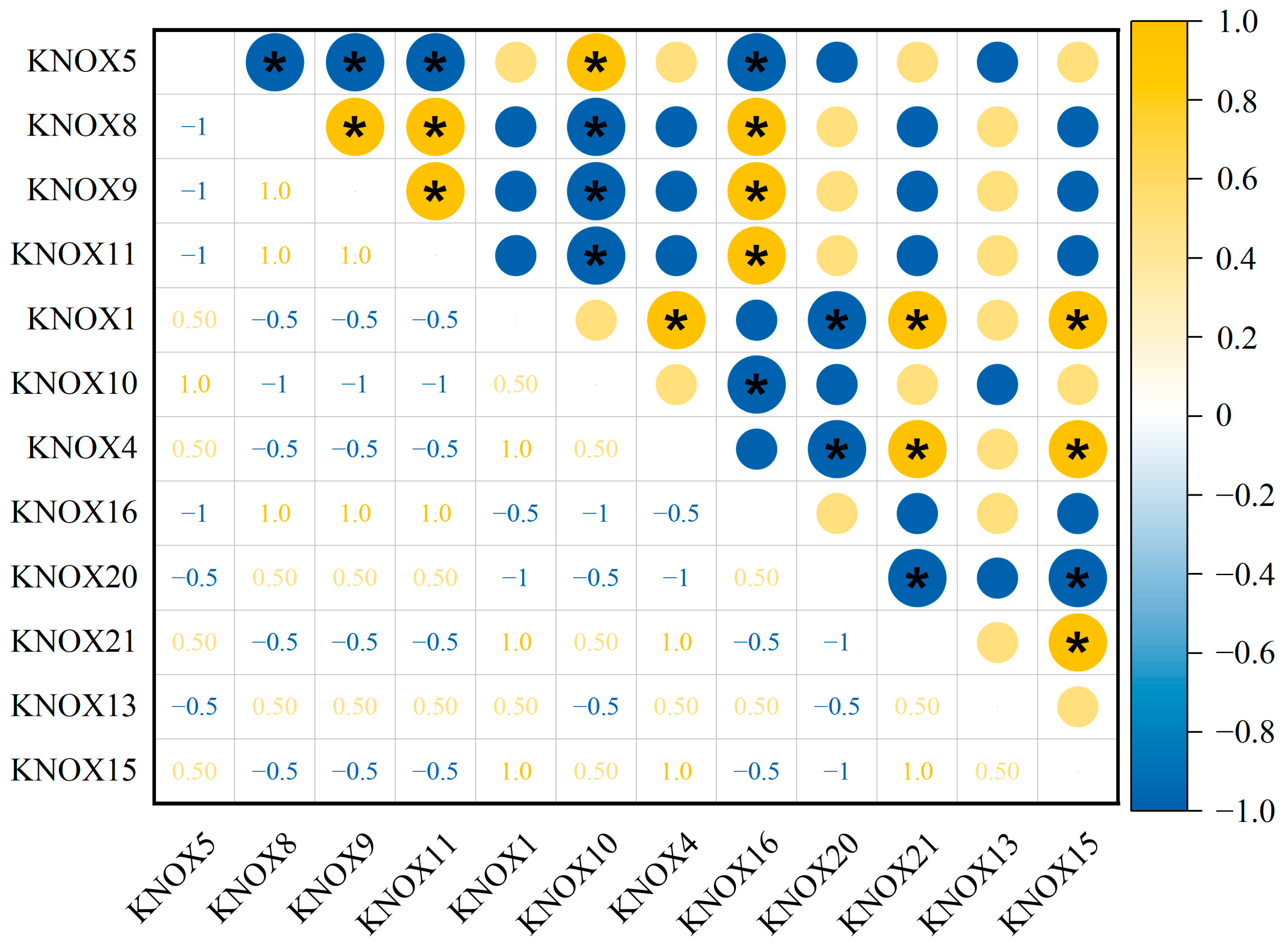
3.6. The Correlation of Expression for 21 TrKNOX Genes of T. repens
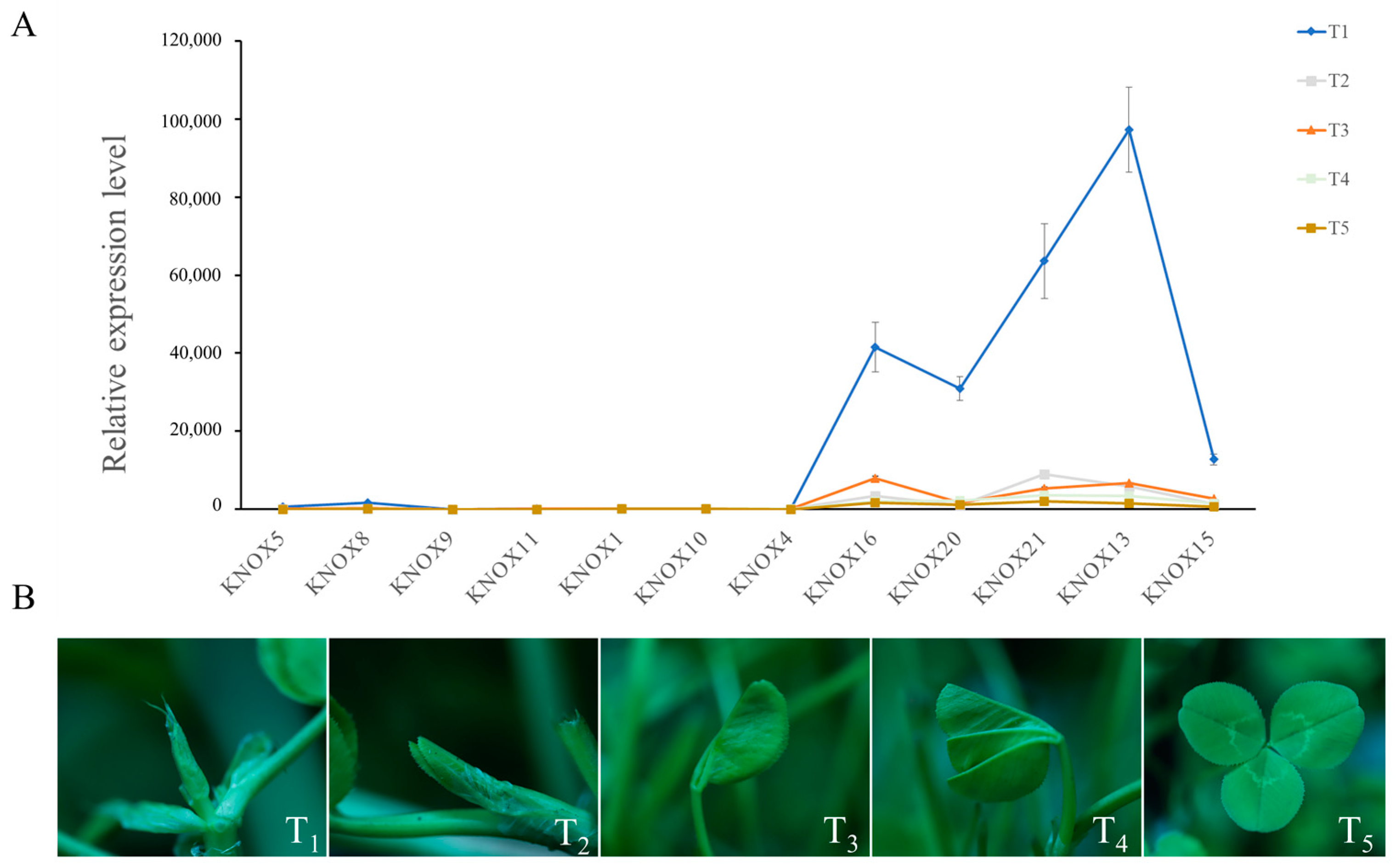
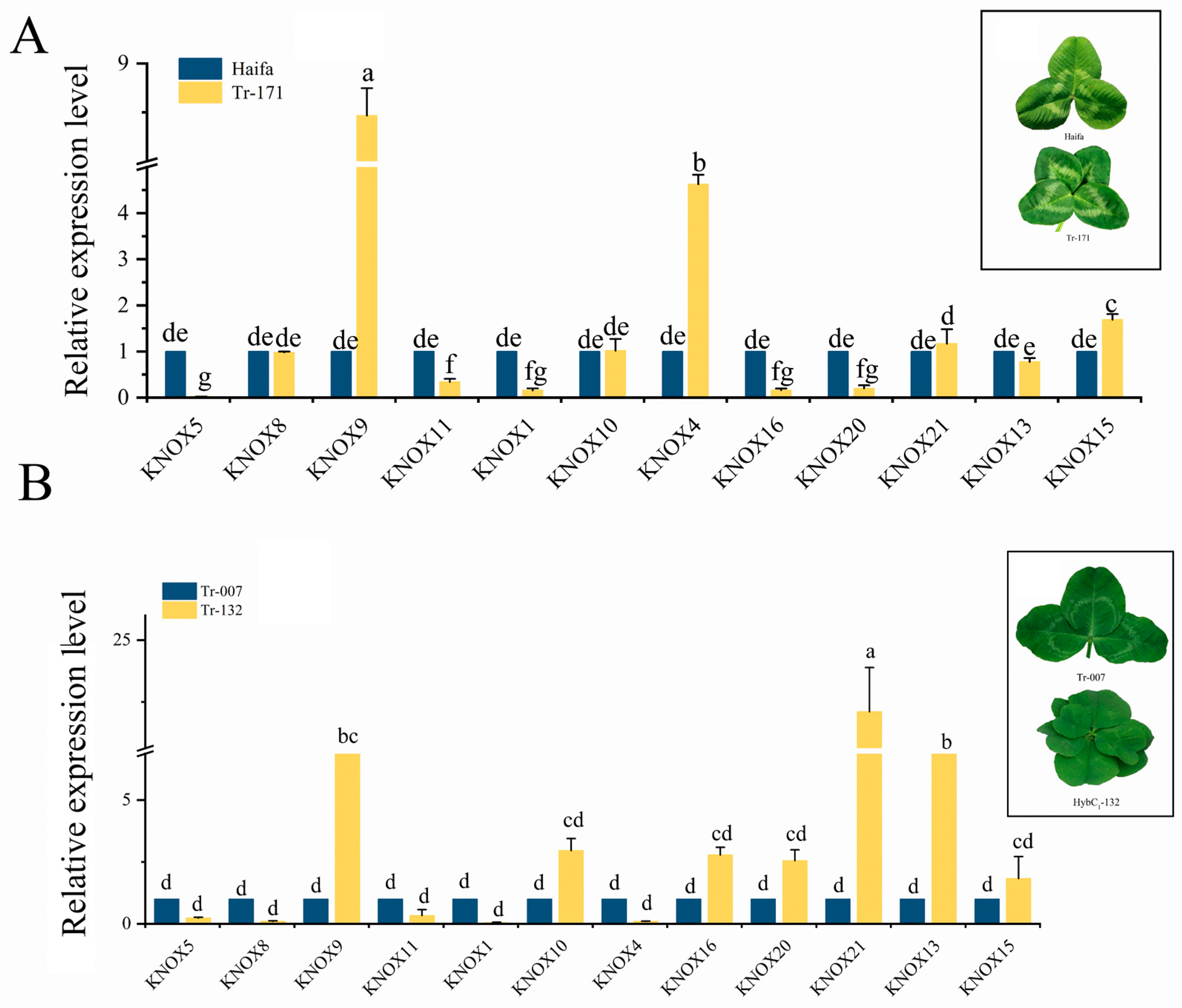
3.7. Expression Patterns of TrKNOX Genes during Leaf Development in T. repens with Different Leaf Types
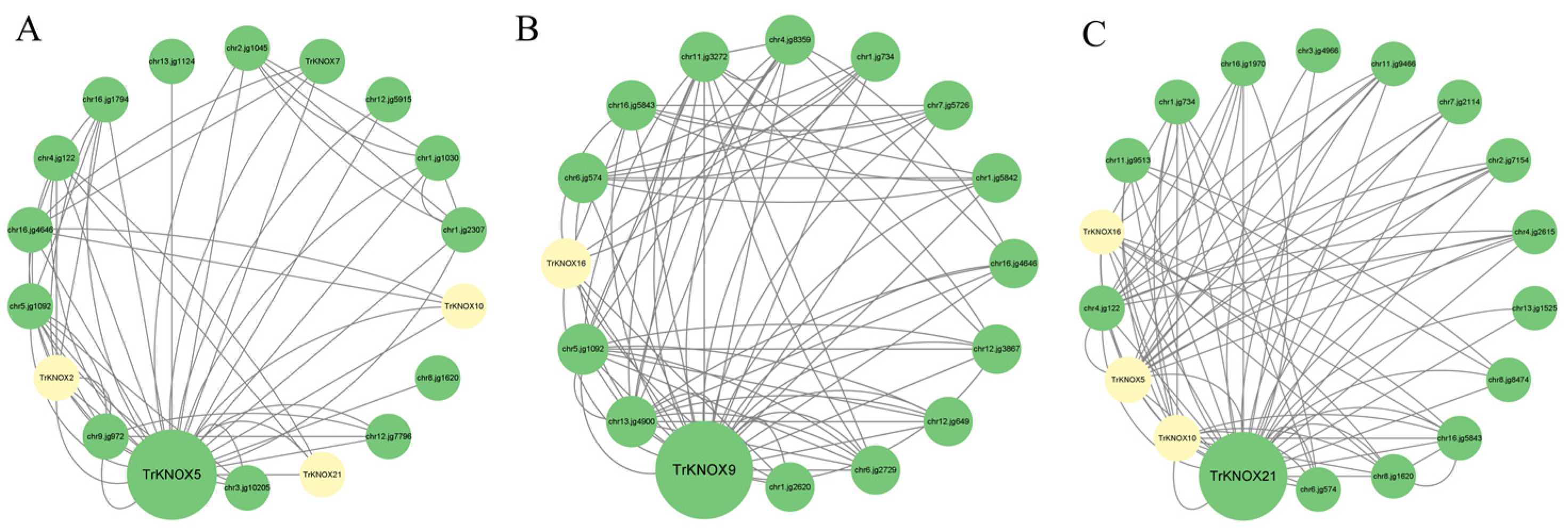
3.8. Protein–Protein Interaction (PPI) Network Prediction of Three TrKNOX Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KNOX | Knotted1-like homeobox |
| IRLC | Inverted repeat-lacking clade |
| STM | SHOOTMERISTEMLESS |
| BP | BREVIPEDICELLUS |
| KNAT | Knotted Arabidopsis Thaliana |
| FCL1 | Fused Compound Leaf1 |
| MW | Molecular weight |
| PI | Isoelectric point |
| NJ | Neighbor-joining |
| PPI | Protein–protein interaction |
References
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The Evolution and Functional Significance of Leaf Shape in the Angiosperms. Funct. Plant Biol. 2011, 38, 353–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, J.F.; Nandra, K.S.; Turner, A.D. A Study of the Nutritive Value of White Clover (Trifolium Repens L.) in Relation to Different Stages of Phenological Maturity in the Primary Growth Phase in Spring. Grass Forage Sci. 1998, 53, 250–259. [Google Scholar] [CrossRef]
- Grev, A.M.; Wells, M.S.; Catalano, D.N.; Martinson, K.L.; Jungers, J.M.; Sheaffer, C.C. Stem and Leaf Forage Nutritive Value and Morphology of Reduced Lignin Alfalfa. Agron. J. 2020, 112, 406–417. [Google Scholar] [CrossRef]
- Cogan, N.O.I.; Abberton, M.T.; Smith, K.F.; Kearney, G.; Marshall, A.H.; Williams, A.; Michaelson-Yeates, T.P.T.; Bowen, C.; Jones, E.S.; Vecchies, A.C.; et al. Individual and Multi-Environment Combined Analyses Identify QTLs for Morphogenetic and Reproductive Development Traits in White Clover (Trifolium repens L.). Theor. Appl. Genet. 2006, 112, 1401–1415. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; He, H.; Liu, Y.; Qi, J.; Zhang, X.; Li, Y.; Mao, Y.; Zhou, S.; Zheng, X.; et al. A Molecular Framework Underlying the Compound Leaf Pattern of Medicago Truncatula. Nat. Plants 2020, 6, 511–521. [Google Scholar] [CrossRef]
- Gehring, W.J.; Affolter, M.; Bürglin, T. Homeodomain Proteins. Annu. Rev. Biochem. 1994, 63, 487–526. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Bürglin, T.R. A Comprehensive Classification and Evolutionary Analysis of Plant Homeobox Genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef] [Green Version]
- Hay, A.; Tsiantis, M. KNOX Genes: Versatile Regulators of Plant Development and Diversity. Development 2010, 137, 3153–3165. [Google Scholar] [CrossRef] [Green Version]
- Rast-Somssich, M.I.; Broholm, S.; Jenkins, H.; Canales, C.; Vlad, D.; Kwantes, M.; Bilsborough, G.; Dello Ioio, R.; Ewing, R.M.; Laufs, P.; et al. Corrigendum: Alternate Wiring of a KNOXI Genetic Network Underlies Differences in Leaf Development of A. Thaliana and C. Hirsuta. Genes Dev. 2016, 30, 132. [Google Scholar]
- Magnani, E.; Hake, S. KNOX Lost the OX: The Arabidopsis KNATM Gene Defines a Novel Class of KNOX Transcriptional Regulators Missing the Homeodomain. Plant Cell 2008, 20, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A Member of the KNOTTED Class of Homeodomain Proteins Encoded by the STM Gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Tsiantis, M. The Genetic Basis for Differences in Leaf Form between Arabidopsis Thaliana and Its Wild Relative Cardamine Hirsuta. Nat. Genet. 2006, 38, 942–947. [Google Scholar] [CrossRef]
- Champagne, C.E.M.; Goliber, T.E.; Wojciechowski, M.F.; Mei, R.W.; Townsley, B.T.; Wang, K.; Paz, M.M.; Geeta, R.; Sinha, N.R. Compound Leaf Development and Evolution in the Legumes. Plant Cell 2007, 19, 3369–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, J.; Gourlay, C.; Michael, A.; Ellis, T.H. Expression of a Class 1 Knotted1-like Homeobox Gene Is down-Regulated in Pea Compound Leaf Primordia. Plant Mol. Biol. 2001, 45, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Han, L.; Li, G.; Chai, M.; Fu, C.; Cheng, X.; Wen, J.; Tang, Y.; Wang, Z.-Y. STM/BP-Like KNOXI Is Uncoupled from ARP in the Regulation of Compound Leaf Development in Medicago Truncatula. Plant Cell 2014, 26, 1464–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Yang, X.; Zhao, W.; Lang, T.; Samuelsson, T. Evolution, Diversification, and Expression of KNOX Proteins in Plants. Front. Plant Sci. 2015, 6, 882. [Google Scholar] [CrossRef] [Green Version]
- Hake, S.; Smith, H.M.S.; Holtan, H.; Magnani, E.; Mele, G.; Ramirez, J. The Role of Knox Genes in Plant Development. Annu. Rev. Cell Dev. Biol. 2004, 20, 125–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Q.; Qin, W.; Gao, H.; Du, J.; Chen, J.; Li, H.; Zhou, G.; Wu, H.; Wu, A.-M. The Class II KNOX Family Members KNAT3 and KNAT7 Redundantly Participate in Arabidopsis Seed Coat Mucilage Biosynthesis. J. Exp. Bot. 2022, 73, 4377–4395. [Google Scholar] [CrossRef]
- Chai, M.; Zhou, C.; Molina, I.; Fu, C.; Nakashima, J.; Li, G.; Zhang, W.; Park, J.; Tang, Y.; Jiang, Q.; et al. A Class II KNOX Gene, KNOX4, Controls Seed Physical Dormancy. Proc. Natl. Acad. Sci. USA 2016, 113, 6997–7002. [Google Scholar] [CrossRef] [Green Version]
- Li, E.; Bhargava, A.; Qiang, W.; Friedmann, M.C.; Forneris, N.; Savidge, R.A.; Johnson, L.A.; Mansfield, S.D.; Ellis, B.E.; Douglas, C.J. The Class II KNOX Gene KNAT7 Negatively Regulates Secondary Wall Formation in Arabidopsis and Is Functionally Conserved in Populus. New Phytol. 2012, 194, 102–115. [Google Scholar] [CrossRef]
- Challa, K.R.; Rath, M.; Sharma, A.N.; Bajpai, A.K.; Davuluri, S.; Acharya, K.K.; Nath, U. Active Suppression of Leaflet Emergence as a Mechanism of Simple Leaf Development. Nat. Plants 2021, 7, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, R. Auxin Efflux Transporter MtPIN10 Regulates Compound Leaf and Flower Development InMedicago Truncatula. Plant Signal. Behav. 2011, 6, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabir, J.; Schwarz, E.; Ellison, N.; Zhang, J.; Baeshen, N.A.; Mutwakil, M.; Jansen, R.; Ruhlman, T. Evolutionary and Biotechnology Implications of Plastid Genome Variation in the Inverted-Repeat-Lacking Clade of Legumes. Plant Biotechnol. J. 2014, 12, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.G.; Moraga, R.; Tausen, M.; Gupta, V.; Bilton, T.P.; Campbell, M.A.; Ashby, R.; Nagy, I.; Khan, A.; Larking, A.; et al. Breaking Free: The Genomics of Allopolyploidy-Facilitated Niche Expansion in White Clover. Plant Cell 2019, 31, 1466–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Hernandez, M.; Berardini, T.Z.; Chen, G.; Crist, D.; Doyle, A.; Huala, E.; Knee, E.; Lambrecht, M.; Miller, N.; Mueller, L.A.; et al. TAIR: A Resource for Integrated Arabidopsis Data. Funct. Integr. Genom. 2002, 2, 239–253. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler-Bauer, A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinform. 2019, 69, e90. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein Identification and Analysis Tools in the ExPASy Server. In The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 1999; pp. 571–601. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. ITAK: A Program for Genome-Wide Prediction and Classification of Plant Transcription Factors, Transcriptional Regulators, and Protein Kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Thanasomboon, R.; Kalapanulak, S.; Netrphan, S.; Saithong, T. Prediction of Cassava Protein Interactome Based on Interolog Method. Sci. Rep. 2017, 7, 17206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2018, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Hou, J.; Iqbal, M.Z.; Zhang, Y.; Cheng, B.; Feng, H.; Li, Z.; Liu, L.; Zhou, J.; Feng, G.; et al. Overexpression of the White Clover TrSAMDC1 Gene Enhanced Salt and Drought Resistance in Arabidopsis Thaliana. Plant Physiol. Biochem. 2021, 165, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yu, J.; Wang, H.; Guo, Y.; Li, G.; Bai, G.; Chen, R. Regulation of Compound Leaf Development in Medicago Truncatula by Fused Compound Leaf1, a Class M KNOX Gene. Plant Cell 2011, 23, 3929–3943. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Shi, A.; Wu, D.; Weng, Y.; Qin, J.; Ravelombola, W.S.; Shu, X.; Zhou, W. Genome-Wide Identification, Classification and Evolutionary Expansion of KNOX Gene Family in Rice (Oryza Sativa) and Populus (Populustrichocarpa). Am. J. Plant Sci. 2018, 9, 1071–1092. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; White, M.J.; MacRae, T.H. Transcription Factors and Their Genes in Higher Plants Functional Domains, Evolution and Regulation. Eur. J. Biochem. 1999, 262, 247–257. [Google Scholar] [CrossRef]
- Bharathan, G.; Goliber, T.E.; Moore, C.; Kessler, S.; Pham, T.; Sinha, N.R. Homologies in Leaf Form Inferred from KNOXI Gene Expression During Development. Science 2002, 296, 1859–1860. [Google Scholar] [CrossRef]
- Wang, H.; Kong, F.; Zhou, C. From Genes to Networks: The Genetic Control of Leaf Development. J. Integr. Plant Biol. 2021, 63, 1181–1196. [Google Scholar] [CrossRef]
- Bar, M.; Ori, N. Compound Leaf Development in Model Plant Species. Curr. Opin. Plant Biol. 2014, 23, 61–69. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Runions, A.; Vuolo, F.; Strauss, S.; Lymbouridou, R.; Routier-Kierzkowska, A.-L.; Wilson-Sánchez, D.; Jenke, H.; Galinha, C.; Mosca, G.; et al. A Growth-Based Framework for Leaf Shape Development and Diversity. Cell 2019, 177, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Furumizu, C.; Alvarez, J.P.; Sakakibara, K.; Bowman, J.L. Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication. PLoS Genet. 2015, 11, e1004980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, K.; Hake, S. Diverse Functions of KNOX Transcription Factors in the Diploid Body Plan of Plants. Curr. Opin. Plant Biol. 2015, 27, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horst, N.A.; Katz, A.; Pereman, I.; Decker, E.L.; Ohad, N.; Reski, R. A Single Homeobox Gene Triggers Phase Transition, Embryogenesis and Asexual Reproduction. Nat. Plants 2016, 2, 15209. [Google Scholar] [CrossRef]
- Ezura, K.; Nakamura, A.; Mitsuda, N. Genome-Wide Characterization of the TALE Homeodomain Family and the KNOX-BLH Interaction Network in Tomato. Plant Mol. Biol. 2022, 109, 799–821. [Google Scholar] [CrossRef] [PubMed]


| Gene | Gene ID | Protein Length | Mw (KDa) | pI | Localization |
|---|---|---|---|---|---|
| TrKNOX1 | chr5.jg1646.t1 | 314 | 36,092.01 | 8.35 | Nuclear |
| TrKNOX2 | chr3.jg10199.t1 | 385 | 43,542.46 | 5.74 | Nuclear |
| TrKNOX3 | chr15.jg975.t1 | 384 | - | - | Nuclear |
| TrKNOX4 | chr4.jg7618.t1 | 234 | 25,798.44 | 4.67 | Nuclear |
| TrKNOX5 | chr1.jg2841.t1 | 134 | 14,759.70 | 4.83 | Nuclear |
| TrKNOX6 | chr8.jg5547.t1 | 333 | 37,894.84 | 5.86 | Nuclear |
| TrKNOX7 | chr9.jg1297.t1 | 437 | 48,822.03 | 5.64 | Nuclear |
| TrKNOX8 | chr4.jg2912.t1 | 309 | 35,077.32 | 5.21 | Nuclear |
| TrKNOX9 | chr10.jg1505.t1 | 385 | 43,606.47 | 6.46 | Nuclear |
| TrKNOX10 | chr12.jg2462.t1 | 344 | 39,142.8 | 6.32 | Nuclear |
| TrKNOX11 | chr11.jg4376.t1 | 317 | 36,226.06 | 6.38 | Nuclear |
| TrKNOX12 | chr8.jg3024.t1 | 165 | 19,418.92 | 8.97 | Nuclear |
| TrKNOX13 | chr1.jg5055.t1 | 358 | 40,653.28 | 5.97 | Nuclear |
| TrKNOX14 | chr6.jg1763.t1 | 318 | 36,055.49 | 5.16 | Nuclear |
| TrKNOX15 | chr1.jg7372.t1 | 384 | 43,455.34 | 5.74 | Nuclear |
| TrKNOX16 | chr1.jg5003.t1 | 301 | 34,059.36 | 6.46 | Nuclear |
| TrKNOX17 | chr1.jg696.t1 | 341 | 38,931.62 | 6.28 | Nuclear |
| TrKNOX18 | chr3.jg10469.t1 | 336 | - | - | Nuclear |
| TrKNOX19 | chr14.jg1682.t1 | 166 | 19,106.12 | 4.61 | Extracellular |
| TrKNOX20 | chr11.jg4105.t1 | 297 | 33,554.85 | 6.31 | Nuclear |
| TrKNOX21 | chr9.jg4282.t1 | 347 | 39,827.41 | 5.69 | Nuclear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Nie, G.; Ma, J.; Hu, R.; He, J.; Wu, F.; Yang, Z.; Ma, S.; Zhang, X.; Zhang, X. The Identification and Characterization of the KNOX Gene Family as an Active Regulator of Leaf Development in Trifolium repens. Genes 2022, 13, 1778. https://doi.org/10.3390/genes13101778
Fan J, Nie G, Ma J, Hu R, He J, Wu F, Yang Z, Ma S, Zhang X, Zhang X. The Identification and Characterization of the KNOX Gene Family as an Active Regulator of Leaf Development in Trifolium repens. Genes. 2022; 13(10):1778. https://doi.org/10.3390/genes13101778
Chicago/Turabian StyleFan, Jinwan, Gang Nie, Jieyu Ma, Ruchang Hu, Jie He, Feifei Wu, Zhongfu Yang, Sainan Ma, Xin Zhang, and Xinquan Zhang. 2022. "The Identification and Characterization of the KNOX Gene Family as an Active Regulator of Leaf Development in Trifolium repens" Genes 13, no. 10: 1778. https://doi.org/10.3390/genes13101778





