Reproductive Cycle and Sexual Group Maturity of Buccinum osagawai (Neogastropoda: Buccinidae)
Abstract
:1. Introduction
2. Materials and Methods
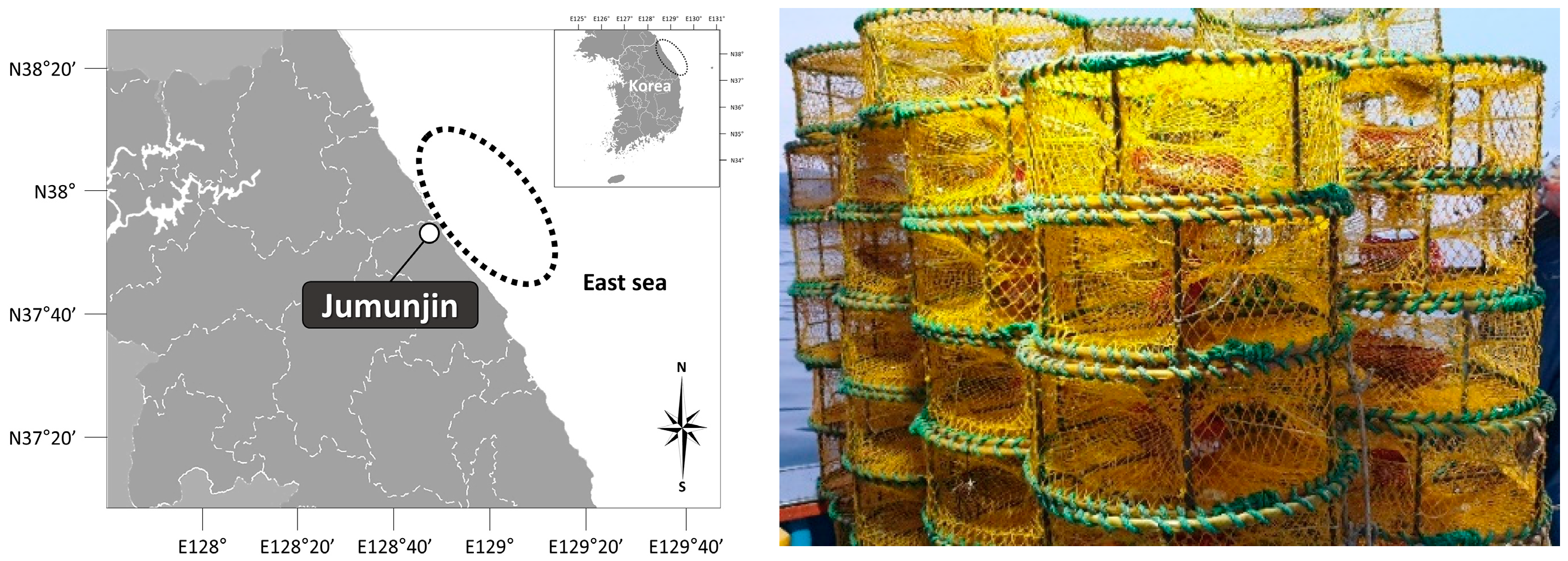
2.1. Sampling
2.2. Environmental Conditions
2.3. Histological Analysis
2.4. Sex Ratio
Female (%) = [Female (n)/Female (n) + male (n)] × 100
2.5. Gonadal Development Stage
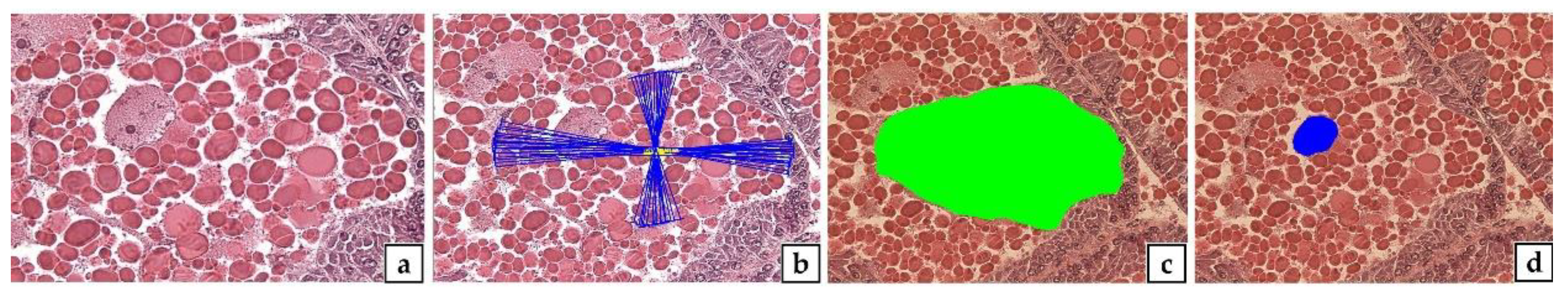
2.6. Gonad Index (GI)
2.7. Meat Weight Rate (MWR)
2.8. Sexual Group Maturity
2.9. Statistical Analysis
3. Results
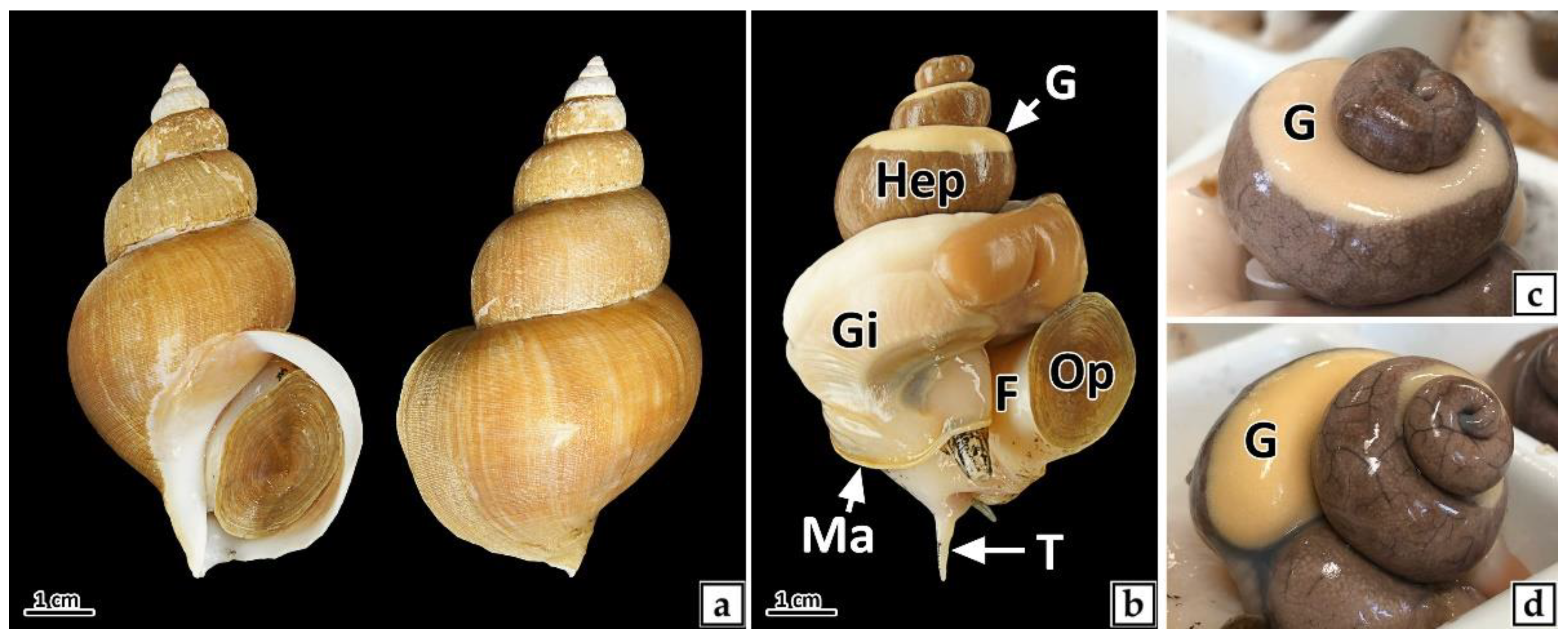
3.1. Sex and Sex Ratio
3.2. Histological Change of Gonad
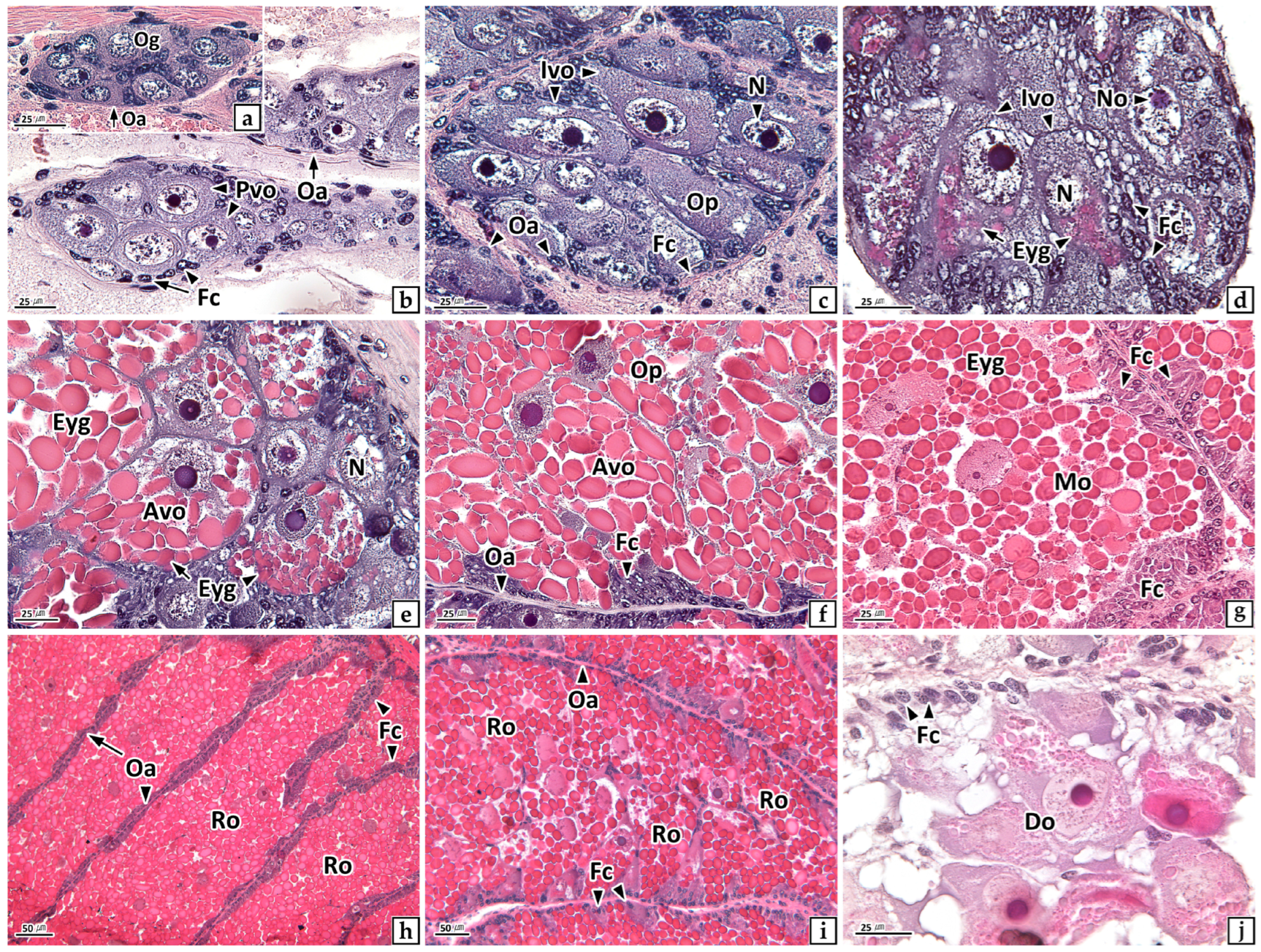
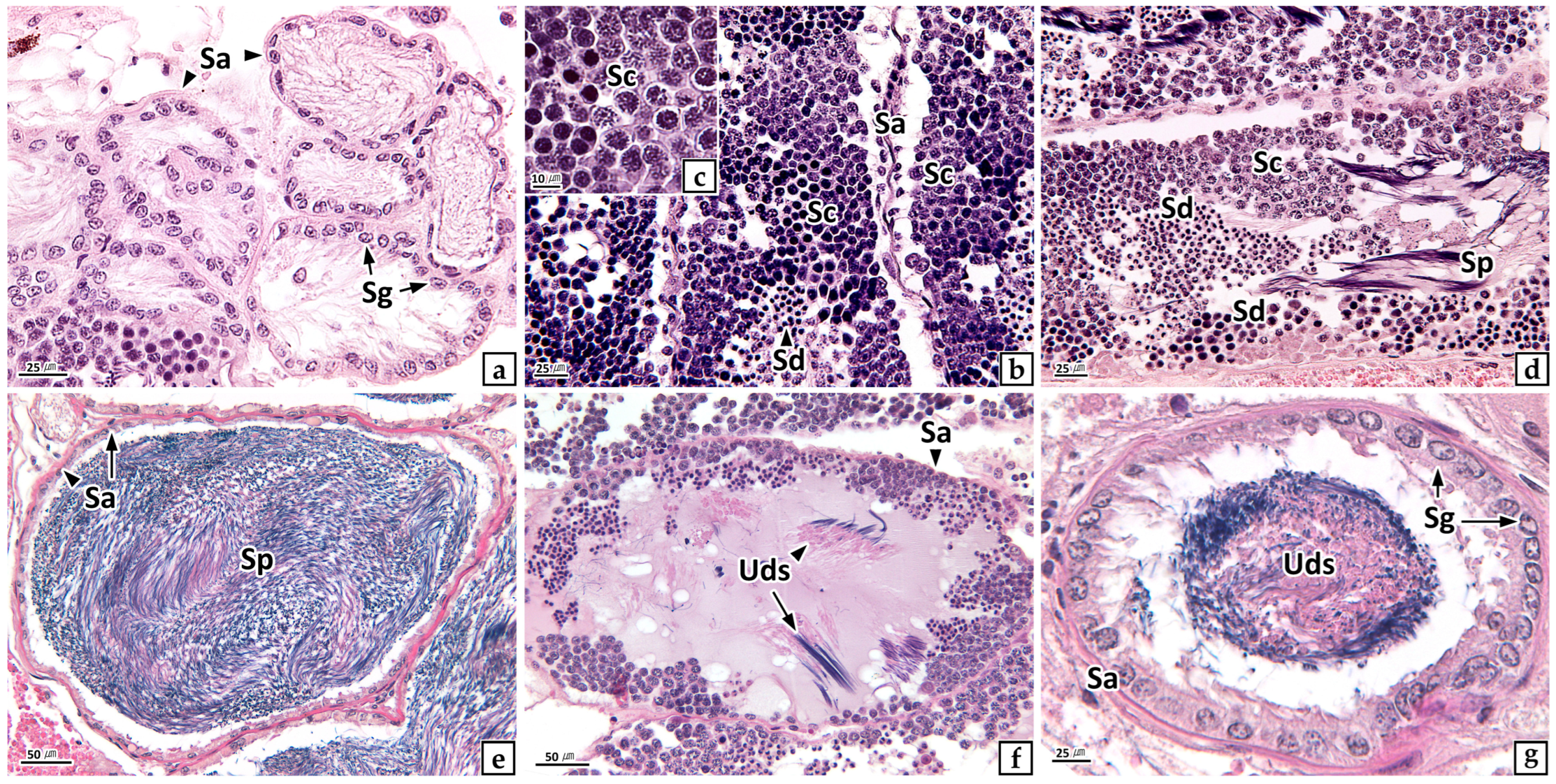
3.2.1. Ovary
3.2.2. Testis
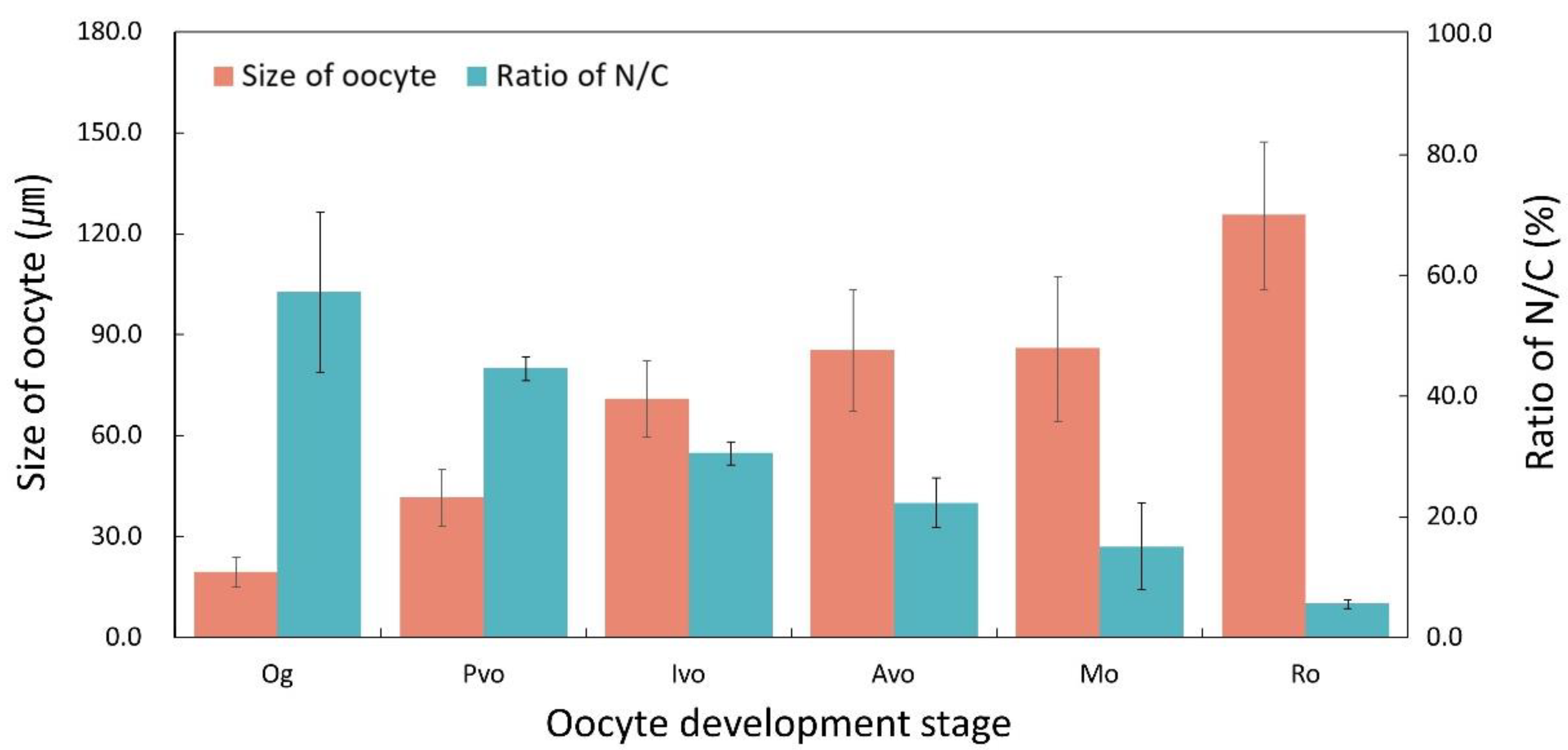
3.3. Gonadal Development
3.3.1. Ovary
3.3.2. Testis
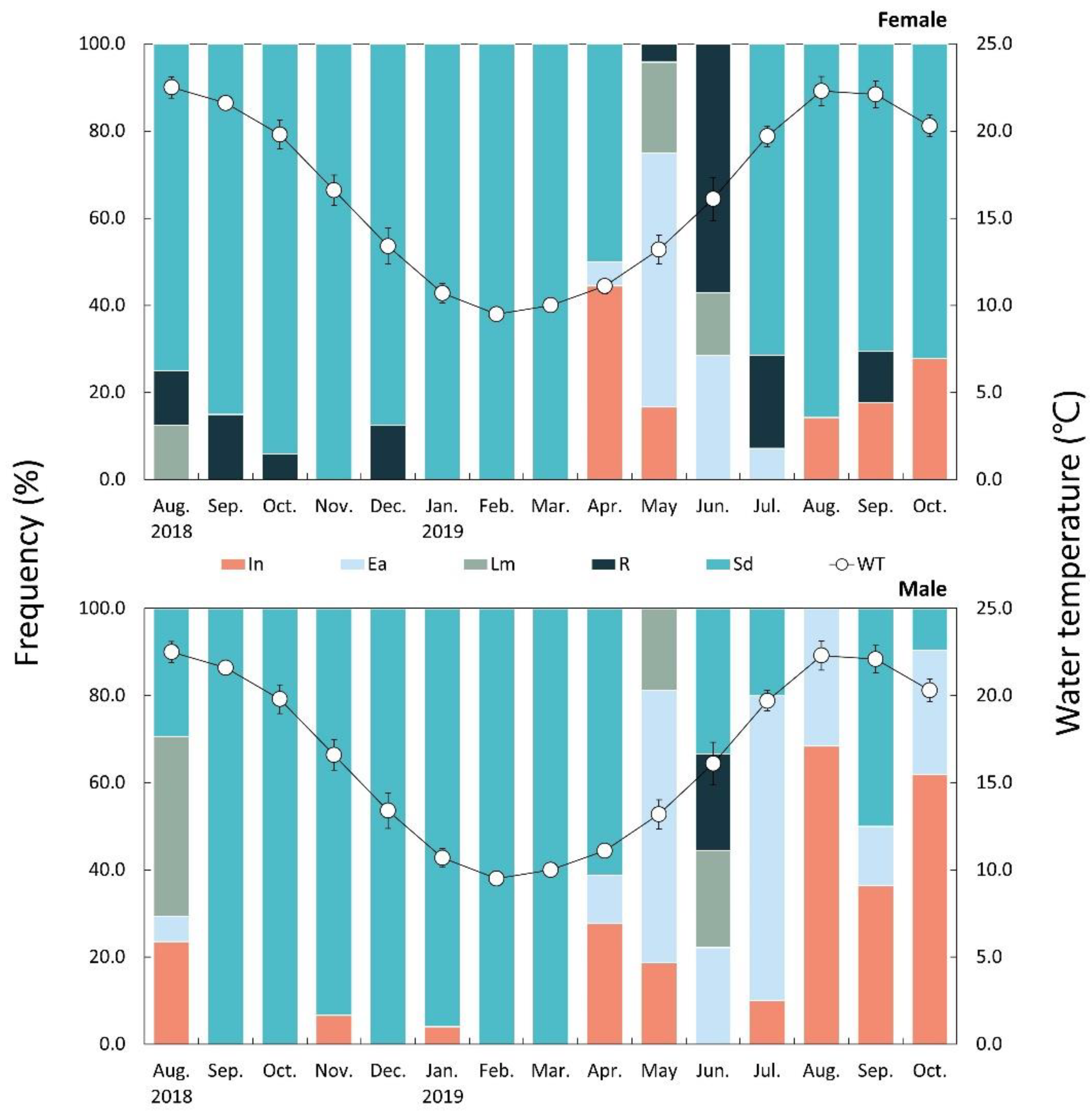
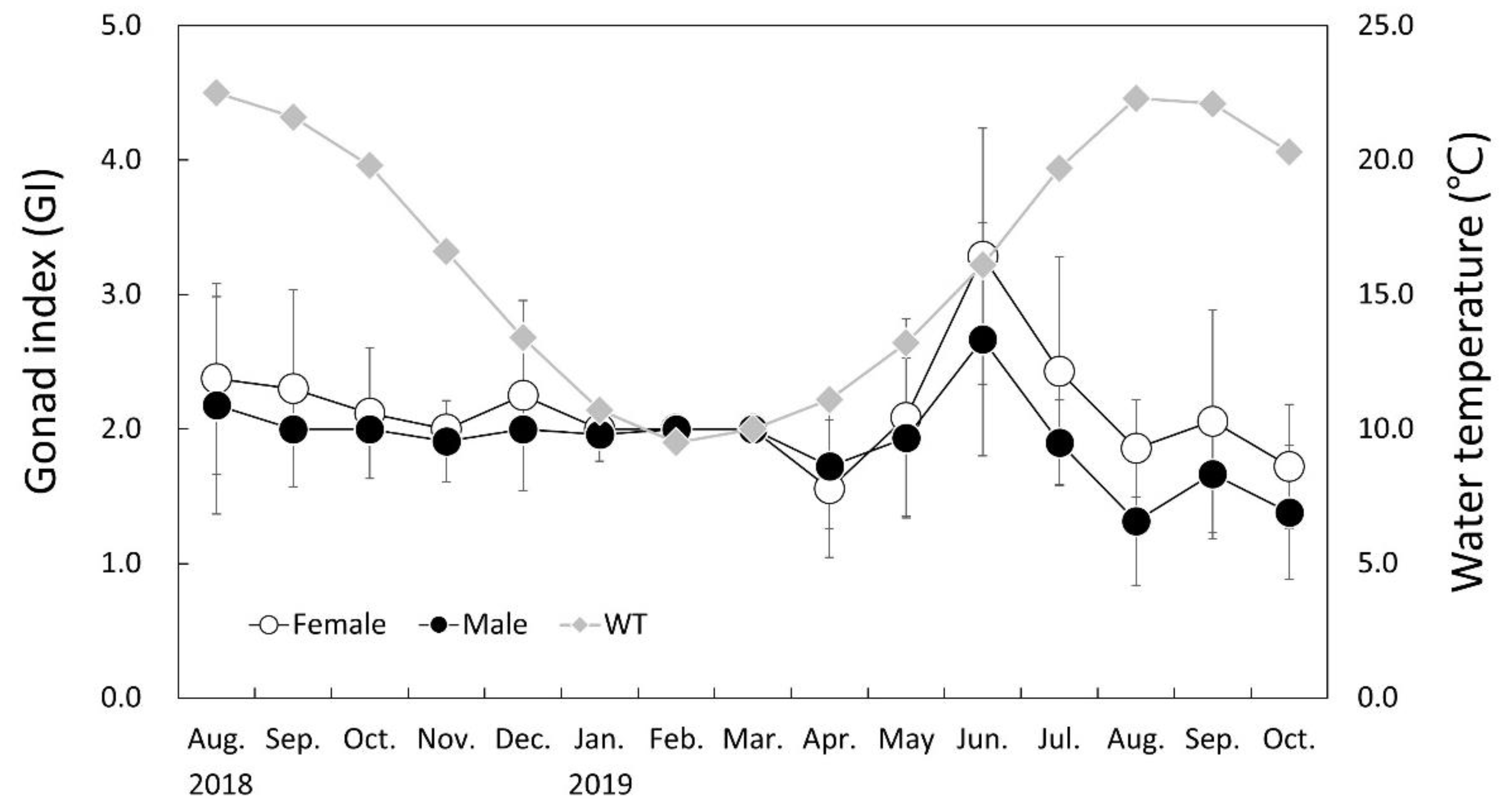
3.4. Environmental Conditions
3.5. Monthly Change of Gonad Index (GI)
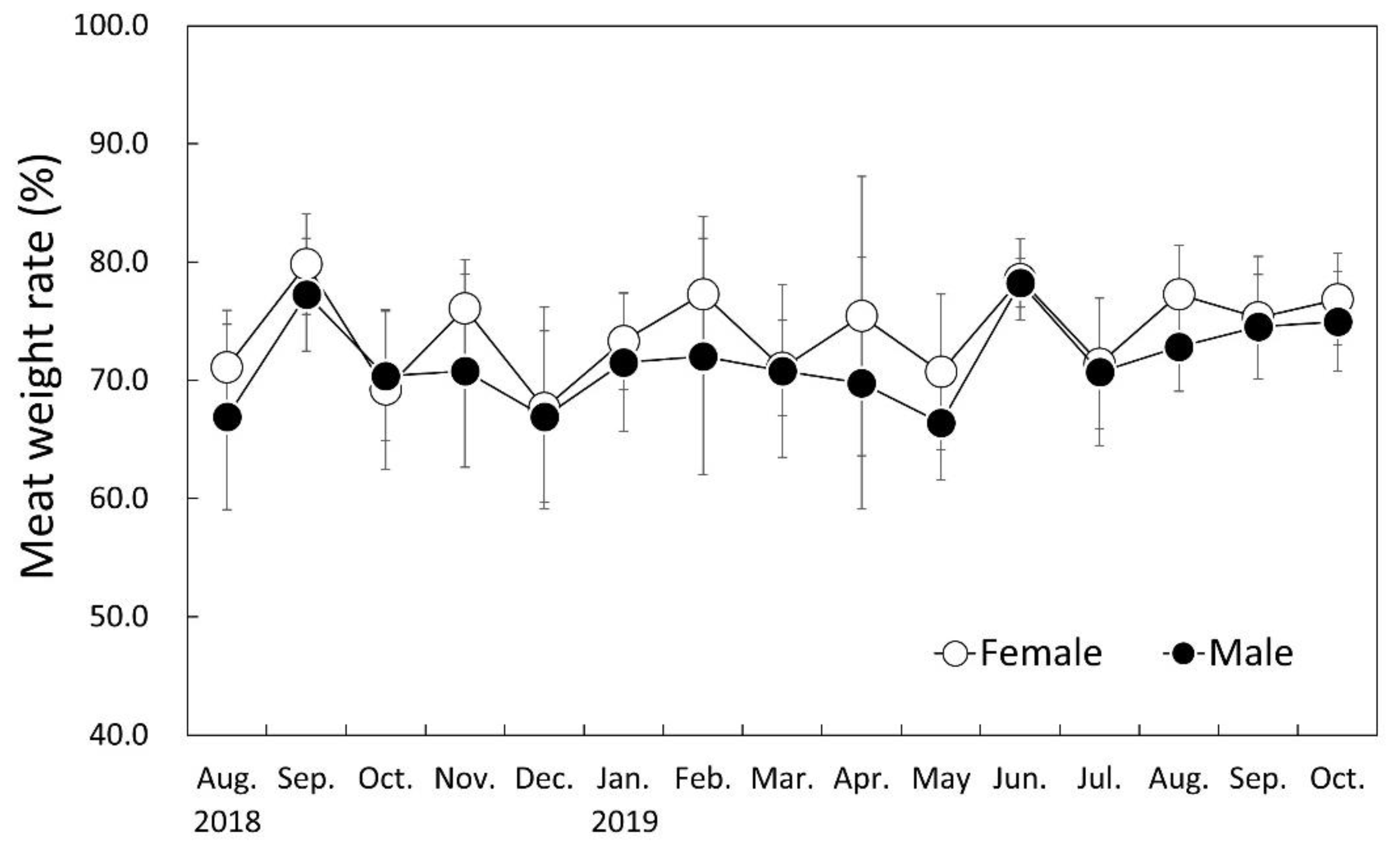
3.6. Monthly Change of Meat Weight Rate (MWR)
3.7. Main Spawning Period
3.8. Sexual Group Maturity
4. Discussion
4.1. Sex and Sex Ratio
4.2. Gonadal Development and Maturity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Head, M.A.; Keller, A.A.; Bradburn, M. Maturity and growth of sablefish, Anoplopoma fimbria, along the U.S. West Coast. Fish. Res. 2014, 159, 56–67. [Google Scholar] [CrossRef]
- Head, M.A.; Stokes, G.L.; Thorson, J.T.; Keller, A.A. Techniques for improving estimates of maturity ogives in groundfish using double-reads and measurement error models. Fish. Res. 2016, 179, 251–258. [Google Scholar] [CrossRef]
- Vaux, F.; Hills, S.F.; Marshall, B.A.; Trewick, S.A.; Morgan-Richards, M.A. A phylogeny of Southern Hemisphere whelks (Gastropoda: Buccinulidae) and concordance with the fossil record. Mol. Phylogenet. Evol. 2017, 114, 367–381. [Google Scholar] [CrossRef] [PubMed]
- NFRDI (National Fisheries Research and Development Institute). Commercial Molluscs from the Freshwater and Continental Shelf in Korea; Kudeok Print Publishing Co.: Busan, Korea, 2000; pp. 1–197.
- Okutani, T. Marine Mollusks in Japan; Tokai Univ. Press: Tokyo, Japan, 2000; pp. 488–489. [Google Scholar]
- Hong, S.Y.; Park, K.Y.; Park, C.W.; Han, C.H.; Suh, H.L.; Yun, S.G.; Song, C.B.; Jo, S.G.; Lim, H.S.; Kang, Y.S.; et al. Marine Invertebrates in Korean Coasts; Academy Publishing Company Inc.: Seoul, Korea, 2006; pp. 1–123. [Google Scholar]
- Martel, A.; Larrivée, D.H.; Klein, K.R.; Himmelman, J.H. Reproductive cycle and seasonal feeding activity of the neogastropod Buccinum undatum. Mar. Biol. 1986, 92, 211–221. [Google Scholar] [CrossRef]
- Kideys, A.E.; Nash, R.D.M.; Hartnoll, R.G. Reproductive cycle and energetic cost of reproduction of the neogastropod Buccinum undatum in the Irish Sea. J. Mar. Biol. Assoc. UK 1993, 73, 391–403. [Google Scholar] [CrossRef]
- Valentinsson, D. Reproductive cycle and maternal effects on offspring size and number in the neogastropod Buccinum undatum (L.). Mar. Biol. 2002, 140, 1139–1147. [Google Scholar] [CrossRef]
- Heude-Berthelin, C.; Hégron-Macé, L.; Legrand, V.; Jouaux, A.; Adeline, B.; Mathieu, M.; Kellner, K. Growth and reproduction of the common whelk Buccinum undatum in west Cotentin (Channel), France. Aquat. Living Resour. 2011, 24, 317–327. [Google Scholar] [CrossRef]
- Ilano, A.S.; Fujinaga, K.; Nakao, S. Reproductive cycle and size at sexual maturity of the commercial whelk Buccinum isaotakii in Funka Bay, Hokkaido, Japan. J. Mar. Biol. Assoc. UK 2003, 83, 1287–1294. [Google Scholar] [CrossRef]
- Lee, J.S.; Min, D.K. A catalogue of molluscan fauna in Korea. Korean J. Malacol. 2002, 18, 93–217. [Google Scholar]
- KHOA (Korea Hydrographic and Oceanographic Agency). Ocean Data in Grid Framework. 2019. Available online: http://www.khoa.go.kr/oceangrid/khoa/intro.do (accessed on 24 August 2021).
- Kim, H.; Kim, H.J.; Kim, Y.S.; Lee, J.S. Microstructural differentiation of the oocyte in the abalone Haliotis discus hannai. Korean J. Fish. Aquat. Sci. 2020, 53, 90–97. [Google Scholar] [CrossRef]
- Shin, S.R.; Kim, H.J.; Lee, D.H.; Kim, H.; Sohn, Y.C.; Kim, J.W.; Lee, J.S. Gonadal maturation and main spawning period of Haliotis gigantea (Gastropoda: Haliotidae). Dev. Reprod. 2020, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Park, J.J.; Jeon, M.A.; Lee, Y.G.; Lee, J.S. Implications for fisheries management: Sex ratio, size at sexual maturity, and reproductive cycle of Saxidomus purpuratus (Bivalvia: Veneridae) in Korea. J. Shellfish Res. 2018, 37, 951–957. [Google Scholar] [CrossRef]
- Jung, G.K.; Park, J.J.; Ju, S.M.; Jin, Y.G.; Lee, J.S. Ovarian structure and oogenesis of the spiny top shell, Batillus cornutus (Lightfoot, 1786) (Gastropoda: Turbinidae). Korean J. Malacol. 2007, 23, 209–216. [Google Scholar]
- Lee, D.H. Maturation and Spawning of Neptunea (Barbitonia) arthritica. Master’s Thesis, Gangneung-Wonju National University, Gangneung, Korea, 2019. [Google Scholar]
- Heller, J. Hermaphroditism in molluscs. Biol. J. Linnean Soc. 1993, 48, 19–42. [Google Scholar] [CrossRef]
- Yusa, Y. Causes of variation in sex ratio and modes of sex determination in the Mollusca—An overview. Am. Malacol. Bull. 2007, 23, 89–98. [Google Scholar] [CrossRef]
- Eversole, A.G. Reproduction in Mercenaria mercenaria. In Biology of the Hard Clam, 1st ed.; Kraeuter, J.N., Castagna, M., Eds.; Elsevier: New York, NY, USA, 2001; pp. 221–260. [Google Scholar]
- Lee, J.S. Sex and sex reversal of bivalves. Korean J. Malacol. 2015, 31, 315–322. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.S.; Shin, Y.K.; Lee, Y.G.; Park, J.J. Sequential hermaphroditism in Manila clam Ruditapes philippinarum (Bivalvia: Veneridae). Invertebr. Reprod. Dev. 2013, 57, 185–188. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.J.; Shin, Y.K.; Kim, H.; Jeon, M.A. Sex change and sequential hermaphroditism in Tegillarca granosa (Bivalvia: Arcidae). Invertebr. Reprod. Dev. 2014, 58, 314–318. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, S.R.; Oh, H.Y.; Kim, J.W.; Lee, J.S. Sex of mussel Mytilus coruscus (Bivalvia: Mytilidae): Sequential hermaphroditism. Dev. Reprod. 2021, 25, 55–57. [Google Scholar] [CrossRef]
- Kamel, S.J.; Oyarzun, F.X.; Grosberg, R.K. Reproductive biology, family conflict, and size of offspring in marine invertebrates. Integr. Comp. Biol. 2010, 50, 619–629. [Google Scholar] [CrossRef]
- Averbuj, A.; Bigatti, G.; Penchaszadeh, P.E. Gametogenic cycle and size at first maturity of the Patagonic edible snail Buccinanops cochlidium from Argentina. Mar. Biol. 2010, 157, 2229–2240. [Google Scholar] [CrossRef]
- Peemoeller, B.J.; Stevens, B.G. Age, size, and sexual maturity of channeled whelk (Busycotypus canaliculatus) in Buzzards Bay, Massachusetts. Fish. Bull. 2013, 111, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.J.; Rymer, T.L.; Congdon, B.C. Sex-ratio bias in Laevistrombus canarium Linné, 1758 (Gastropoda: Strombidae) from Far North Queensland, Australia. Mem. Queensl. Mus. 2017, 60, 133–138. [Google Scholar] [CrossRef]
- Olabarria, C.; Ramirez-Llodra, E. Reproductive strategies of two deep-sea gastropod species from the Porcupine Seabight (Northeast Atlantic). Mar. Biol. 2004, 145, 541–549. [Google Scholar] [CrossRef]
- Avaca, M.S.; Narvarte, M.; Martin, P. Age, growth and mortality in Buccinanops globulosus (Gastropoda: Nassariidae) from Golfo Nuevo (Argentina). Mar. Biol. Res. 2013, 9, 208–219. [Google Scholar] [CrossRef]
- Flores-Garza, R.; González, A.V.; Flores-Rodríguez, P.; Cruz-Ramirez, N.L. Density, sex ratio, size, weight, and recruitment of Plicopurpura pansa (Gastropoda: Muricidae) in Costa Chica, Guerrero, Mexico. Open J. Mar. Sci. 2012, 2, 157. [Google Scholar] [CrossRef]
- Elhasni, K.; Vasconcelos, P.; Ghorbel, M.; Jarboui, O. Reproductive cycle of Bolinus brandaris (Gastropoda: Muricidae) in the Gulf of Gabès (southern Tunisia). Mediterr. Mar. Sci. 2013, 14, 24–35. [Google Scholar] [CrossRef]
- Bondarev, I.P. Dynamics of Rapana venosa (Valenciennes, 1846) (Gastropoda: Muricidae) population in the Black Sea. Int. J. Mar. Sci. 2014, 4, 46–60. [Google Scholar] [CrossRef]
- Saglam, H.; Kutlu, S.; Dagtekin, M.; Bascinar, S.; Sahin, A.; Selen, H.; Duzgunes, E. Population biology of Rapana venosa (Valenciennes, 1846) (Gastropoda: Neogastropoda) in the south-eastern Black Sea of Turkey. Cah. Biol. Mar. 2015, 56, 363–368. [Google Scholar]
- Hua, N.P.; Nguyen, T.X.T.; Mai, D.M.; Phan, D.H.; Kieu, T.Y. Spawning characteristics of Babylonia areolata (Neogastropoda: Buccinidae). Spec. Publ. Phuket Mar. Biol. Cent. 2001, 25, 151–165. [Google Scholar]
- Beams, H.W.; Sekhon, S.S. Electron microscope studies on the oocyte of the fresh-water mussel (Anodonta), with special reference to the stalk and mechanism of yolk deposition. J. Morphol. 1966, 119, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Lee, J.Y.; Lee, J.S.; Chang, Y.J. Gonadal development and gametogenic cycle of the equilateral venus, Gomphina veneriformis (Bivalvia: Veneridae). Korean J. Fish. Aqua. Sci. 2003, 36, 352–357. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Moura, P.; Barroso, C.M.; Gaspar, M.B. Reproductive cycle of Bolinus brandaris (Gastropoda: Muricidae) in the Ria Formosa lagoon (southern Portugal). Aquat. Biol. 2012, 16, 69–83. [Google Scholar] [CrossRef]
- Hodgson, A.N.; Eckelbarger, K.J. Ultrastructure of the ovary and oogenesis in six species of patellid limpets (Gastropoda: Patellogastropoda) from South Africa. Invertebr. Biol. 2000, 119, 265–277. [Google Scholar] [CrossRef]
- Arrighetti, F.; Penchaszadeh, P.E. Gametogenesis, seasonal reproduction and imposex of Adelomelon beckii (Neogastropoda: Volutidae) in Mar del Plata, Argentina. Aquat. Biol. 2010, 9, 63–75. [Google Scholar] [CrossRef]
- Ilano, A.S.; Fujinaga, K.; Nakao, S. Mating, development and effects of female size on offspring number and size in the neogastropod Buccinum isaotakii (Kira, 1959). J. Molluscan Stud. 2004, 70, 277–282. [Google Scholar] [CrossRef]
- Chan, K.; Morton, B. The reproductive biology of Nassarius festivus (Powys, 1835) (Gastropoda: Nassariidae) in relation to seasonal changes in temperature and salinity in subtropical Hong Kong. Aquat. Ecol. 2005, 39, 213–228. [Google Scholar] [CrossRef]
- Luz, Í.O.D. Morfologia Radular e Descrição da Estrutura Populacional e Reprodutiva do Molusco Gastrópode Hastula cinerea (BORN, 1778) (Conoidea: Terebridae). Ph.D. Thesis, Universidade federal do Ceará, Fortaleza, Brazil, 2021. [Google Scholar]
- Marcus, E.; Marcus, E. On Hastula cinerea. Bol. Fac. Filos. Cienc. Let. Univ. São Paulo Zool. 1960, 23, 25–65. [Google Scholar] [CrossRef]
- Radwan, N.; Mohammad, S.; Mohamed, S.; Yaseen, A. Reproduction and gonad development of gastropod Thais carinifera in Lake Timsah, Suez Canal, Egypt. Egypt J. Aquat. Biol. Fish. 2009, 13, 53–67. [Google Scholar] [CrossRef]
- Spight, T.M. Ecology of hatching size for marine snails. Oecologia 1976, 24, 283–294. [Google Scholar] [CrossRef]
- Arrighetti, F.; Penchaszadeh, P.E. Size and age at first sexual maturity of the edible giant snail Adelomelon beckii (Neogastropoda: Volutidae) from Mar del Plata, Argentina. Malacologia 2010, 53, 193–197. [Google Scholar] [CrossRef]
- Cledón, M.; Arntz, W.; Penchaszadeh, P.E. Gonadal cycle in an Adelomelon brasiliana (Neogastropoda: Volutidae) population of Buenos Aires province, Argentina. Mar. Biol. 2005, 147, 439–445. [Google Scholar] [CrossRef]
- Luzzatto, D.C. The biology and ecology of the giant free egg capsules of Adelomelon brasiliana Lamarck, 1811 (Gastropoda: Volutidae). Malacologia 2006, 49, 107–119. [Google Scholar] [CrossRef]
- Bigatti, G.; Marzinelli, E.M.; Penchaszadeh, P.E. Seasonal reproduction and sexual maturity in Odontocymbiola magellanica (Neogastropoda, Volutidae). Inverteb. Biol. 2008, 127, 314–326. [Google Scholar] [CrossRef]
- Giménez, J.; Penchaszadeh, P. Reproductive cycle of Zidona dufresnei (Caenogastropoda: Volutidae) from the southwestern Atlantic Ocean. Mar. Biol. 2002, 140, 755–761. [Google Scholar] [CrossRef]
- Eckelbarger, K.J.; Watling, L. Role of phylogenetic constraints in determining reproductive patterns in deep-sea invertebrates. Invertebr. Biol. 1995, 114, 256–269. [Google Scholar] [CrossRef]
- Wallace, R.A.; Selmen, K. Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 1981, 21, 325–343. [Google Scholar] [CrossRef]
- Ward, D.W.; Davis, A.R. Reproduction of the turban shell Turbo torquatus Gmelin 1791 (Mollusca: Gastropoda), in New South Wales, Australia. Mar. Freshw. Res. 2002, 53, 85–91. [Google Scholar] [CrossRef]
- Fretter, V.; Graham, A. Reproduction. In Physiology of Mollusca; Wilbur, K.M., Yonge, C.M., Eds.; Academic Press: New York, NY, USA, 1964; pp. 127–164. [Google Scholar]
- Lee, J.H. Gametogenesis and reproductive cycle of the murex shell Ceratostoma rorifluum (Neogastropoda: Muricidae). J. Kor. Fish. Soc. 2008, 41, 253–260. [Google Scholar] [CrossRef]
- Villamora, S.; Yamamoto, T. Reproductive seasonality of Monetaria annulus (Linnaeus, 1758) (Mollusca: Gastropoda: Cypraeidae) in a temperate area. Molluscan Res. 2015, 35, 95–101. [Google Scholar] [CrossRef]

| SH (mm) | Total | Female | Male | Sex Ratio (F %) | Chi-Square | p Value |
|---|---|---|---|---|---|---|
| ≤50.0 | 12 | 5 | 7 | 1:1.4 (F 41.7%) | - | - |
| 50.1–60.0 | 73 | 36 | 37 | 1:1.0 (F 49.3%) | 0.014 | 0.907 |
| 60.1–70.0 | 133 | 52 | 81 | 1:1.6 (F 39.1%) | 6.323 | 0.012 |
| 70.1–80.0 | 133 | 57 | 76 | 1:1.3 (F 42.9%) | 2.714 | 0.099 |
| 80.1–90.0 | 101 | 47 | 54 | 1:1.1 (F 46.5%) | 0.485 | 0.486 |
| 90.1–100.0 | 68 | 33 | 35 | 1:1.1 (F 48.5%) | 0.059 | 0.808 |
| 100.1–110.0 | 24 | 16 | 8 | 1:0.5 (F 66.7%) | - | - |
| 110.1≤ | 5 | 4 | 1 | 1:0.3 (F 80.0%) | - | - |
| Total/Mean | 549 | 250 | 299 | 1:1.2 (F 45.5%) | 4.373 | 0.37 |
| SH (mm) | Female | Male | ||||
|---|---|---|---|---|---|---|
| Total | Mature | Maturity (%) | Total | Mature | Maturity (%) | |
| ≤50.0 | 5 | 2 | 40.0 | 7 | 2 | 28.6 |
| 50.1–60.0 | 36 | 31 | 86.1 | 37 | 29 | 78.4 |
| 60.1–70.0 | 52 | 46 | 88.5 | 81 | 49 | 60.5 |
| 70.1–80.0 | 57 | 42 | 73.7 | 76 | 54 | 71.1 |
| 80.1–90.0 | 47 | 39 | 83.0 | 54 | 39 | 72.2 |
| 90.1–100.0 | 33 | 26 | 78.8 | 35 | 26 | 74.3 |
| 100.1–110.0 | 16 | 15 | 93.8 | 8 | 6 | 75.0 |
| 110.1≤ | 4 | 4 | 100.0 | 1 | 1 | 100.0 |
| Total/Mean | 250 | 205 | 80.5 | 299 | 206 | 70.0 |
| Order | Family/Species | Egg Stalk | Egg Size (μm) | Citation |
|---|---|---|---|---|
| Vetigastropoda | Haliotidae | |||
| Haliotis discus hannai | + | 202.9 × 142.1 | [14] | |
| Haliotis gigantea | + | 200 | [15] | |
| Turbinidae | ||||
| Batillus cornutus | + | 140 | [17] | |
| Neogatropoda | Buccinanopsidae | |||
| Buccinanops cochlidium | - | 220 | [27] | |
| Buccinidae | ||||
| Buccinum isaotakii | - | 240 ± 40 | [11,42] | |
| Buccinum osagawai | - | 82.3 × 125.5 | Present study | |
| Busyconidae | ||||
| Busycotypus canaliculatus | - | - | [28] | |
| Columbellidae | ||||
| Amphissa acutecostata | - | 99.06 | [30] | |
| Nassariidae | ||||
| Nassarius festivus | - | 160 | [43] | |
| Terebridae | ||||
| Hastula cinerea | - | 300 × 150 × 100 | [44,45] | |
| Raphitomidae | ||||
| Gymnobela subaraneosa | - | 114.82 | [30] | |
| Muricidae | ||||
| Bolinus brandaris | - | - | [39] | |
| Thais carinifera | - | 200–220 | [46,47] | |
| Volutidae | ||||
| Adelomelon beckii | - | 222 | [48] | |
| Adelomelon brasiliana | - | 180 ± 8 | [49,50] | |
| Odontocymbiola magellanica | - | - | [51] | |
| Zidona dufresnei | - | - | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.J.; Kim, H.J.; Shin, S.R.; Jin, Y.G.; Kim, J.W.; Lee, J.S. Reproductive Cycle and Sexual Group Maturity of Buccinum osagawai (Neogastropoda: Buccinidae). Fishes 2022, 7, 267. https://doi.org/10.3390/fishes7050267
Park JJ, Kim HJ, Shin SR, Jin YG, Kim JW, Lee JS. Reproductive Cycle and Sexual Group Maturity of Buccinum osagawai (Neogastropoda: Buccinidae). Fishes. 2022; 7(5):267. https://doi.org/10.3390/fishes7050267
Chicago/Turabian StylePark, Jung Jun, Hyeon Jin Kim, So Ryung Shin, Young Guk Jin, Jae Won Kim, and Jung Sick Lee. 2022. "Reproductive Cycle and Sexual Group Maturity of Buccinum osagawai (Neogastropoda: Buccinidae)" Fishes 7, no. 5: 267. https://doi.org/10.3390/fishes7050267
APA StylePark, J. J., Kim, H. J., Shin, S. R., Jin, Y. G., Kim, J. W., & Lee, J. S. (2022). Reproductive Cycle and Sexual Group Maturity of Buccinum osagawai (Neogastropoda: Buccinidae). Fishes, 7(5), 267. https://doi.org/10.3390/fishes7050267







