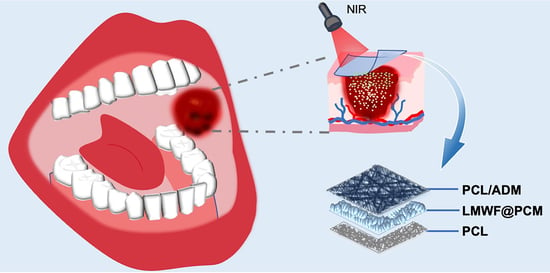
On-Demand Release of Fucoidan from a Multilayered Nanofiber Patch for the Killing of Oral Squamous Cancer Cells and Promotion of Epithelial Regeneration
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Reagents
2.2. Fabrication and Characterization of the Nanofiber Patch
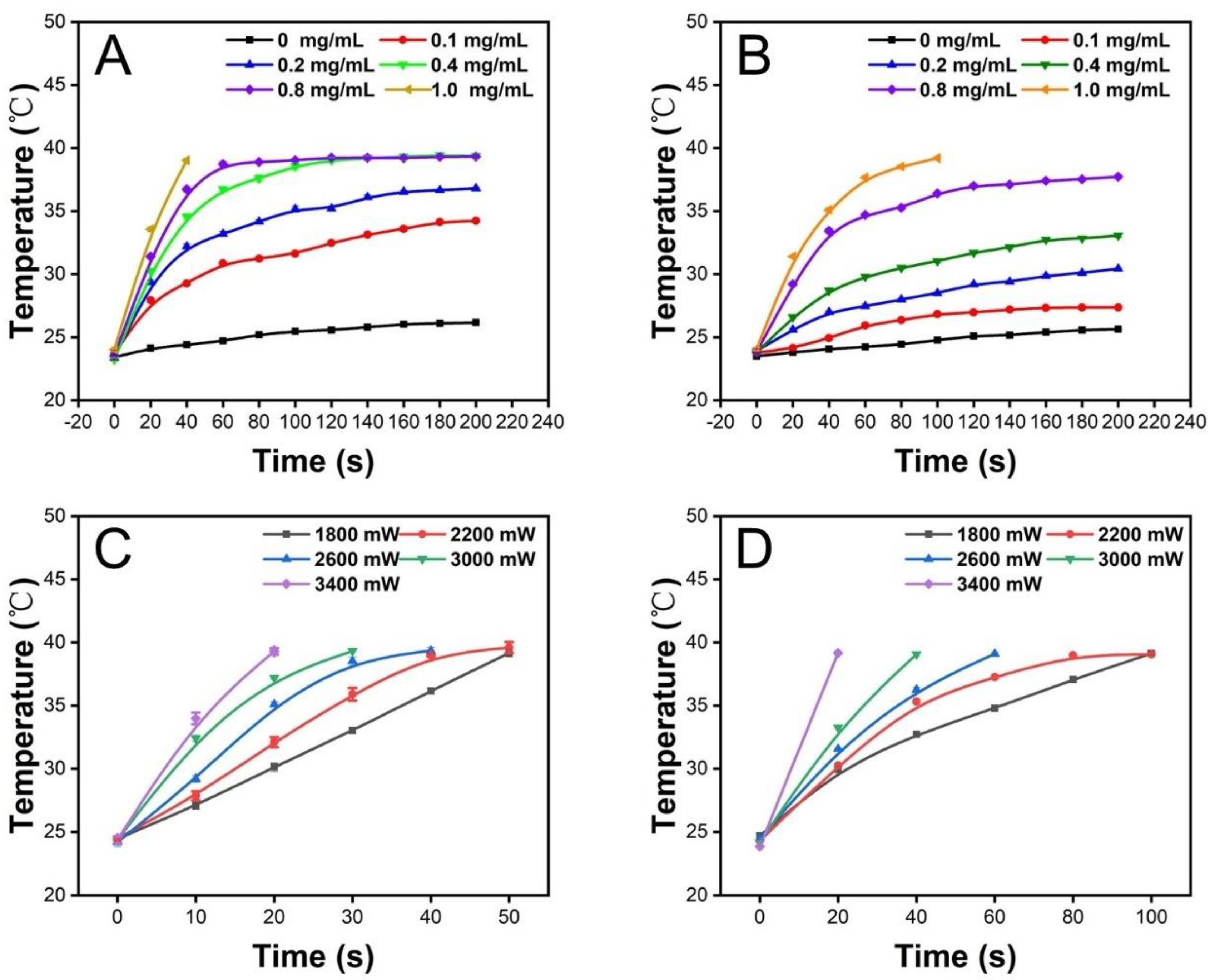
2.3. Photothermal Performance of the Sandwiched PCM Microparticles
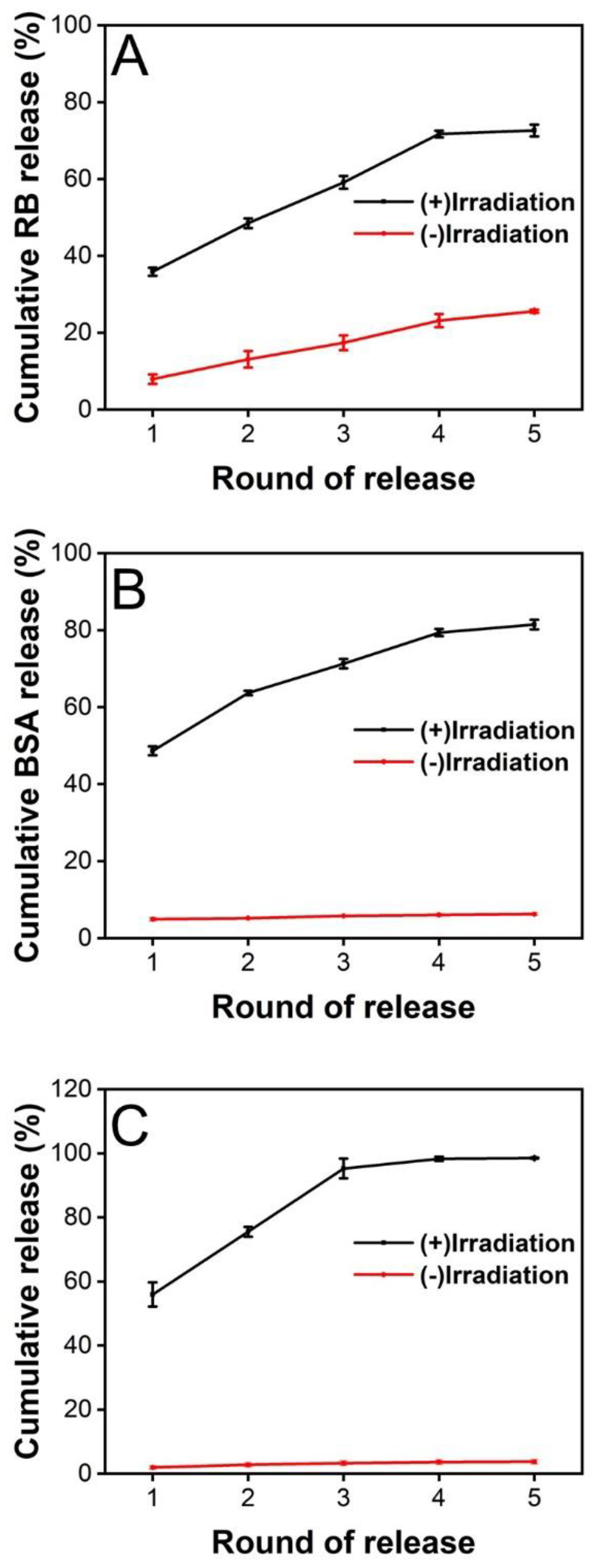
2.4. In Vitro Release of LMWF under NIR Irradiation
2.4.1. Release of Rhodamine B from PCM upon Heating
2.4.2. Release of BSA from PCM under NIR Irradiation
2.4.3. Release of LMWF from PCM under NIR Irradiation
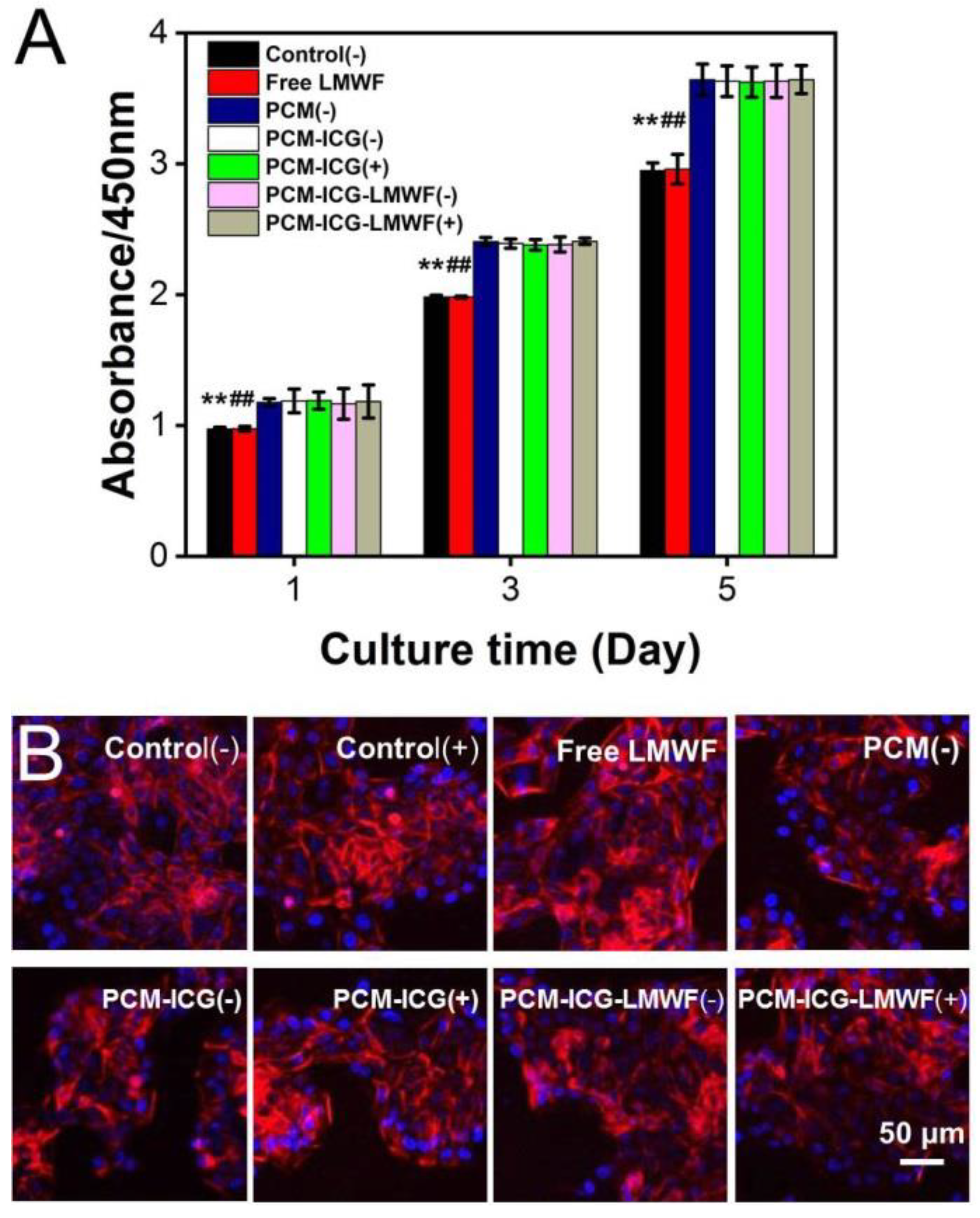
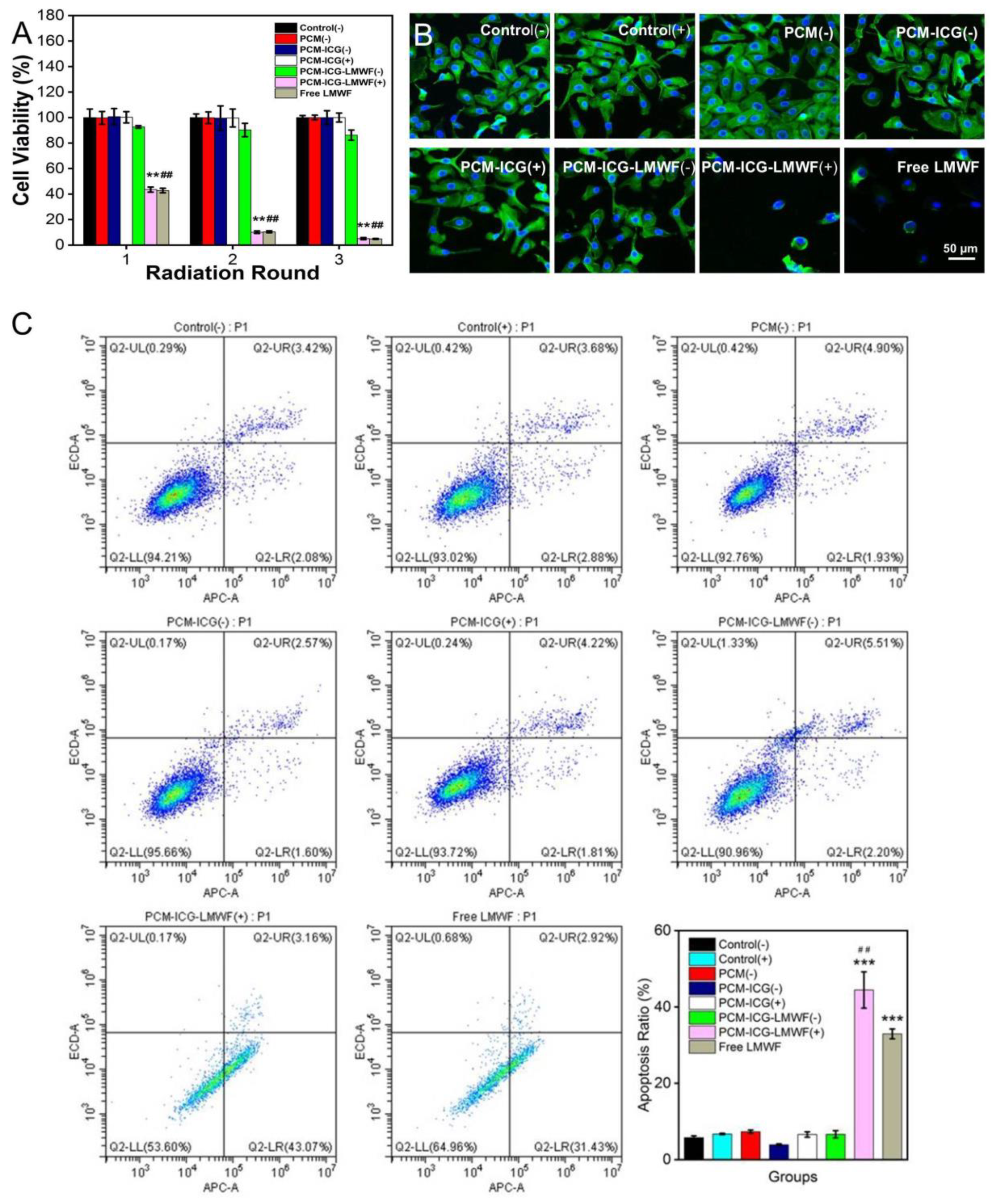
2.5. In Vitro Cell Viability Assay
2.6. Cell Apoptosis Detection Assay
2.7. Characterization
2.8. Statistical Analysis
3. Results and Discussion
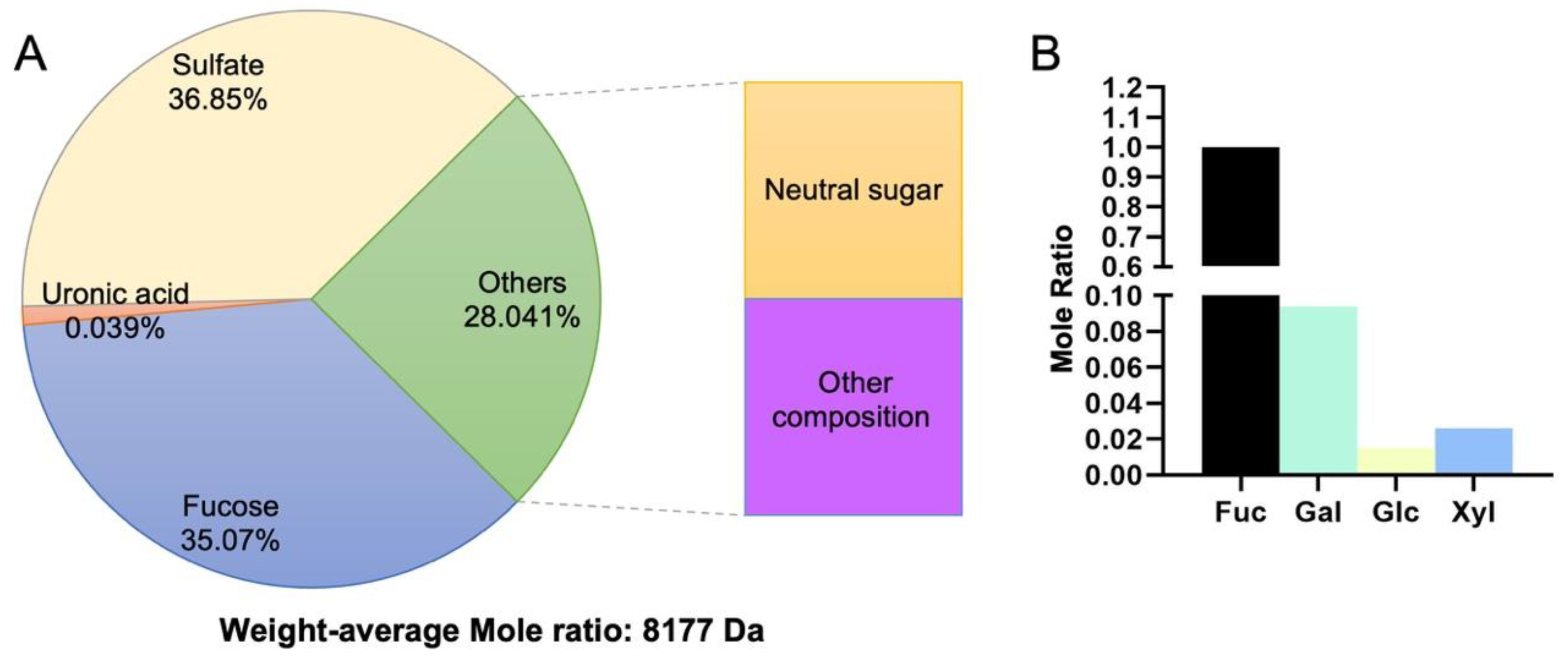
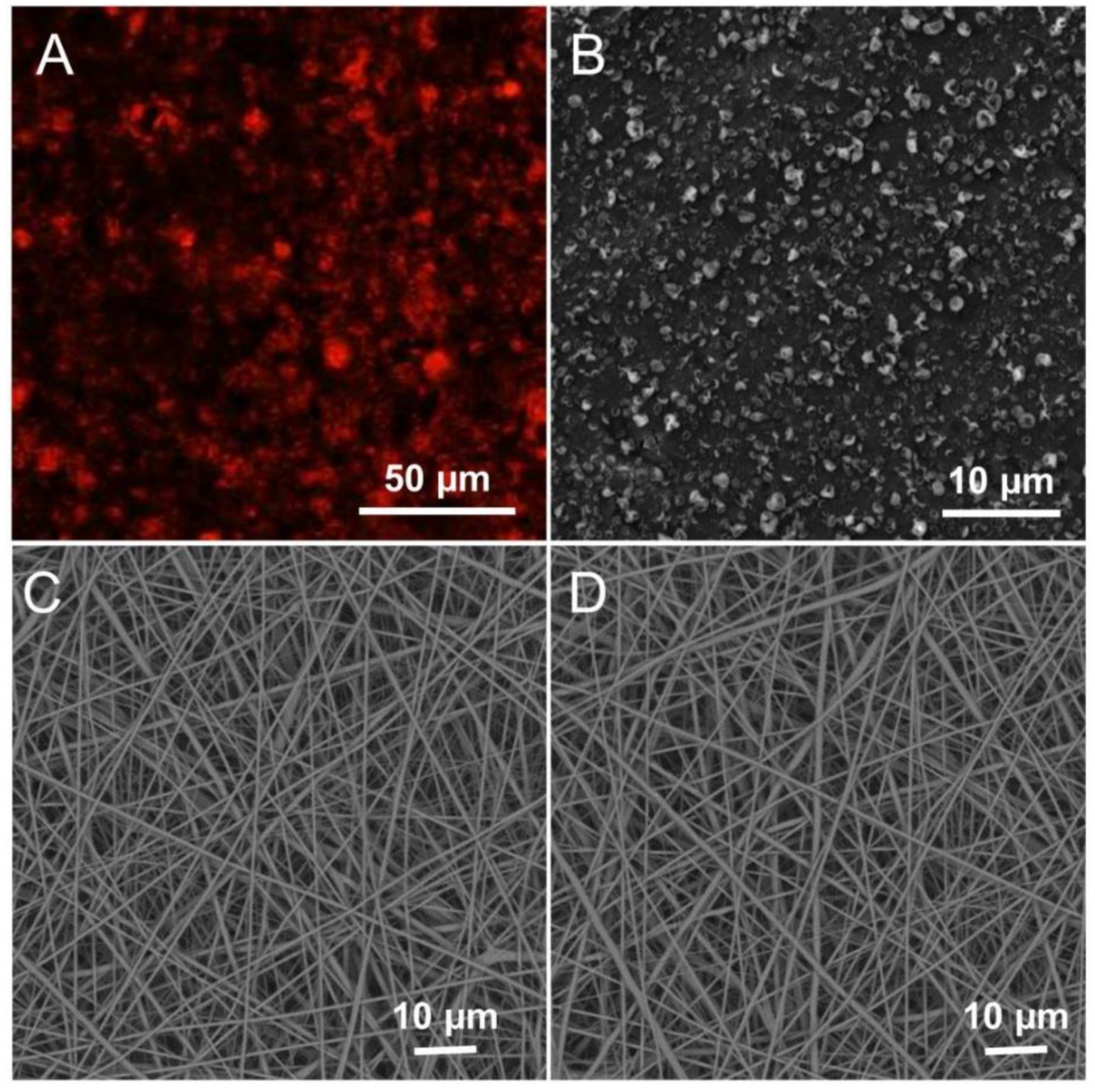
3.1. Fabrication and Characterization of LMWF and the Scaffold
3.2. Photothermal Properties of the Scaffold
3.3. Release of Substances from the PCM Microparticles
3.4. Evaluation of the Treatment Effect on HOK and SCC-9 Cells
3.5. Detection of Cancer Cell Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Aparna, M.; Rao, L.; Kunhikatta, V.; Radhakrishnan, R. The role of MMP-2 and MMP-9 as prognostic markers in the early stages of tongue squamous cell carcinoma. J. Oral Pathol. Med. 2015, 44, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Rêgo, D.F.; Assad, D.X.; Coletta, R.D.; De Luca Canto, G.; Guerra, E.N. In vivo and in vitro effects of curcumin on head and neck carcinoma: A systematic review. J. Oral Pathol. Med. 2017, 46, 3–20. [Google Scholar] [CrossRef]
- Gharat, S.A.; Momin, M.; Bhavsar, C. Oral Squamous Cell Carcinoma: Current Treatment Strategies and Nanotechnology-Based Approaches for Prevention and Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 363–400. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Will, O.M.; Purcz, N.; Chalaris, A.; Heneweer, C.; Boretius, S.; Purcz, L.; Nikkola, L.; Ashammakhi, N.; Kalthoff, H.; Gluer, C.C.; et al. Increased survival rate by local release of diclofenac in a murine model of recurrent oral carcinoma. Int. J. Nanomed. 2016, 11, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial Chitosan based formulations with impact on different biomedical applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Moroșan, A.; Tihăuan, B.; Oprea, O.; Motelică, L.; Trușcă, R.; Nicoară, A.I.; Popescu, R.C.; Savu, D.; Mihăiescu, D.E.; et al. Novel Graphene Oxide/Quercetin and Graphene Oxide/Juglone Nanostructured Platforms as Effective Drug Delivery Systems with Biomedical Applications. Nanomaterials 2022, 12, 1943. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhang, Z.; Zhang, Y.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Use of asymmetric multilayer polylactide nanofiber mats in controlled release of drugs and prevention of liver cancer recurrence after surgery in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1047–1056. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, W. Functional Electrospun Fibers for Local Therapy of Cancer. Adv. Fiber Mater. 2020, 2, 229–245. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Di Santo, R.; Tai, W.; Gu, Z. ATP-triggered anticancer drug delivery. Nat. Commun. 2014, 5, 3364. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.S.; Wang, K.; Wang, Y.X.; Liu, Y. Cholinesterase-responsive supramolecular vesicle. J. Am. Chem. Soc. 2012, 134, 10244–10250. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, Y.; Pena, D.A.; Peng, L.; Yu, G. Thermally Responsive Hydrogel Blends: A General Drug Carrier Model for Controlled Drug Release. Angew. Chem. 2015, 54, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Al-Jamal, W.T.; Bossche, J.V.; Bui, T.T.; Drake, A.F.; Mason, A.J.; Kostarelos, K. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 2012, 6, 9335–9346. [Google Scholar] [CrossRef]
- Santini, J.T., Jr.; Cima, M.J.; Langer, R. A controlled-release microchip. Nature 1999, 397, 335–338. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef]
- Singh, A.V.; Chandrasekar, V.; Laux, P.; Luch, A.; Dakua, S.P.; Zamboni, P.; Shelar, A.; Yang, Y.; Pandit, V.; Tisato, V.; et al. Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells 2022, 11, 2801. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, Y.; Shi, X. Construction of Electrospun Organic/Inorganic Hybrid Nanofibers for Drug Delivery and Tissue Engineering Applications. Adv. Fiber Mater. 2019, 1, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Moon, G.D.; Choi, S.W.; Cai, X.; Li, W.; Cho, E.C.; Jeong, U.; Wang, L.V.; Xia, Y. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc. 2011, 133, 4762–4765. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and near-IR triggered release from polymeric nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G.; et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-responsive nanomaterials for biomedical applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef]
- Choi, S.W.; Zhang, Y.; Xia, Y. A temperature-sensitive drug release system based on phase-change materials. Angew. Chem. 2010, 49, 7904–7908. [Google Scholar] [CrossRef]
- Hyun, D.C.; Lu, P.; Choi, S.I.; Jeong, U.; Xia, Y. Microscale polymer bottles corked with a phase-change material for temperature-controlled release. Angew. Chem. 2013, 52, 10468–10471. [Google Scholar] [CrossRef]
- Hyun, D.C.; Levinson, N.S.; Jeong, U.; Xia, Y. Emerging applications of phase-change materials (PCMs): Teaching an old dog new tricks. Angew. Chem. 2014, 53, 3780–3795. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Jiang, H.; Wang, J.; Zhong, W.; Xue, K.; Zhu, C. Transporting mitochondrion-targeting photosensitizers into cancer cells by low-density lipoproteins for fluorescence-feedback photodynamic therapy. Nanoscale 2021, 13, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Lv, S.; Zhu, C. Bringing naturally-occurring saturated fatty acids into biomedical research. J. Mater. Chem. B 2021, 9, 6973–6987. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Wang, C.; Wang, J.; Lv, S.; Hao, B.; Zhu, C.; Tang, B.Z. A Sensitive and Reliable Organic Fluorescent Nanothermometer for Noninvasive Temperature Sensing. J. Am. Chem. Soc. 2021, 143, 14147–14157. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhu, C.; Huo, D.; Yang, M.; Xue, J.; Xia, Y. A Hybrid Nanomaterial for the Controlled Generation of Free Radicals and Oxidative Destruction of Hypoxic Cancer Cells. Angew. Chem. 2017, 56, 8801–8804. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, C.; Li, J.; Li, H.; Xia, Y. Integration of Phase-Change Materials with Electrospun Fibers for Promoting Neurite Outgrowth under Controlled Release. Adv. Funct. Mater. 2018, 28, 1705563. [Google Scholar] [CrossRef]
- Cheng, H.; Huo, D.; Zhu, C.; Shen, S.; Wang, W.; Li, H.; Zhu, Z.; Xia, Y. Combination cancer treatment through photothermally controlled release of selenous acid from gold nanocages. Biomaterials 2018, 178, 517–526. [Google Scholar] [CrossRef]
- Zhu, C.; Huo, D.; Chen, Q.; Xue, J.; Shen, S.; Xia, Y. A Eutectic Mixture of Natural Fatty Acids Can Serve as the Gating Material for Near-Infrared-Triggered Drug Release. Adv. Mater. 2017, 29, 1703702. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, S.; Wang, J.; Li, F.; Chen, A.; Li, B. A comparative study of antithrombotic and antiplatelet activities of different fucoidans from Laminaria japonica. Thromb. Res. 2012, 129, 771–778. [Google Scholar] [CrossRef]
- Yang, W.; Yu, X.; Zhang, Q.; Lu, Q.; Wang, J.; Cui, W.; Zheng, Y.; Wang, X.; Luo, D. Attenuation of streptozotocin-induced diabetic retinopathy with low molecular weight fucoidan via inhibition of vascular endothelial growth factor. Exp. Eye Res. 2013, 115, 96–105. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Ushakova, N.A.; Zyuzina, K.A.; Bilan, M.I.; Elizarova, A.L.; Somonova, O.V.; Madzhuga, A.V.; Krylov, V.B.; Preobrazhenskaya, M.E.; Usov, A.I.; et al. Influence of fucoidans on hemostatic system. Mar. Drugs 2013, 11, 2444–2458. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Li, Y.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested Fucoidan Extracts Derived from Seaweed Mozuku of Cladosiphon novae-caledoniae kylin Inhibit Invasion and Angiogenesis of Tumor Cells. Cytotechnology 2005, 47, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Induction of apoptosis by low-molecular-weight fucoidan through calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci. Biotechnol. Biochem. 2013, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.J.; Li, L.X.; Zhang, J.W.; Yang, Z.S.; Shi, D.M.; Yang, Y.K.; Wu, W.Z. Antimetastatic Effect of Fucoidan-Sargassum against Liver Cancer Cell Invadopodia Formation via Targeting Integrin αVβ3 and Mediating αVβ3/Src/E2F1 Signaling. J. Cancer 2019, 10, 4777–4792. [Google Scholar] [CrossRef] [PubMed]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr. Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Wang, Y.; Liu, M.; Deng, S.; Yu, X.; Cong, B.; Wang, W. Effects of Low Molecular Weight Fucoidan on the Proliferation and Apoptosis of Dysplastic Oral Keratinocyte and Oral Squamous Cell Carcinoma Cells. Nat. Prod. Commun. 2020, 15, 1934578X20921681. [Google Scholar] [CrossRef]
- Singh, A.V.; Kayal, A.; Malik, A.; Maharjan, R.S.; Dietrich, P.; Thissen, A.; Siewert, K.; Curato, C.; Pande, K.; Prahlad, D.; et al. Interfacial Water in the SARS Spike Protein: Investigating the Interaction with Human ACE2 Receptor and In Vitro Uptake in A549 Cells. Langmuir 2022, 38, 7976–7988. [Google Scholar] [CrossRef]
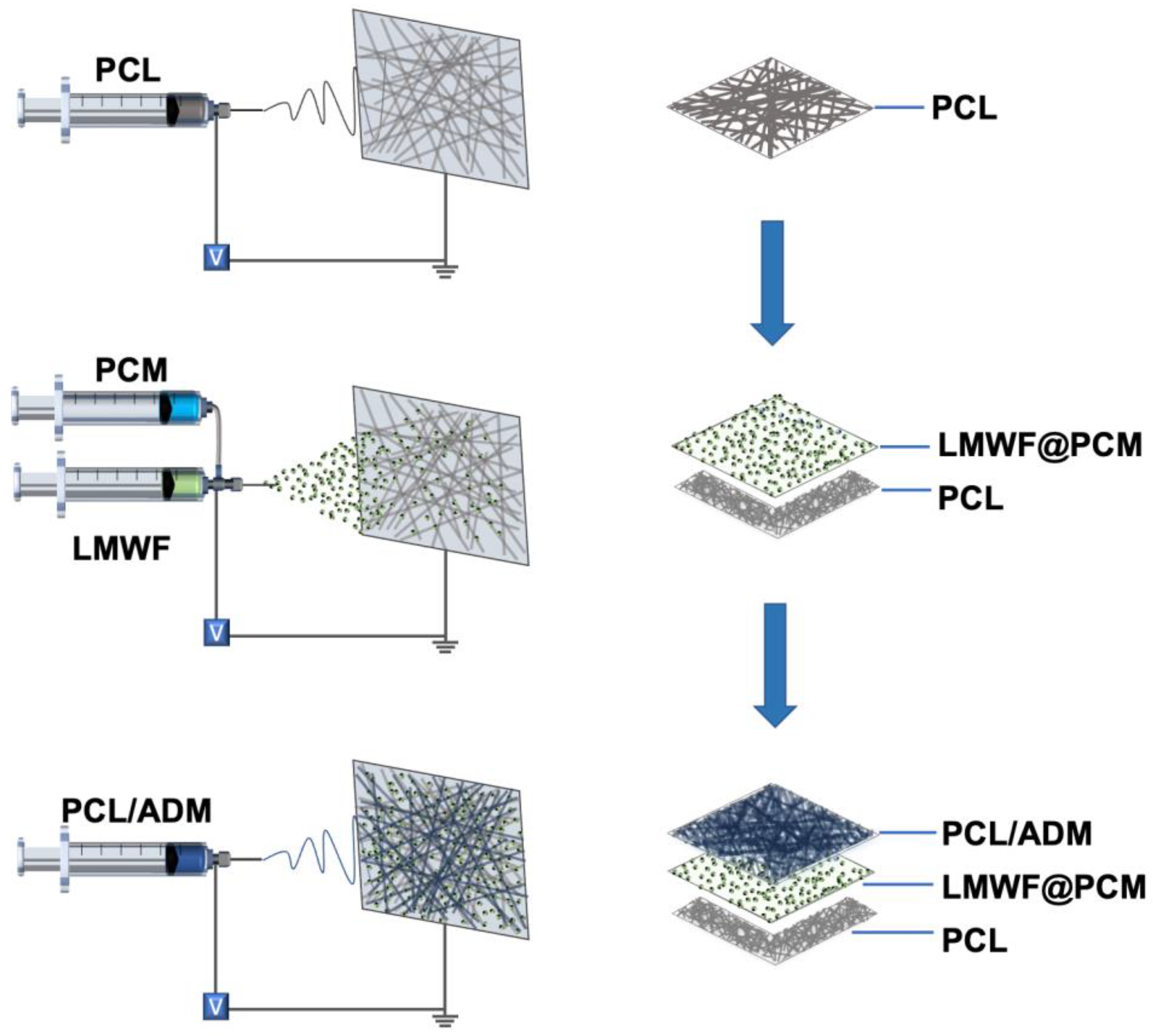

| Syringe Diameter (mL) | Gauge | Voltage (kV) | Flow Rate (mL·h−1) | Time (min) | Distance (cm) | |
|---|---|---|---|---|---|---|
| PCL | 5 | 22 | +15/−3 | 2.0 | 30 | 15 |
| PCL/ADM | 5 | 22 | +20/−3 | 2.0 | 60 | 15 |
| PCM | 5 | 19 | +15/−3 | 3.0 (outer) 1.0 (inner) | 10 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, Y.; Zhang, X.; Liu, N.; Cong, B.; Sun, Y.; Guo, M.; Liu, Z.; Jiang, L.; Wang, W.; et al. On-Demand Release of Fucoidan from a Multilayered Nanofiber Patch for the Killing of Oral Squamous Cancer Cells and Promotion of Epithelial Regeneration. J. Funct. Biomater. 2022, 13, 167. https://doi.org/10.3390/jfb13040167
Liu Y, Xu Y, Zhang X, Liu N, Cong B, Sun Y, Guo M, Liu Z, Jiang L, Wang W, et al. On-Demand Release of Fucoidan from a Multilayered Nanofiber Patch for the Killing of Oral Squamous Cancer Cells and Promotion of Epithelial Regeneration. Journal of Functional Biomaterials. 2022; 13(4):167. https://doi.org/10.3390/jfb13040167
Chicago/Turabian StyleLiu, Yingnan, Yingjie Xu, Xiaopei Zhang, Na Liu, Beibei Cong, Yu Sun, Mingxia Guo, Zeyu Liu, Le Jiang, Wanchun Wang, and et al. 2022. "On-Demand Release of Fucoidan from a Multilayered Nanofiber Patch for the Killing of Oral Squamous Cancer Cells and Promotion of Epithelial Regeneration" Journal of Functional Biomaterials 13, no. 4: 167. https://doi.org/10.3390/jfb13040167
APA StyleLiu, Y., Xu, Y., Zhang, X., Liu, N., Cong, B., Sun, Y., Guo, M., Liu, Z., Jiang, L., Wang, W., Wu, T., & Wang, Y. (2022). On-Demand Release of Fucoidan from a Multilayered Nanofiber Patch for the Killing of Oral Squamous Cancer Cells and Promotion of Epithelial Regeneration. Journal of Functional Biomaterials, 13(4), 167. https://doi.org/10.3390/jfb13040167