Design of a Composite Based on Polyamide Fabric-Hydrogel-Zinc Oxide Particles to Act as Adsorbent and Photocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
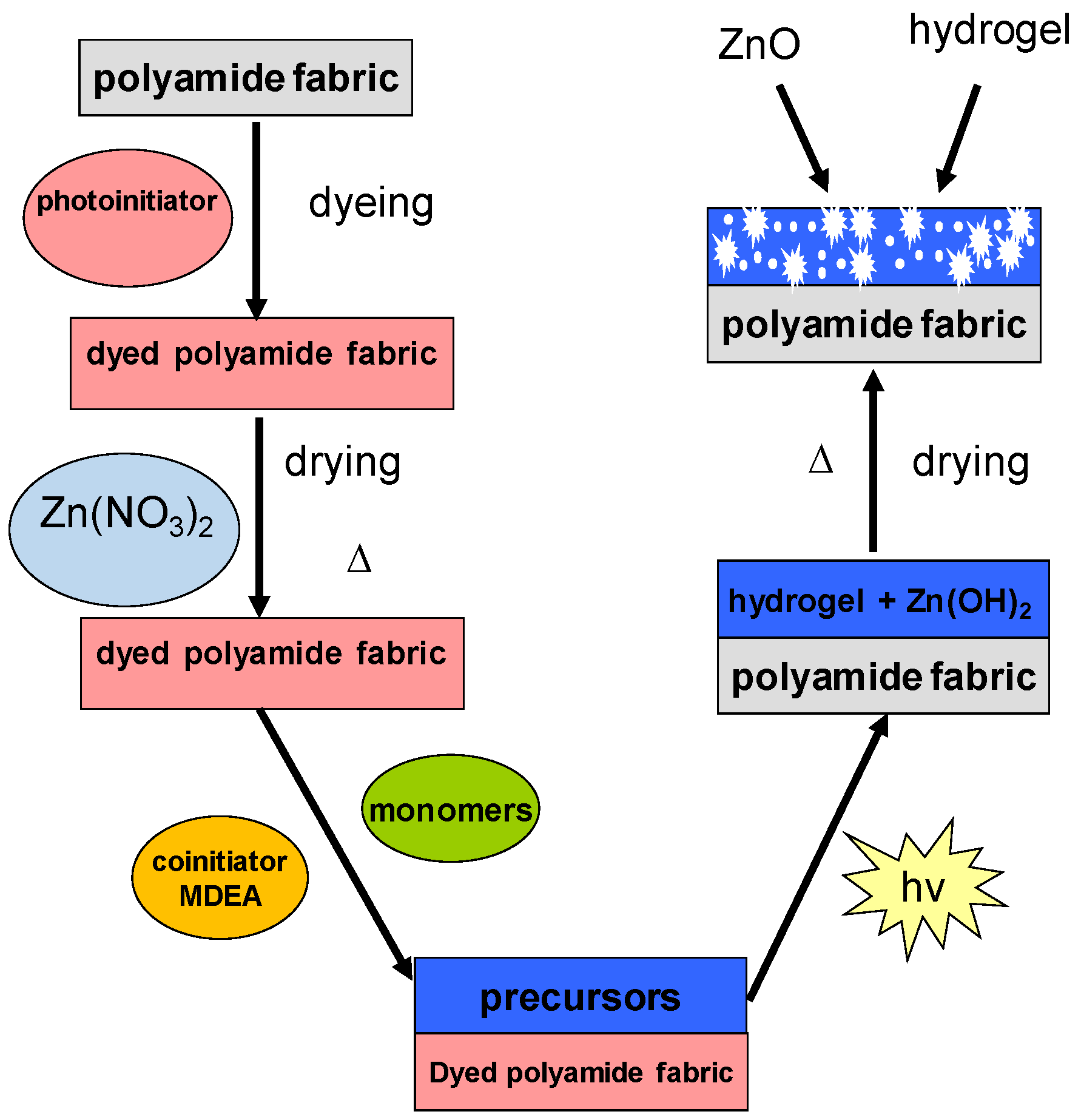
2.2. Preparation of Composite Materials ZnO Nanoparticles-Hydrogel-Polyamide Fabric
2.3. Characterization of the Prepared Composite Materials
2.3.1. Gravimetric Analysis
2.3.2. Structural Characterization
2.4. Study of the Obtained Material as an Adsorbent and Photocatalyst in the Decolorization of Solutions of Reactive Dye Drimaren Rot K-7B
2.4.1. Adsorption Experiment
2.4.2. Photocatalytic Testing of Samples
2.5. Antibacterial Assay of the Composite Material PA30
3. Results and Discussion
3.1. Gravimetric Analysis
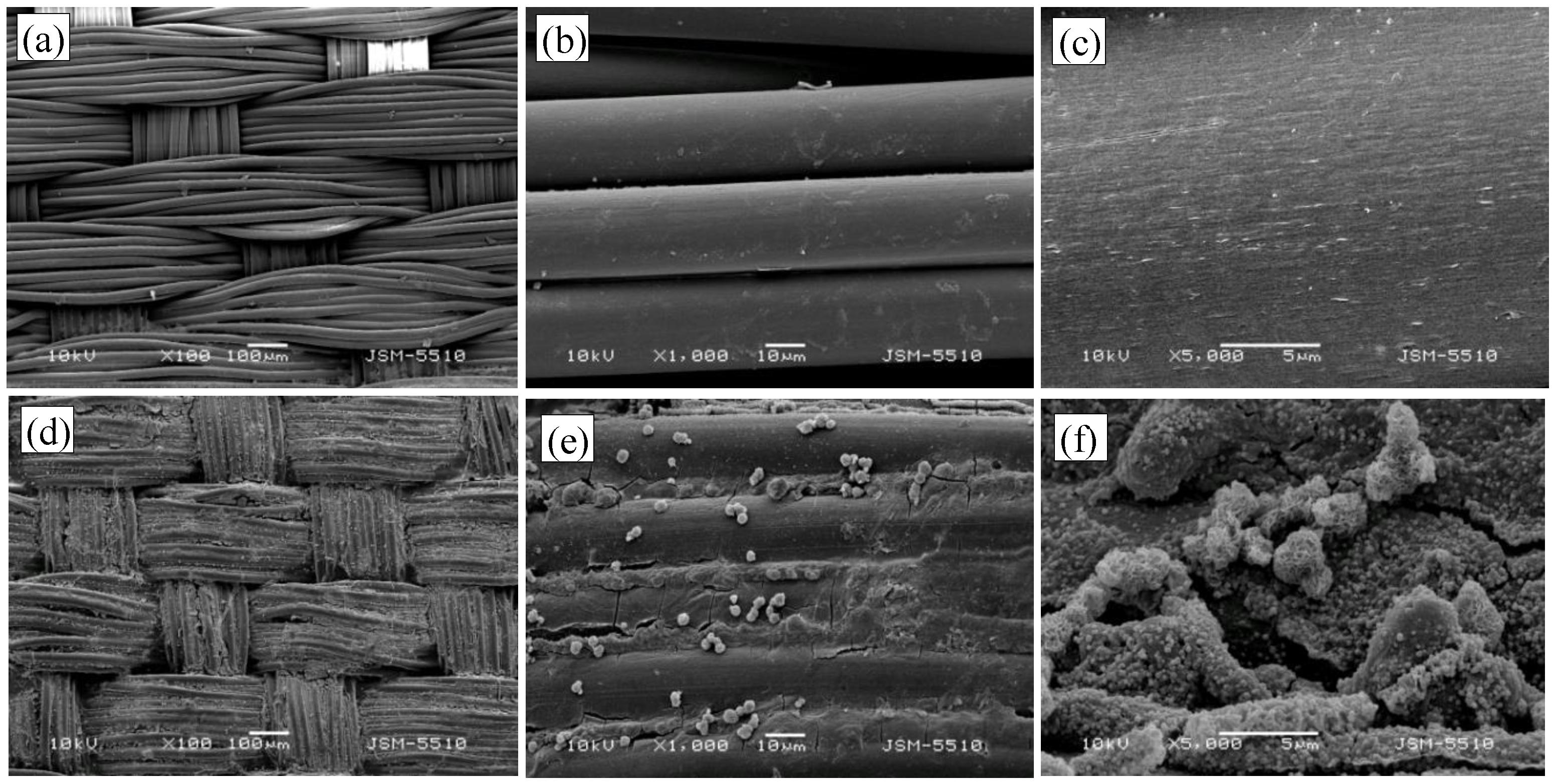
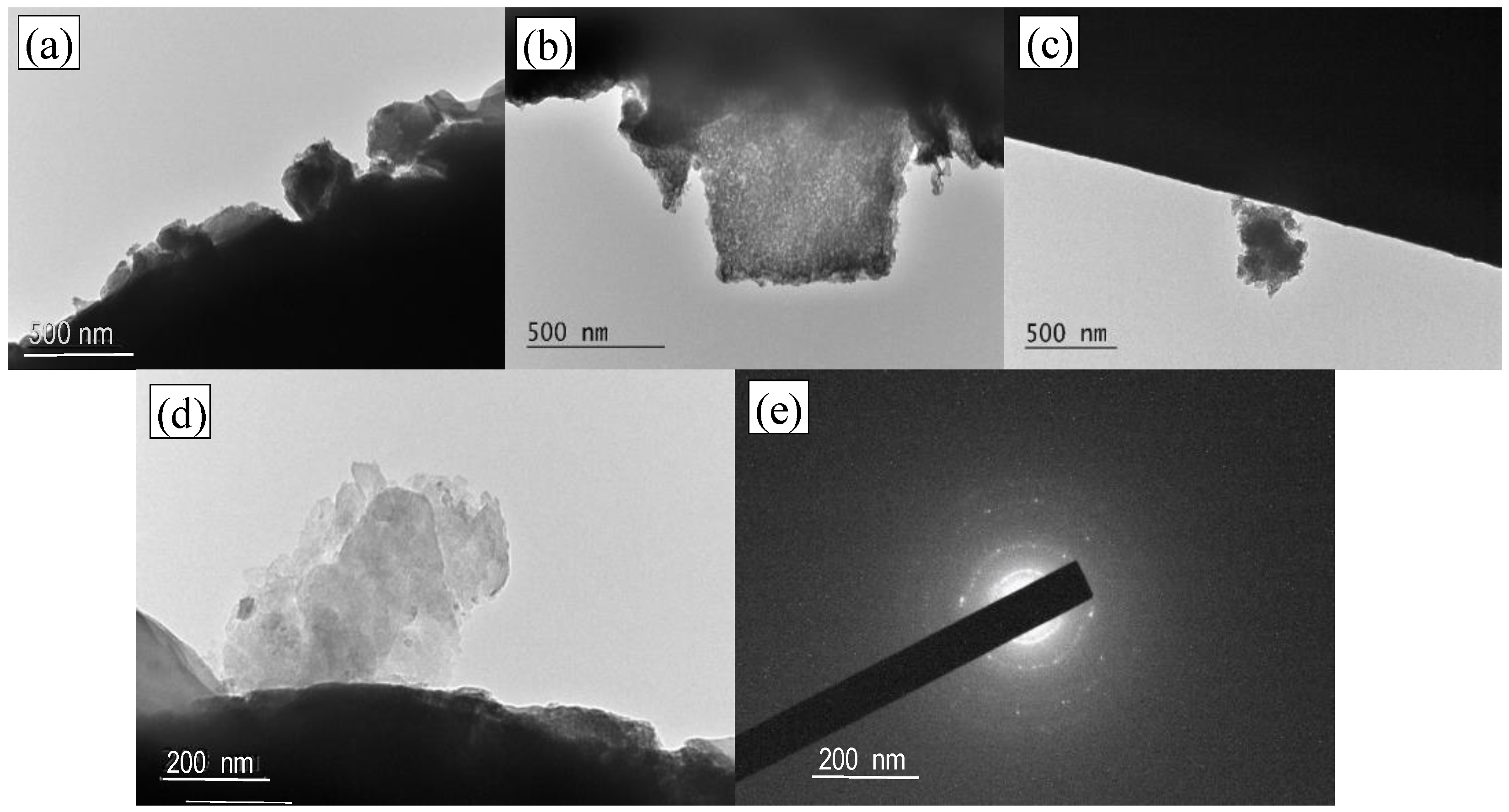
3.2. Characterization by SEM and TEM
3.3. Investigations on Composite Material PA30 as an Adsorbent for Drimaren Rot K-7B Dye
3.3.1. The Effect of Adsorbent Amount
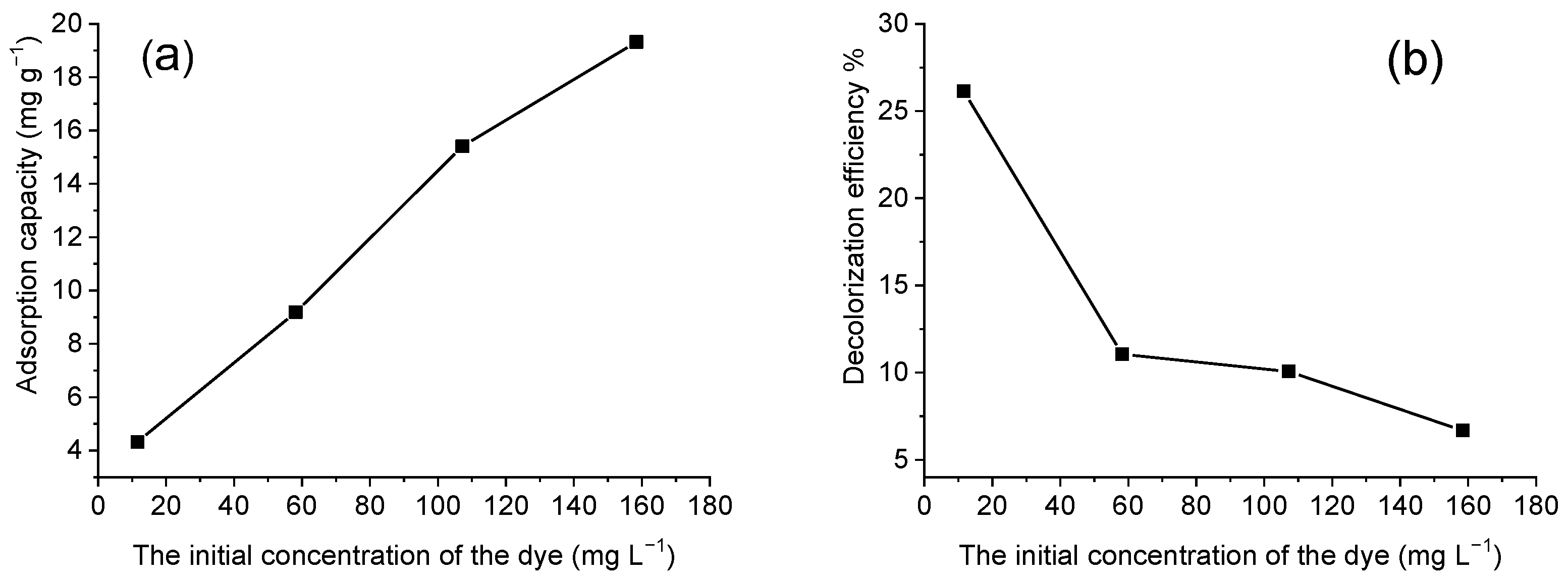
3.3.2. The Effect of Dye Concentration
3.3.3. Adsorption Isotherms
- Langmuir isotherm
- Freundlich isotherm
3.3.4. Temperature Effect
3.3.5. Kinetic Models and Mechanism
- Pseudo-first-order
- Pseudo-second-order
- Model of kinetic diffusion of the dye adsorption onto the composite material
3.3.6. Thermodynamic Adsorption Studies
3.3.7. The Effect of the Processing Time on Dye Adsorption
3.3.8. Investigations on the Composite Material Polyamide Fabric-Hydrogel-Zinc Oxide Nanoparticles as a Photocatalyst for Decolorization of Dye Aqueous Solutions
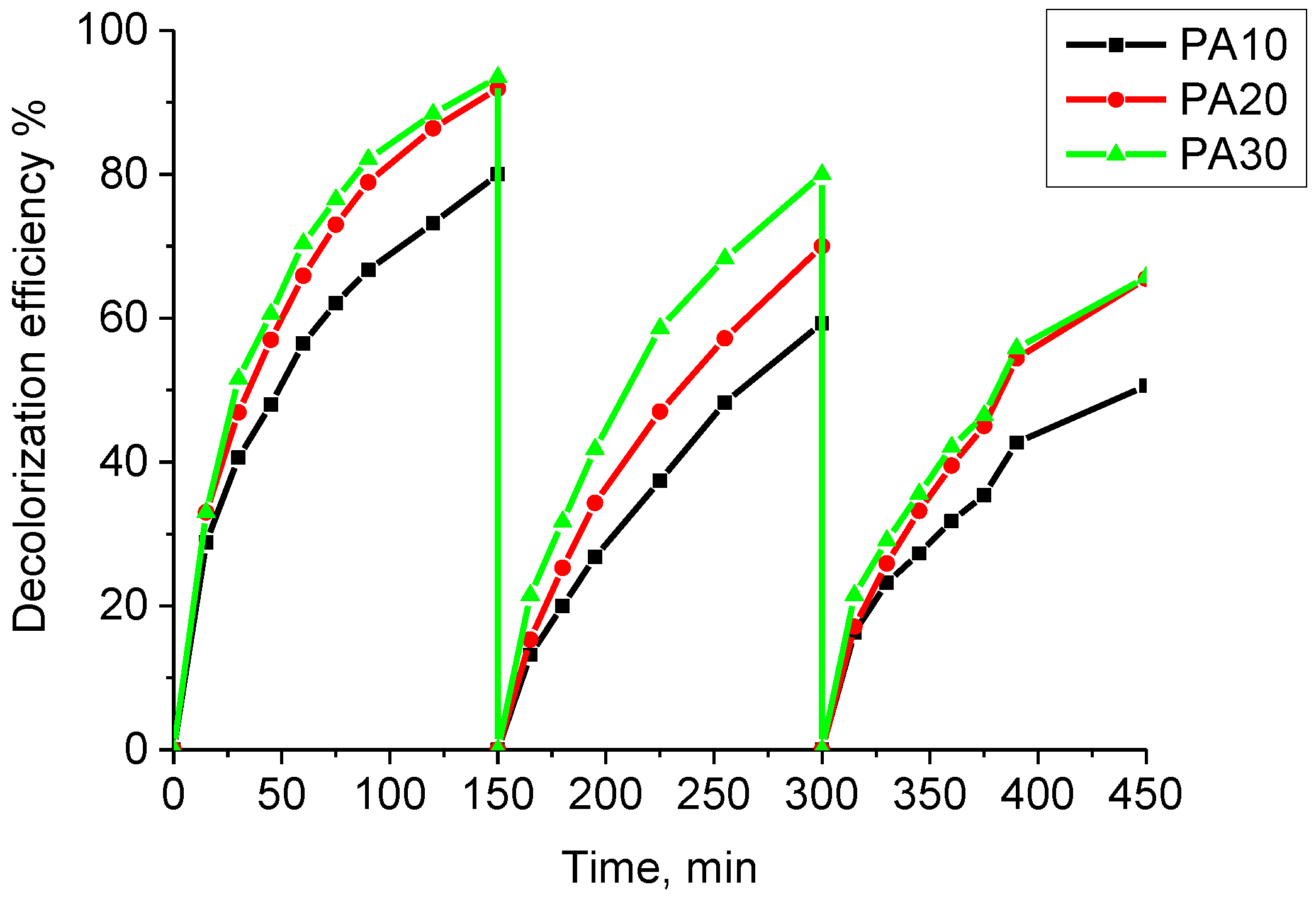
- Repeated use of the resulting composite materials
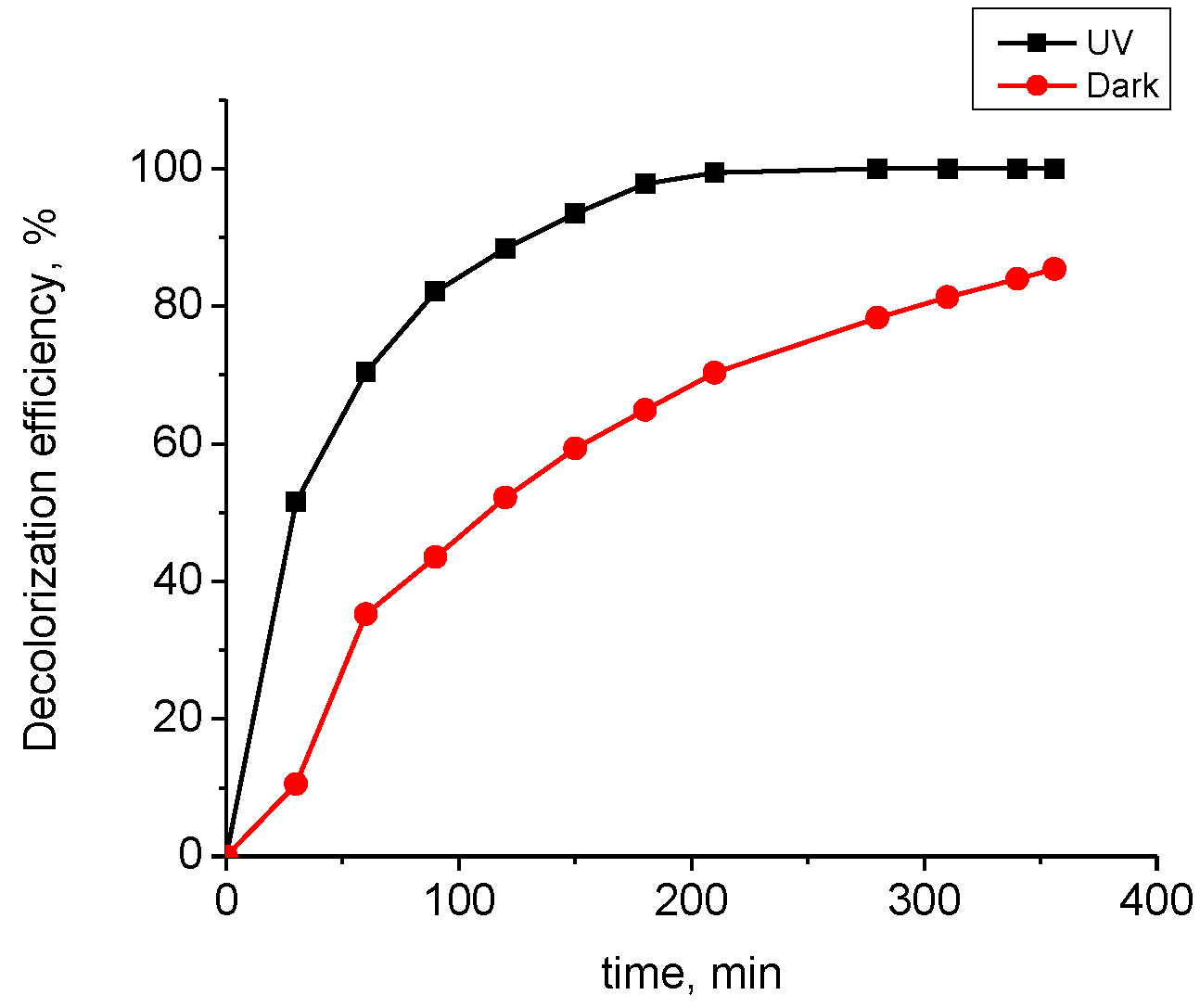
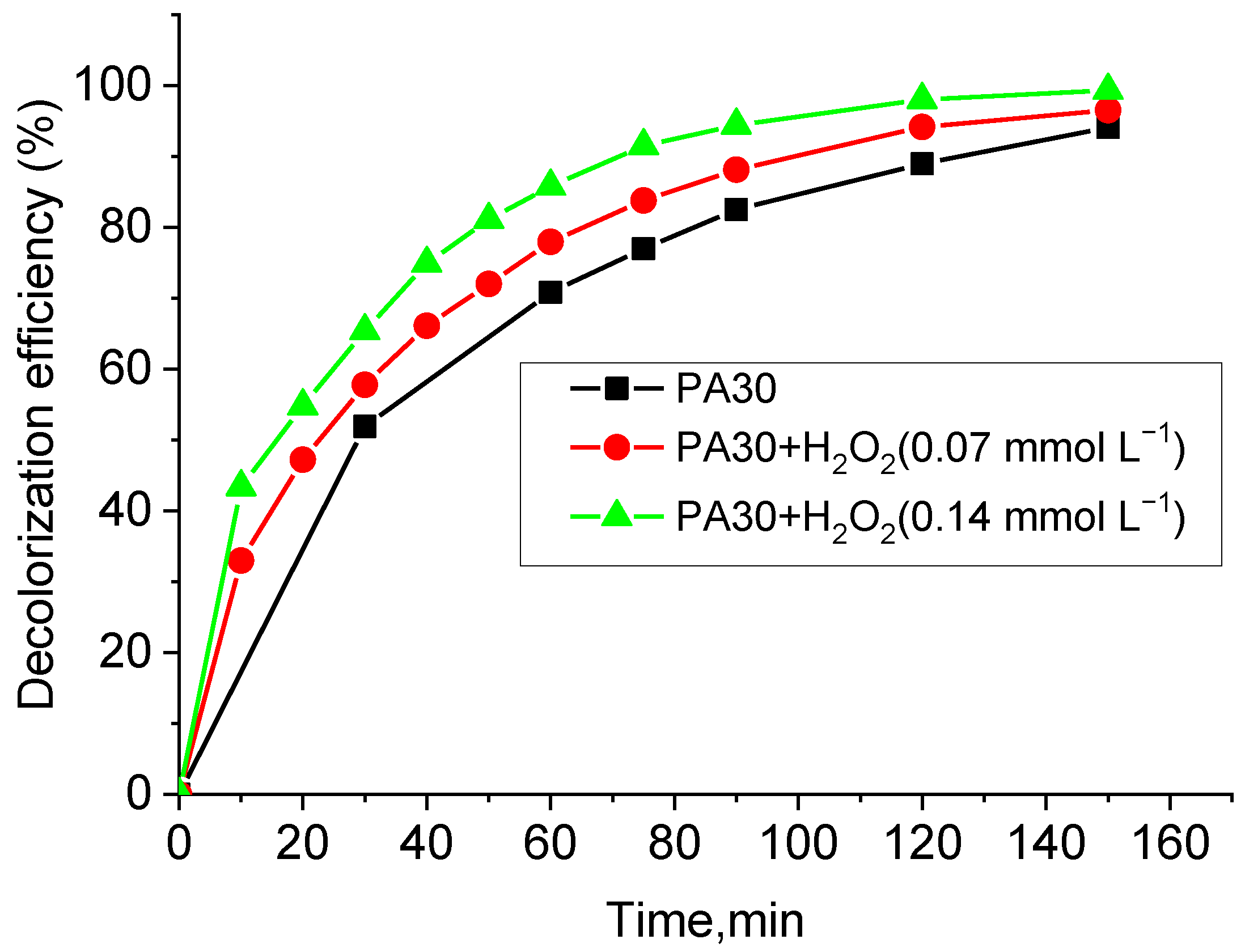
- Accelerated decolorization of the dye solution when exposed to UV light and upon the addition of hydrogen peroxide
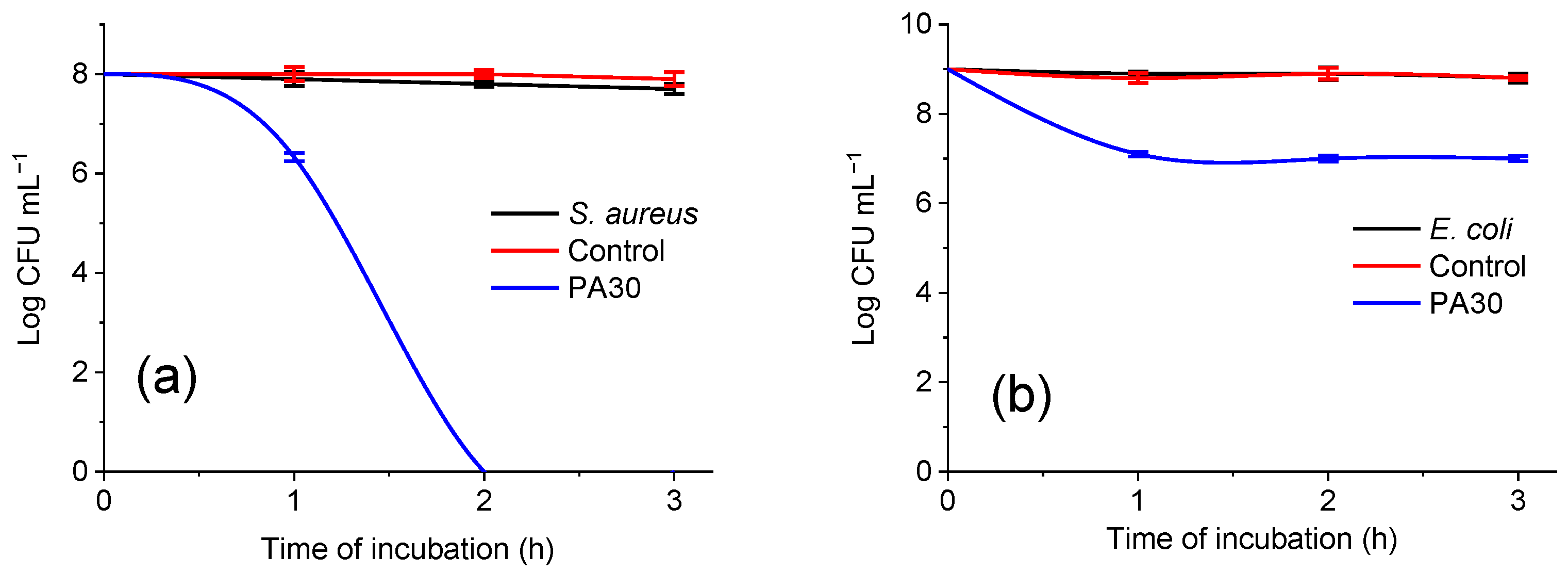
3.3.9. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Color. Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Malladi, R.S.; Shetty, N.S.; Reddy, K.R.; Aminabhavi, T.M. Synthesis of Ca-doped ZnO nanoparticles and its application as highly efficient electrochemical sensor for the determination of anti-viral drug, acyclovir. J. Mol. Liq. 2021, 322, 114552. [Google Scholar] [CrossRef]
- Prabhu, K.; Malode, S.J.; Shetti, N.P.; Kulkarni, R.M. Analysis of herbicide and its applications through a sensitive electrochemical technique based on MWCNTs/ZnO/CPE fabricated sensor. Chemosphere 2022, 287, 132086. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.; McKinley, A.; Tsuzuki, T.; Saunders, M. A comparative evaluation of the photocatalytic and optical properties of nanoparticulate ZnO synthesized by mechanochemical processing. J. Nanopart. Res. 2008, 10, 243–248. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Zinc oxide nanostructures: Synthesis and properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573. [Google Scholar] [CrossRef]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.N.; Yetmez, M. ZnO Applications and Challenges. Curr. Org. Chem. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Motelica, L.; Popescu, A.; Răzvan, A.G.; Oprea, O.; Truşcă, R.D.; Vasile, B.S.; Dumitru, F.; Holban, A.M. Facile Use of ZnO Nanopowders to Protect Old Manual Paper Documents. Materials 2020, 13, 5452. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.I.; Trușcă, R.D.; Oprea, O.C.; Surdu, V.A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Staneva, D.; Atanasova, D.; Vasileva-Tonkova, E.; Lukanova, V.; Grabchev, I. A cotton fabric modified with a hydrogel containing ZnO nanoparticles. Preparation and properties study. Appl. Surf. Sci. 2015, 345, 72–80. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Deng, D.; Scot, M.; Ramanathan, S. Synthesis and characterization of one-dimensional flat ZnO nanotower arrays as high-efficiency adsorbents for the photocatalytic remediation of water pollutants. Nanoscale 2010, 2, 2685–2691. [Google Scholar] [CrossRef]
- Pronin, I.; Kaneva, N.; Bozhinova, A.; Averin, I.; Papazova, K.; Dimitrov, D.; Moshnikov, V. Photocatalytic Oxidation of Pharmaceuticals on Thin Nanostructured Zinc Oxide Films. Kinet. Catal. 2014, 55, 1–5. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of Integrated Photocatalyst Adsorbents for Wastewater Treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Zarkogianni, M.; Coutouli-Argyropoulou, E.; Samara, C.; Anthemidis, A.; Nikolaidis, N.; Tsatsaroni, E. A novel synthesis, characterization and application of an anionic Cr-complexed azo dye based on environmental considerations. Text. Res. J. 2012, 82, 2054–2061. [Google Scholar] [CrossRef]
- Weerasundara, L.; Gabriele, B.; Figoli, A.; Ok, Y.S.; Bundschuh, J. Hydrogels: Novel materials for contaminant removal in water—A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1970–2014. [Google Scholar] [CrossRef]
- Atanasova, D.; Staneva, D.; Grabchev, I. Textile with a hydrogel and iron oxide nanoparticles for wastewater treatment after reactive dyeing. J. Appl. Polym. Sci. 2021, 138, 49954. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent Hydrogel–Textile Composite Material Synthesized by Photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847. [Google Scholar] [CrossRef][Green Version]
- Espitia, P.J.P.; Soares, N.F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y. Facile Synthesis and Enhanced Photocatalytic Performance of Flower-like ZnO Hierarchical Microstructures. J. Phys. Chem. C 2010, 114, 890–896. [Google Scholar] [CrossRef]
- Krishnapriya, R.; Praneetha, S.; Vadivel Murugan, A. Energy efficient, microwave-hydro-/solvothermal synthesis of hierarchical flowers and rice-grain like ZnO nanocrystals as photoanodes for high performance dye-sensitized solar cells. Cryst. Eng. Comm. 2015, 17, 8353–8367. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Vadivelan, V.; Vasanth Kumar, K. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Angaye, S.S.; Wankasi, D.; Dikio, E.D. Synthesis, characterization and application of Mg/Al layered double hydroxide for the degradation of congo red in aqueous solution. Open J. Physic. Chem. 2015, 5, 56–70. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Srivastava, R.; Rupainwar, D.C. Eucalyptus bark powder as an effective adsorbent: Evaluation of adsorptive characteristics for various dyes. Desalin. Water Treatment. 2009, 11, 302–313. [Google Scholar] [CrossRef]
- Khan, T.; Dahiy, S.; Ali, I. Removal of Direct Red 81 dye from aqueous solution by native and citric acid modified bamboo sawdust-Kinetic study and equilibrium isotherm analyses. Gazi. Univ. J. Sci. 2012, 25, 59–87. [Google Scholar]
- Abdeen, Z.; Mohammad, S. Study of the adsorption efficiency of an eco-friendly carbohydrate polymer for contaminated aqueous solution by organophosphorus pesticide. Open J. Organic Polym. Mater. 2014, 4, 16–28. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloester stoffe. Veternskaps Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Koynucu, H. Adsorption kinetics of 3-Hydroxybenzaldehyde on native and activated bentonite. Appl. Clay Sci. 2008, 38, 279–287. [Google Scholar] [CrossRef]
- Sismanoglu, T.; Ercag, A.; Pura, S.; Ercag, E. Kinetics and isotherms of dazomet adsorption on natural adsorbents. J. Braz. Chem. Soc. 2004, 15, 669–675. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Adsorption of Congo red by three Australian kaolins. Appl. Clay Sci. 2009, 43, 465–472. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behaviour of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Adam, F.; Chua, J.H. The adsorption of palmytic acid on rice husk ash chemically modified with Al(III) ion using the sol-gel technique. J. Colloid Interface Sci. 2004, 280, 55–61. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Adebowale, K.O.; Olu-Owolabi, B.I. Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay. J. Hazard. Mater. 2007, 144, 386–395. [Google Scholar] [CrossRef]
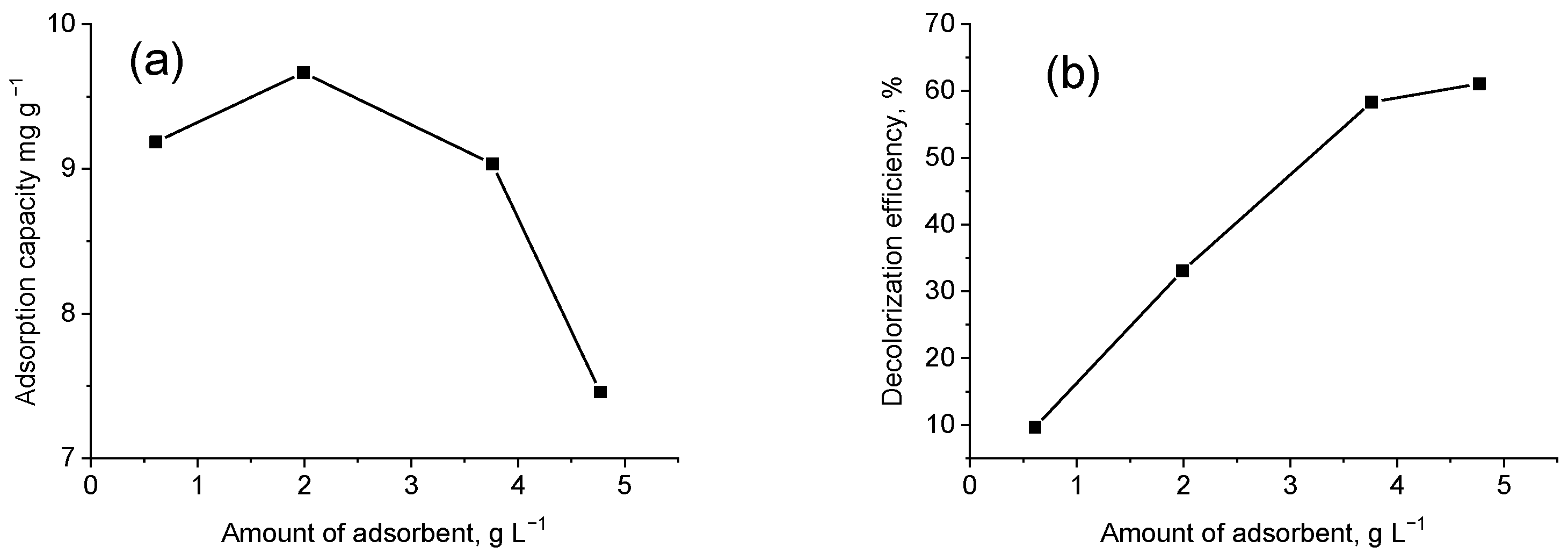

| Sample | Treatment Conditions, Zn2+ (%) | Gel Fraction, (%) | Fabric Weight Gain, (%) |
|---|---|---|---|
| PA10 | 10 | 81.1 | 13.8 |
| PA20 | 20 | 75.4 | 15.9 |
| PA30 | 30 | 73.2 | 21.7 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | RL | R2 | KF (mg g−1) | n | R2 |
| 26.6 | 0.015 | 0.53 | 0.789 | 1.030 | 1.905 | 0.964 |
| Temperature (K) | Pseudo-First-Order | Pseudo-Second-Order | Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg g−1) | R2 | K2 (g mg−1 min−1) | qe (mg g−1) | R2 | Kid (mg g−1min−0.5) | C (mg g−1) | R2 | |
| 298 | 0.0074 | 3.38 | 0.877 | 0.0042 | 4.92 | 0.944 | 0.1763 | 1.14 | 0.936 |
| 308 | 0.0106 | 6.13 | 0.956 | 0.0022 | 8.00 | 0.984 | 0.3573 | 0.43 | 0.995 |
| 323 | 0.0080 | 7.37 | 0.927 | 0.0018 | 10.42 | 0.962 | 0.4417 | 1.28 | 0.956 |
| T (K) | LnKD | ∆S° (kJ mol−1 K−1) | ∆H° (kJ mol−1) | ∆G° (kJ mol−1) | Ea (kJ mol−1) |
|---|---|---|---|---|---|
| 298 | −2.40 | 0.047 | 19.74 | 5.936 | 28.58 |
| Number of Uses | Sample | First-Order | Second-Order | ||
|---|---|---|---|---|---|
| K1 | R2 | K2 | R2 | ||
| First | PA10 | 0.00995 | 0.969 | 5.14 × 10−4 | 0.982 |
| PA20 | 0.01581 | 0.995 | 1.37 × 10−3 | 0.883 | |
| PA30 | 0.01719 | 0.994 | 1.71 × 10−3 | 0.867 | |
| Second | PA10 | 0.00585 | 0.993 | 1.97 × 10−4 | 0.989 |
| PA20 | 0.00777 | 0.996 | 3.07 × 10−4 | 0.975 | |
| PA30 | 0.01039 | 0.996 | 5.21 × 10−4 | 0.958 | |
| Third | PA10 | 0.00437 | 0.943 | 1.34 × 10−4 | 0.985 |
| PA20 | 0.00679 | 0.987 | 2.51 × 10−4 | 0.989 | |
| PA30 | 0.00667 | 0.973 | 2.49 × 10−4 | 0.992 | |
| H2O2 Concentration, mmol L−1 | K1 | R2 | K2 | R2 |
|---|---|---|---|---|
| 0.07 | 0.02356 | 0.997 | 0.004 | 0.790 |
| 0.14 | 0.02937 | 0.997 | 0.011 | 0.734 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasova, D.; Irikova, M.; Staneva, D.; Grabchev, I. Design of a Composite Based on Polyamide Fabric-Hydrogel-Zinc Oxide Particles to Act as Adsorbent and Photocatalyst. Materials 2022, 15, 6649. https://doi.org/10.3390/ma15196649
Atanasova D, Irikova M, Staneva D, Grabchev I. Design of a Composite Based on Polyamide Fabric-Hydrogel-Zinc Oxide Particles to Act as Adsorbent and Photocatalyst. Materials. 2022; 15(19):6649. https://doi.org/10.3390/ma15196649
Chicago/Turabian StyleAtanasova, Daniela, Miglena Irikova, Desislava Staneva, and Ivo Grabchev. 2022. "Design of a Composite Based on Polyamide Fabric-Hydrogel-Zinc Oxide Particles to Act as Adsorbent and Photocatalyst" Materials 15, no. 19: 6649. https://doi.org/10.3390/ma15196649
APA StyleAtanasova, D., Irikova, M., Staneva, D., & Grabchev, I. (2022). Design of a Composite Based on Polyamide Fabric-Hydrogel-Zinc Oxide Particles to Act as Adsorbent and Photocatalyst. Materials, 15(19), 6649. https://doi.org/10.3390/ma15196649








