Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial
Abstract
1. Introduction
1.1. Children’s Eating Behaviors and Parental Concerns
1.2. Structure around Family Meals
1.3. Communication around Food
1.4. Parental Involvement in Food-Based Interventions
2. Materials and Methods
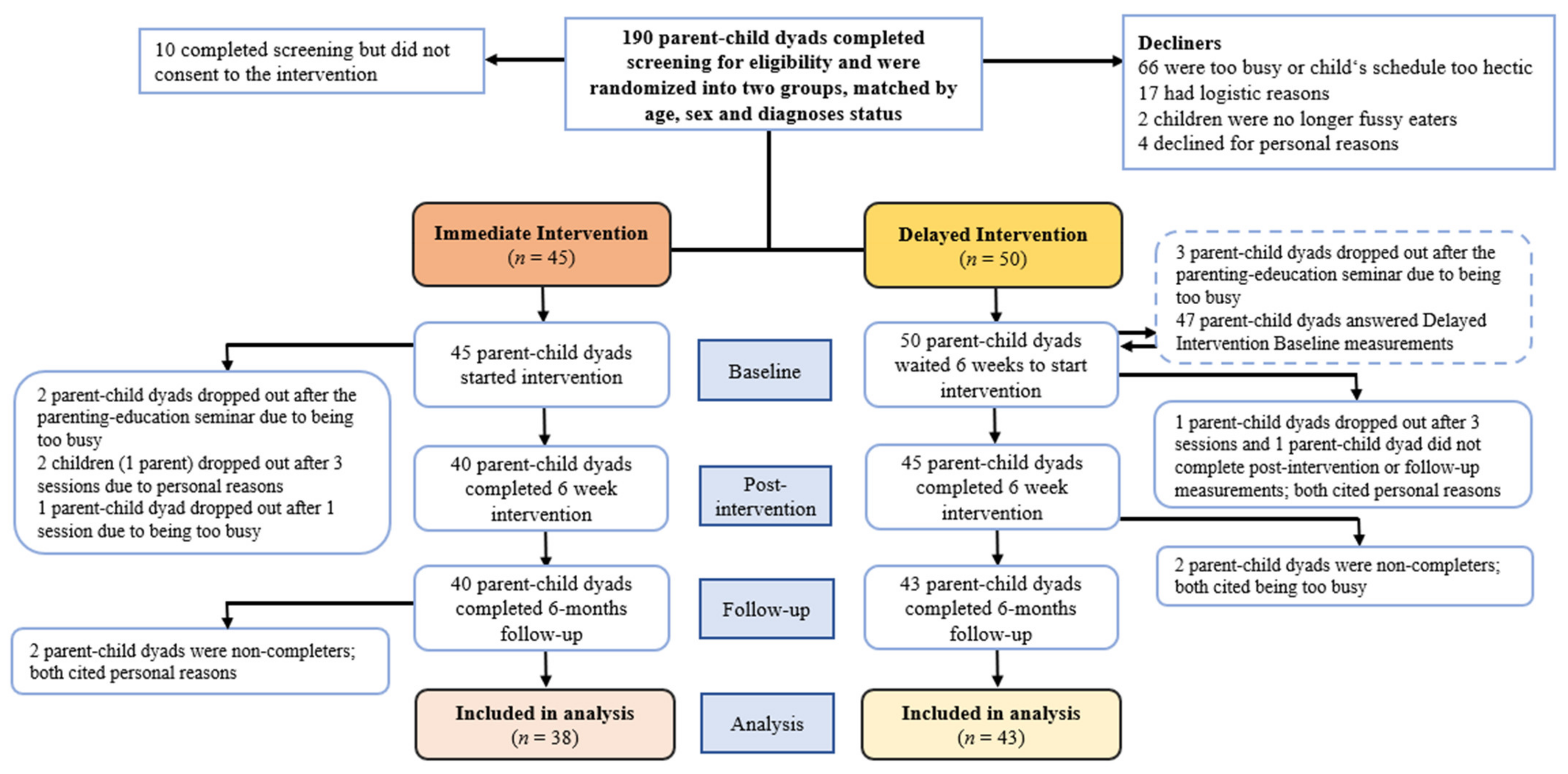
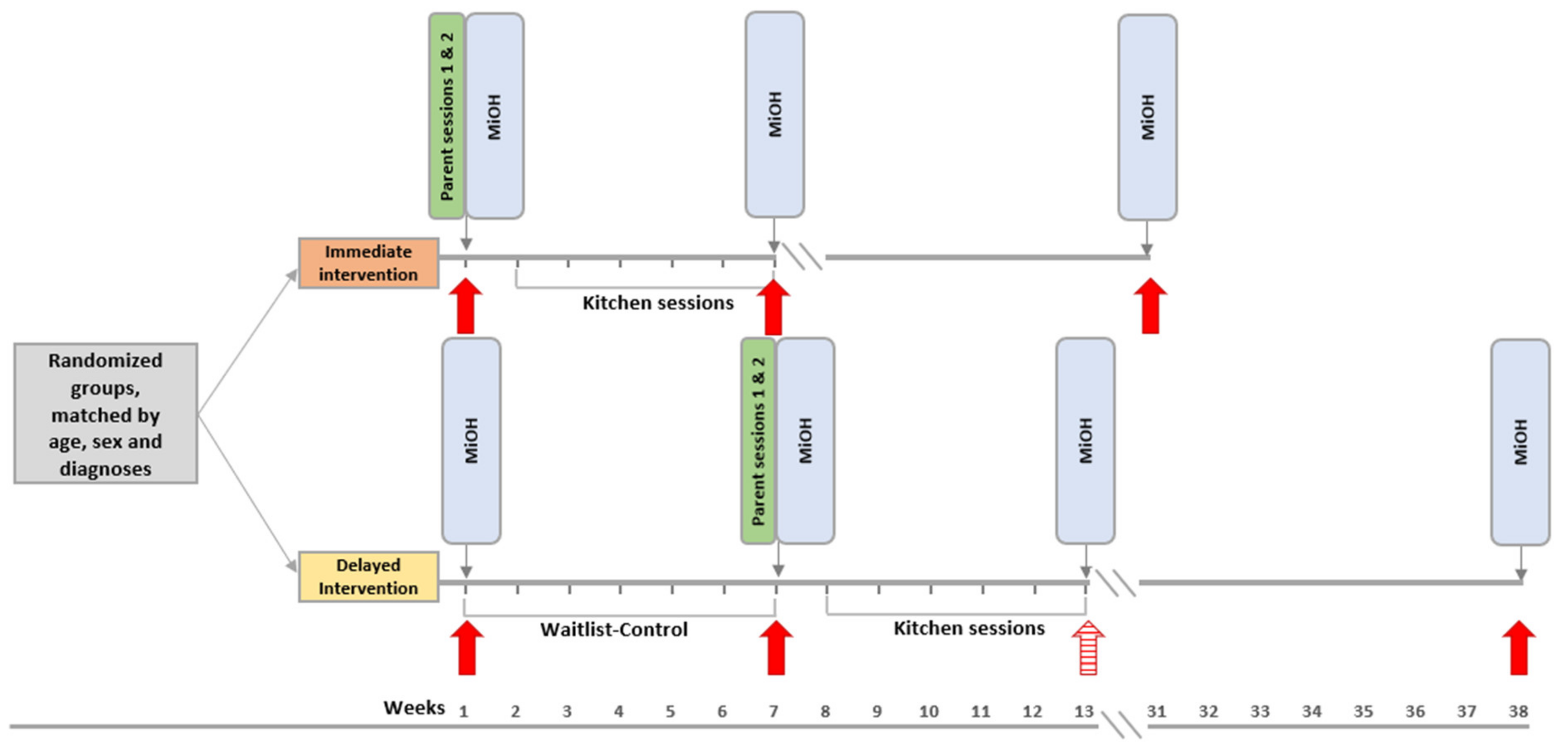
2.1. Study Design and Randomization
2.2. Study Setting
2.3. Measures
2.4. Recruitment
2.5. Participants
2.6. Procedures
2.7. The Taste Education Parenting-Education Sessions
2.8. The Taste Education Sessions
Instructions for Trainers
2.9. Statistical Analyses
3. Results
3.1. Sample Characteristics
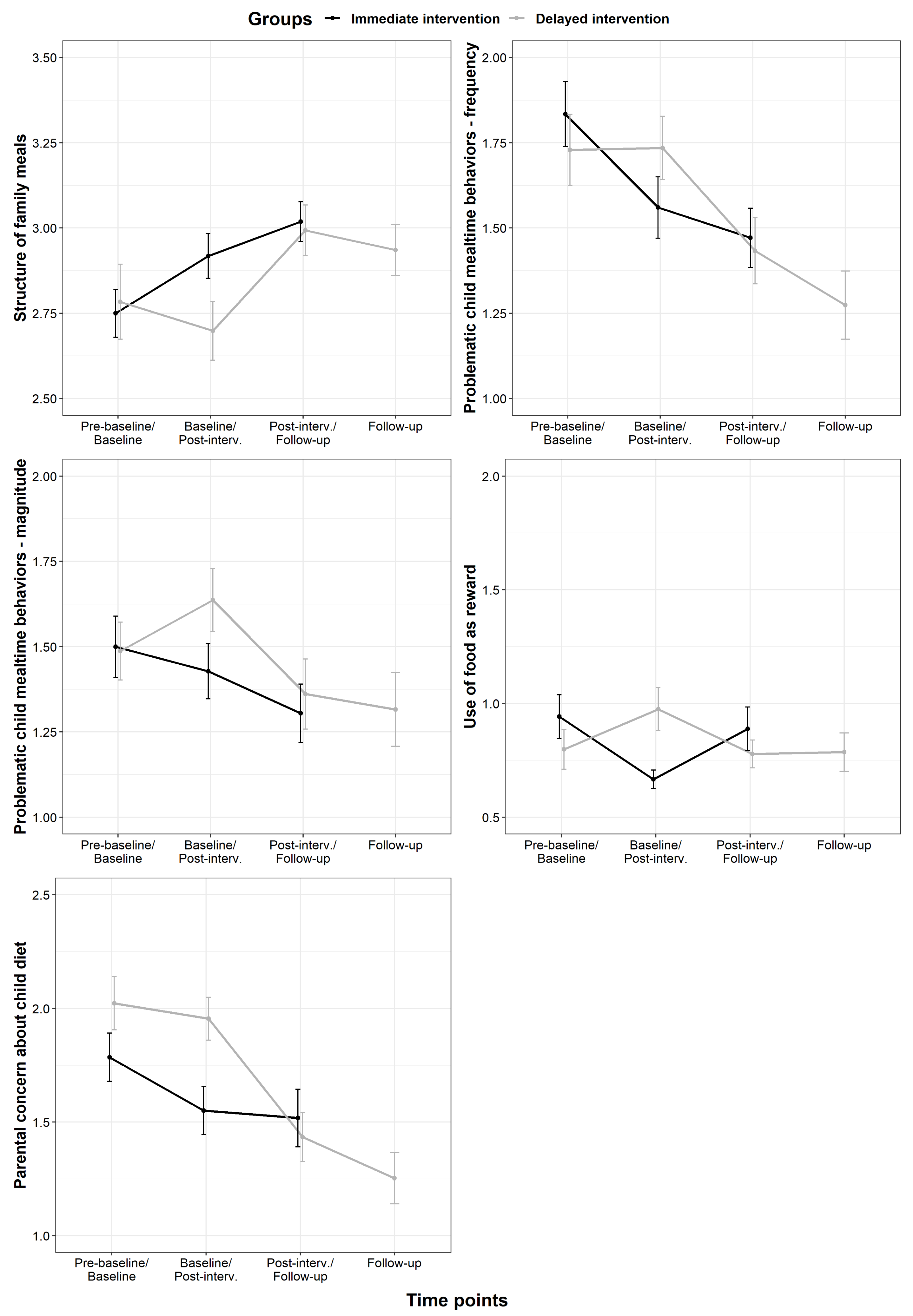
3.2. MiOH Scores
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeCosta, P.; Møller, P.; Frøst, M.B.; Olsen, A. Changing children’s eating behavior—A review of experimental research. Appetite 2017, 113, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A. Reflections on current practice for taste learning in children. Int. J. Gastron. Food Sci. 2019, 15, 26–29. [Google Scholar] [CrossRef]
- Dovey, T.M.; Kumari, V.; Blissett, J. Eating behaviour, behavioural problems and sensory profiles of children with avoidant/restrictive food intake disorder (ARFID), autistic spectrum disorders or picky eating: Same or different? Eur. Psychiatry 2019, 61, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dovey, T.M.; Staples, P.A.; Gibson, E.L.; Halford, J.C. Food neophobia and ‘picky/fussy’ eating in children: A review. Appetite 2008, 50, 181–193. [Google Scholar] [CrossRef]
- Lim, P.S.; Balistreri, K.A.; Silverman, A.H.; Davies, W.H. Disrupted mealtime interactions are associated with stress and internalizing symptoms in caregivers of school-age children. Child. Health Care 2021, 50, 432–451. [Google Scholar] [CrossRef]
- Taylor, C.M.; Wernimont, S.M.; Northstone, K.; Emmett, P.M. Picky/fussy eating in children: Review of definitions, assessment, prevalence and dietary intakes. Appetite 2015, 95, 349–359. [Google Scholar] [CrossRef]
- Lafraire, J.; Rioux, C.; Giboreau, A.; Picard, D. Food rejections in children: Cognitive and social/environmental factors involved in food neophobia and picky/fussy eating behavior. Appetite 2016, 96, 347–357. [Google Scholar] [CrossRef]
- Antoniou, E.E.; Roefs, A.; Kremers, S.P.J.; Jansen, A.; Gubbels, J.S.; Sleddens, E.F.C.; Thijs, C. Picky eating and child weight status development: A longitudinal study. J. Hum. Nutr. Diet. 2016, 29, 298–307. [Google Scholar] [CrossRef]
- Barnhill, K.; Gutierrez, A.; Ghossainy, M.; Marediya, Z.; Devlin, M.; Sachdev, P.; Marti, C.N.; Hewitson, L. Dietary status and nutrient intake of children with autism spectrum disorder: A case-control study. Res. Autism Spectr. Disord. 2018, 50, 51–59. [Google Scholar] [CrossRef]
- Güngör, S.; Celiloğlu, S.; Raif, S.G.; Özcan, Ö.Ö.; Selimoğlu, M.A. Malnutrition and Obesity in Children with ADHD. J. Atten. Disord. 2016, 20, 647–652. [Google Scholar] [CrossRef]
- Scaglioni, S.; Arrizza, C.; Vecchi, F.; Tedeschi, S. Determinants of children’s eating behavior. Am. J. Clin. Nutr. 2011, 94, 2006S–2011S. [Google Scholar] [CrossRef]
- Boswell, N.; Byrne, R.; Davies, P.S. Eating behavior traits associated with demographic variables and implications for obesity outcomes in early childhood. Appetite 2018, 120, 482–490. [Google Scholar] [CrossRef]
- Carnell, S.; Wardle, J. Appetite and adiposity in children: Evidence for a behavioral susceptibility theory of obesity. Am. J. Clin. Nutr. 2008, 88, 22–29. [Google Scholar] [CrossRef]
- Demir, D.; Bektas, M. The effect of childrens’ eating behaviors and parental feeding style on childhood obesity. Eat. Behav. 2017, 26, 137–142. [Google Scholar] [CrossRef]
- Fulkerson, J.A.; Telke, S.; Larson, N.; Berge, J.; Sherwood, N.E.; Neumark-Sztainer, D. A healthful home food environment: Is it possible amidst household chaos and parental stress? Appetite 2019, 142, 104391. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.L.; Farrow, C.; Haycraft, E.; Meyer, C. Parental influences on children’s eating behaviour and characteristics of successful parent-focussed interventions. Appetite 2013, 60, 85–94. [Google Scholar] [CrossRef]
- White, H.J.; Meyer, C.; Palfreyman, Z.; Haycraft, E. Family mealtime emotions and food parenting practices among mothers of young children: Development of the Mealtime Emotions Measure for Parents (MEM-P). Matern. Child Nutr. 2022, 18, e13346. [Google Scholar] [CrossRef]
- Adamson, M.; Morawska, A.; Sanders, M.R. Childhood Feeding Difficulties: A Randomized Controlled Trial of a Group-Based Parenting Intervention. J. Dev. Behav. Pediatrics 2013, 34, 293–302. [Google Scholar] [CrossRef]
- Schreck, K.A.; Williams, K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res. Dev. Disabil. 2006, 27, 353–363. [Google Scholar] [CrossRef]
- Cole, N.C.; An, R.; Lee, S.-Y.; Donovan, S.M. Correlates of picky eating and food neophobia in young children: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 516–532. [Google Scholar] [CrossRef]
- Anderson, S.E.; Must, A.; Curtin, C.; Bandini, L.G. Meals in Our Household: Reliability and Initial Validation of a Questionnaire to Assess Child Mealtime Behaviors and Family Mealtime Environments. J. Acad. Nutr. Diet. 2012, 112, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.; Hubbard, K.L.; Anderson, S.E.; Mick, E.O.; Must, A.; Bandini, L.G. Food Selectivity, Mealtime Behavior Problems, Spousal Stress, and Family Food Choices in Children with and without Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Neumark-Sztainer, D.; Larson, N.I.; Fulkerson, J.A.; Eisenberg, M.E.; Story, M. Family meals and adolescents: What have we learned from Project EAT (Eating Among Teens)? Public Health Nutr. 2010, 13, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Boswell, N.; Byrne, R.; Davies, P.S.W. An examination of children’s eating behaviours as mediators of the relationship between parents’ feeding practices and early childhood body mass index z-scores. Obes. Sci. Pract. 2019, 5, 168–176. [Google Scholar] [CrossRef]
- Daniels, L.A. Feeding Practices and Parenting: A Pathway to Child Health and Family Happiness. Ann. Nutr. Metab. 2019, 74, 29–42. [Google Scholar] [CrossRef]
- Nadon, G.; Feldman, D.E.; Dunn, W.; Gisel, E. Mealtime problems in children with Autism Spectrum Disorder and their typically developing siblings: A comparison study. Autism 2010, 15, 98–113. [Google Scholar] [CrossRef]
- Hubbard, K.L.; Anderson, S.E.; Curtin, C.; Must, A.; Bandini, L.G. A Comparison of Food Refusal Related to Characteristics of Food in Children with Autism Spectrum Disorder and Typically Developing Children. J. Acad. Nutr. Diet. 2014, 114, 1981–1987. [Google Scholar] [CrossRef]
- Hutchison, L.; Feder, M.; Abar, B.; Winsler, A. Relations between Parenting Stress, Parenting Style, and Child Executive Functioning for Children with ADHD or Autism. J. Child Fam. Stud. 2016, 25, 3644–3656. [Google Scholar] [CrossRef]
- Margari, L.; Marzulli, L.; Gabellone, A.; de Giambattista, C. Eating and Mealtime Behaviors in Patients with Autism Spectrum Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 2083–2102. [Google Scholar] [CrossRef]
- APA (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Ellis, J.M.; Galloway, A.T.; Webb, R.M.; Martz, D.M.; Farrow, C.V. Recollections of pressure to eat during childhood, but not picky eating, predict young adult eating behavior. Appetite 2016, 97, 58–63. [Google Scholar] [CrossRef]
- Galloway, A.T.; Fiorito, L.; Lee, Y.; Birch, L.L. Parental pressure, dietary patterns, and weight status among girls who are “picky eaters”. J. Am. Diet. Assoc. 2005, 105, 541–548. [Google Scholar] [CrossRef]
- Houldcroft, L.; Farrow, C.; Haycraft, E. Perceptions of parental pressure to eat and eating behaviours in preadolescents: The mediating role of anxiety. Appetite 2014, 80, 61–69. [Google Scholar] [CrossRef]
- Jansen, P.W.; de Barse, L.M.; Jaddoe, V.W.; Verhulst, F.C.; Franco, O.H.; Tiemeier, H. Bi-directional associations between child fussy eating and parents’ pressure to eat: Who influences whom? Physiol. Behav. 2017, 176, 101–106. [Google Scholar] [CrossRef]
- Taylor, C.M.; Emmett, P.M. Picky eating in children: Causes and consequences. Proc. Nutr. Soc. 2019, 78, 161–169. [Google Scholar] [CrossRef]
- Galloway, A.T.; Fiorito, L.M.; Francis, L.A.; Birch, L.L. ‘Finish your soup’: Counterproductive effects of pressuring children to eat on intake and affect. Appetite 2006, 46, 318–323. [Google Scholar] [CrossRef]
- Ledford, J.R.; Whiteside, E.; Severini, K.E. A systematic review of interventions for feeding-related behaviors for individuals with autism spectrum disorders. Res. Autism Spectr. Disord. 2018, 52, 69–80. [Google Scholar] [CrossRef]
- Marshall, J.V.; Ware, R.; Ziviani, J.M.; Hill, R.; Dodrill, P.M.M. Efficacy of interventions to improve feeding difficulties in children with autism spectrum disorders: A systematic review and meta-analysis. Child Care Health Dev. 2015, 41, 278–302. [Google Scholar] [CrossRef]
- Klintwall, L.; Holm, A.; Eriksson, M.; Carlsson, L.H.; Olsson, M.B.; Hedvall, Å.; Gillberg, C.; Fernell, E. Sensory abnormalities in autism: A brief report. Res. Dev. Disabil. 2011, 32, 795–800. [Google Scholar] [CrossRef]
- Crowe, T.K.; Freeze, B.; Provost, E.; King, L.; Sanders, M. Maternal perceptions of nutrition, stress, time, and assistance during mealtimes: Similarities and differences between mothers of children with autism spectrum disorders and mothers of children with typical development. J. Occup. Ther. Sch. Early Interv. 2016, 9, 242–257. [Google Scholar] [CrossRef]
- Johnson, C.R.; Brown, K.; Hyman, S.L.; Brooks, M.M.; Aponte, C.; Levato, L.; Schmidt, B.; Evans, V.; Huo, Z.; Bendixen, R.; et al. Parent Training for Feeding Problems in Children with Autism Spectrum Disorder: Initial Randomized Trial. J. Pediatric Psychol. 2019, 44, 164–175. [Google Scholar] [CrossRef]
- Murphy, J.; Zlomke, K.; VanOrmer, J.; Swingle, H. Impact of Disruptive Behavior in Childhood Feeding Difficulties. J. Clin. Psychol. Med Settings 2020, 27, 406–415. [Google Scholar] [CrossRef]
- Bowling, A.; Davison, K.; Haneuse, S.; Beardslee, W.; Miller, D.P. ADHD Medication, Dietary Patterns, Physical Activity, and BMI in Children: A Longitudinal Analysis of the ECLS-K Study. Obesity 2017, 25, 1802–1808. [Google Scholar] [CrossRef]
- Fries, L.R.; Martin, N.; van der Horst, K. Parent-child mealtime interactions associated with toddlers’ refusals of novel and familiar foods. Physiol. Behav. 2017, 176, 93–100. [Google Scholar] [CrossRef]
- Zohar, A.H.; Pick, S.; Lev-Ari, L.; Bachner-Melman, R. A longitudinal study of maternal feeding and children’s picky eating. Appetite 2020, 154, 104804. [Google Scholar] [CrossRef]
- Devine, C.M.; Farrell, T.J.; Blake, C.E.; Jastran, M.; Wethington, E.; Bisogni, C.A. Work Conditions and the Food Choice Coping Strategies of Employed Parents. J. Nutr. Educ. Behav. 2009, 41, 365–370. [Google Scholar] [CrossRef]
- Berge, J.M.; Tate, A.; Trofholz, A.; Fertig, A.; Crow, S.; Neumark-Sztainer, D.; Miner, M. Examining within- and across-day relationships between transient and chronic stress and parent food-related parenting practices in a racially/ethnically diverse and immigrant population. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 7. [Google Scholar] [CrossRef]
- Rockett, H.R.H. Family Dinner: More than Just a Meal. J. Am. Diet. Assoc. 2007, 107, 1498–1501. [Google Scholar] [CrossRef]
- Jones, B.L. Making time for family meals: Parental influences, home eating environments, barriers and protective factors. Physiol. Behav. 2018, 193, 248–251. [Google Scholar] [CrossRef]
- Hammons, A.J.; Fiese, B.H. Is Frequency of Shared Family Meals Related to the Nutritional Health of Children and Adolescents? Pediatrics 2011, 127, e1565–e1574. [Google Scholar] [CrossRef]
- Berge, J.M.; Wall, M.; Hsueh, T.-F.; Fulkerson, J.A.; Larson, N.; Neumark-Sztainer, D. The Protective Role of Family Meals for Youth Obesity: 10-Year Longitudinal Associations. J. Pediatrics 2014, 166, 296–301. [Google Scholar] [CrossRef]
- Eisenberg, M.E.; Olson, R.E.; Neumark-Sztainer, D.; Story, M.; Bearinger, L.H. Correlations Between Family Meals and Psychosocial Well-being Among Adolescents. Arch. Pediatrics Adolesc. Med. 2004, 158, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Sen, B. The relationship between frequency of family dinner and adolescent problem behaviors after adjusting for other family characteristics. J. Adolesc. 2010, 33, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Franko, D.L.; Thompson, D.; Affenito, S.G.; Barton, B.A.; Striegel-Moore, R.H. What mediates the relationship between family meals and adolescent health issues. Health Psychol. 2008, 27, S109–S117. [Google Scholar] [CrossRef]
- Searle, B.-R.E.; Harris, H.A.; Thorpe, K.; Jansen, E. What children bring to the table: The association of temperament and child fussy eating with maternal and paternal mealtime structure. Appetite 2020, 151, 104680. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Marx, J.M.; Musher-Eizenman, D.R. Using food as a reward: An examination of parental reward practices. Appetite 2018, 120, 318–326. [Google Scholar] [CrossRef]
- Fedewa, A.L.; Davis, M.C. How Food as a Reward Is Detrimental to Children’s Health, Learning, and Behavior. J. Sch. Health 2015, 85, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Holub, S.C. Children’s self-regulation in eating: Associations with inhibitory control and parents’ feeding behavior. J. Pediatric Psychol. 2011, 36, 340–345. [Google Scholar] [CrossRef]
- Jansen, P.W.; Derks, I.P.; Mou, Y.; van Rijen, E.H.; Gaillard, R.; Micali, N.; Voortman, T.; Hillegers, M.H. Associations of parents’ use of food as reward with children’s eating behaviour and BMI in a population-based cohort. Pediatric Obes. 2020, 15, e12662. [Google Scholar]
- Farrow, C.V.; Coulthard, H. Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite 2012, 58, 842–846. [Google Scholar] [CrossRef]
- Kerr-Gaffney, J.; Harrison, A.; Tchanturia, K. Social anxiety in the eating disorders: A systematic review and meta-analysis. Psychol. Med. 2018, 48, 2477–2491. [Google Scholar] [CrossRef]
- Sege, C.T.; Bradley, M.M.; Lang, P.J. Avoidance and escape: Defensive reactivity and trait anxiety. Behav. Res. Ther. 2018, 104, 62–68. [Google Scholar] [CrossRef]
- Pace, U.; D’Urso, G.; Zappulla, C. Negative eating attitudes and behaviors among adolescents: The role of parental control and perceived peer support. Appetite 2018, 121, 77–82. [Google Scholar] [CrossRef]
- Batsell, W.R., Jr.; Brown, A.S.; Ansfield, M.E.; Paschall, G.Y. “You Will Eat All of That!”: A retrospective analysis of forced consumption episodes. Appetite 2002, 38, 211–219. [Google Scholar] [CrossRef]
- Polfuss, M.; Simpson, P.; Greenley, R.N.; Zhang, L.; Sawin, K.J. Parental Feeding Behaviors and Weight-Related Concerns in Children with Special Needs. West. J. Nurs. Res. 2017, 39, 1070–1093. [Google Scholar] [CrossRef]
- Beckerman, J.P.; Alike, Q.; Lovin, E.; Tamez, M.; Mattei, J. The Development and Public Health Implications of Food Preferences in Children. Front. Nutr. 2017, 4, 66. [Google Scholar] [CrossRef]
- Sharp, W.G.; Burrell, T.L.; Jaquess, D.L. The Autism MEAL Plan: A parent-training curriculum to manage eating aversions and low intake among children with autism. Autism 2014, 18, 712–722. [Google Scholar] [CrossRef]
- Gunnarsdottir, T.; Sigurdardottir, Z.G.; Njardvik, U.; Olafsdottir, A.S.; Bjarnason, R. A randomized-controlled pilot study of Epstein’s family-based behavioural treatment for childhood obesity in a clinical setting in Iceland. Nord. Psychol. 2011, 63, 6–19. [Google Scholar] [CrossRef]
- Gunnarsdottir, T.; Njardvik, U.; Olafsdottir, A.S.; Craighead, L.; Bjarnason, R. Childhood obesity and co-morbid problems: Effects of Epstein’s family-based behavioural treatment in an Icelandic sample. J. Eval. Clin. Pract. 2012, 18, 465–472. [Google Scholar] [CrossRef]
- Harris, H.; Jansen, E.; Mallan, K.M.; Daniels, L.; Thorpe, K. Do Dads Make a Difference? Family Feeding Dynamics and Child Fussy Eating. J. Dev. Behav. Pediatrics 2018, 39, 415–423. [Google Scholar] [CrossRef]
- Holley, C.E.; Haycraft, E.; Farrow, C. ‘Why don’t you try it again?’ A comparison of parent led, home based interventions aimed at increasing children’s consumption of a disliked vegetable. Appetite 2015, 87, 215–222. [Google Scholar] [CrossRef]
- Wardle, J.; Cooke, L.J.; Gibson, E.L.; Sapochnik, M.; Sheiham, A.; Lawson, M. Increasing children’s acceptance of vegetables; a randomized trial of parent-led exposure. Appetite 2003, 40, 155–162. [Google Scholar] [CrossRef]
- Fildes, A.; van Jaarsveld, C.H.; Wardle, J.; Cooke, L. Parent-Administered Exposure to Increase Children’s Vegetable Acceptance: A Randomized Controlled Trial. J. Acad. Nutr. Diet. 2014, 114, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, P.; Aggarwal, A.; Drewnowski, A. Time Spent on Home Food Preparation and Indicators of Healthy Eating. Am. J. Prev. Med. 2014, 47, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Holley, C.E.; Farrow, C.V.; Haycraft, E. A Systematic Review of Methods for Increasing Vegetable Consumption in Early Childhood. Curr. Nutr. Rep. 2017, 6, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.V.; Husby, S.; Skov, L.R.; Perez-Cueto, F.J.A. A systematic review of types of healthy eating interventions in preschools. Nutr. J. 2014, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Eto, K.; Miyoshi, M.; Yokoyama, T.; Haraikawa, M.; Yoshiike, N. Parent–child cooking meal together may relate to parental concerns about the diets of their toddlers and preschoolers: A cross-sectional analysis in Japan. Nutr. J. 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Balantekin, K.N.; Anzman-Frasca, S.; Francis, L.A.; Ventura, A.K.; Fisher, J.O.; Johnson, S.L. Positive parenting approaches and their association with child eating and weight: A narrative review from infancy to adolescence. Pediatric Obes. 2020, 15, e12722. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Burrell, T.L.; Berry, R.C.; Stubbs, K.H.; McCracken, C.E.; Gillespie, S.E.; Scahill, L. The Autism Managing Eating Aversions and Limited Variety Plan vs Parent Education: A Randomized Clinical Trial. J. Pediatrics 2019, 211, 185–192. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Olsen, A.; Olafsdottir, A. Fussy Eating among Children and Their Parents: Associations in Parent-Child Dyads, in a Sample of Children with and without Neurodevelopmental Disorders. Nutrients 2021, 13, 2196. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Haraldsson, H.; Olafsdottir, A.S. Taste education—A food-based intervention in a school setting, focusing on children with and without neurodevelopmental disorders and their families. A randomized controlled trial. Appetite 2021, 167, 105623. [Google Scholar] [CrossRef]
- Bandini, L.G.; Anderson, S.E.; Curtin, C.; Cermak, S.; Evans, E.W.; Scampini, R.; Maslin, M.; Must, A. Food Selectivity in Children with Autism Spectrum Disorders and Typically Developing Children. J. Pediatrics 2010, 157, 259–264. [Google Scholar] [CrossRef]
- Steingrímsdóttir, L.; Thorkelsson, G.; Eythórsdóttir, E. Chapter 6—Food, Nutrition, and Health in Iceland. In Nutritional and Health Aspects of Food in Nordic Countries; Andersen, V., Bar, E., Wirtanen, G., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–177. [Google Scholar]
- Blissett, J.; Bennett, C.; Fogel, A.; Harris, G.; Higgs, S. Parental modelling and prompting effects on acceptance of a novel fruit in 2–4-year-old children are dependent on children’s food responsiveness. Br. J. Nutr. 2015, 115, 554–564. [Google Scholar] [CrossRef]
- R CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- O’Connell, N.S.; Dai, L.; Jiang, Y.; Speiser, J.L.; Ward, R.; Wei, W.; Carroll, R.; Gebregziabher, M. Methods for Analysis of Pre-Post Data in Clinical Research: A Comparison of Five Common Methods. J. Biom. Biostat. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Koller, M. robustlmm: An R Package for Robust Estimation of Linear Mixed-Effects Models. J. Stat. Softw. 2016, 75, 1–24. [Google Scholar] [CrossRef]
- Forehand, R.; Jones, D.J.; Parent, J. Behavioral parenting interventions for child disruptive behaviors and anxiety: What’s different and what’s the same. Clin. Psychol. Rev. 2013, 33, 133–145. [Google Scholar] [CrossRef]
- Daley, D.; Van Der Oord, S.; Ferrin, M.; Cortese, S.; Danckaerts, M.; Doepfner, M.; Van Den Hoofdakker, B.J.; Coghill, D.; Thompson, M.; Asherson, P.; et al. Practitioner Review: Current best practice in the use of parent training and other behavioural interventions in the treatment of children and adolescents with attention deficit hyperactivity disorder. J. Child Psychol. Psychiatry 2018, 59, 932–947. [Google Scholar] [CrossRef]
- Mata, J.; Scheibehenne, B.; Todd, M.P. Predictin children’s meal preferences: How much do parents know? Appetite 2008, 50, 367–375. [Google Scholar] [CrossRef]
- Råstam, M.; Täljemark, J.; Tajnia, A.; Lundström, S.; Gustafsson, P.; Lichtenstein, P.; Gillberg, C.; Anckarsäter, H.; Kerekes, N. Eating Problems and Overlap with ADHD and Autism Spectrum Disorders in a Nationwide Twin Study of 9- and 12-Year-Old Children. Sci. World J. 2013, 2013, 315429. [Google Scholar] [CrossRef]
- Smith, B.; Rogers, S.L.; Blissett, J.; Ludlow, A.K. The relationship between sensory sensitivity, food fussiness and food preferences in children with neurodevelopmental disorders. Appetite 2020, 150, 104643. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Olafsdottir, A.S.; Brynjolfsdottir, B.; Bjarnason, R.; Njardvik, U. Odds of fussy eating are greater among children with obesity and anxiety. Obes. Sci. Pract. 2021, 8, 91–100. [Google Scholar] [CrossRef]
- Bandini, L.G.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 439–446. [Google Scholar] [CrossRef] [PubMed]

| Overall n = 81 | Immediate Intervention n = 38 | Delayed Intervention n = 43 | |
|---|---|---|---|
| Child | |||
| Children’s age in years, Mean (SD) | 10.4 (1.43) | 10.4 (1.23) | 10.4 (1.47) |
| Female, n (%) | 34 (41.9) | 18 (47.4) | 16 (37.2) |
| Diagnoses, n (%) | |||
| ND (ADHD, Autism, or both) | 31 (38.2) | 16 (42.1) | 15 (34.9) |
| ADHD, primarily | 12 (14.8) | 5 (13.2) | 7 (16.3) |
| Autism, primarily | 7 (8.6) | 4 (10.5) | 3 (7.0) |
| Anxiety | 12 (14.8) | 4 (10.5) | 8 (18.6) |
| Other | 18 (22.2) | 11 (28.9) | 10 (23.2) |
| No diagnoses | 39 (48.1) | 17 (44.7) | 22 (51.2) |
| Overall ‡n = 77 | Immediate Intervention n = 37 | Delayed Intervention n = 40 | |
| Parent, n (%) | |||
| Mother | 71 (92.2) | 35 (94.6) | 36 (90.0) |
| Education, n (%) | |||
| No higher education | 3 (3.9) | 1 (2.7) | 2 (5.0) |
| Vocational education | 13 (16.9) | 8 (21.6) | 5 (12.5) |
| University level | 61 (79.2) | 28 (75.7) | 33 (82.5) |
| Single parent household, n (%) | 10 (13.0) | 6 (16.2) | 4 (10.0) |
| Occupational status, n (%) | |||
| Full-time occupation | 58 (75.3) | 29 (78.4) | 29 (72.5) |
| Part-time occupation | 10 (13.0) | 4 (10.8) | 6 (15.0) |
| Student | 9 (11.7) | 4 (10.8) | 5 (12.5) |
| Other | 8 (10.4) | 2 (5.4) | 6 (15.0) |
| Children in household, n (%) | |||
| 1 | 9 (11.7) | 7 (18.9) | 2 (5.0) |
| 2 | 34 (44.1) | 16 (43.2) | 18 (45.0) |
| 3 | 26 (33.8) | 10 (27.0) | 16 (40.0) |
| 4 or more | 8 (10.4) | 4 (10.8) | 4 (10.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Olafsdottir, A.S. Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients 2022, 14, 4000. https://doi.org/10.3390/nu14194000
Thorsteinsdottir S, Njardvik U, Bjarnason R, Olafsdottir AS. Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients. 2022; 14(19):4000. https://doi.org/10.3390/nu14194000
Chicago/Turabian StyleThorsteinsdottir, Sigrun, Urdur Njardvik, Ragnar Bjarnason, and Anna S. Olafsdottir. 2022. "Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial" Nutrients 14, no. 19: 4000. https://doi.org/10.3390/nu14194000
APA StyleThorsteinsdottir, S., Njardvik, U., Bjarnason, R., & Olafsdottir, A. S. (2022). Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients, 14(19), 4000. https://doi.org/10.3390/nu14194000





