Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis
Abstract
1. Introduction
2. Results
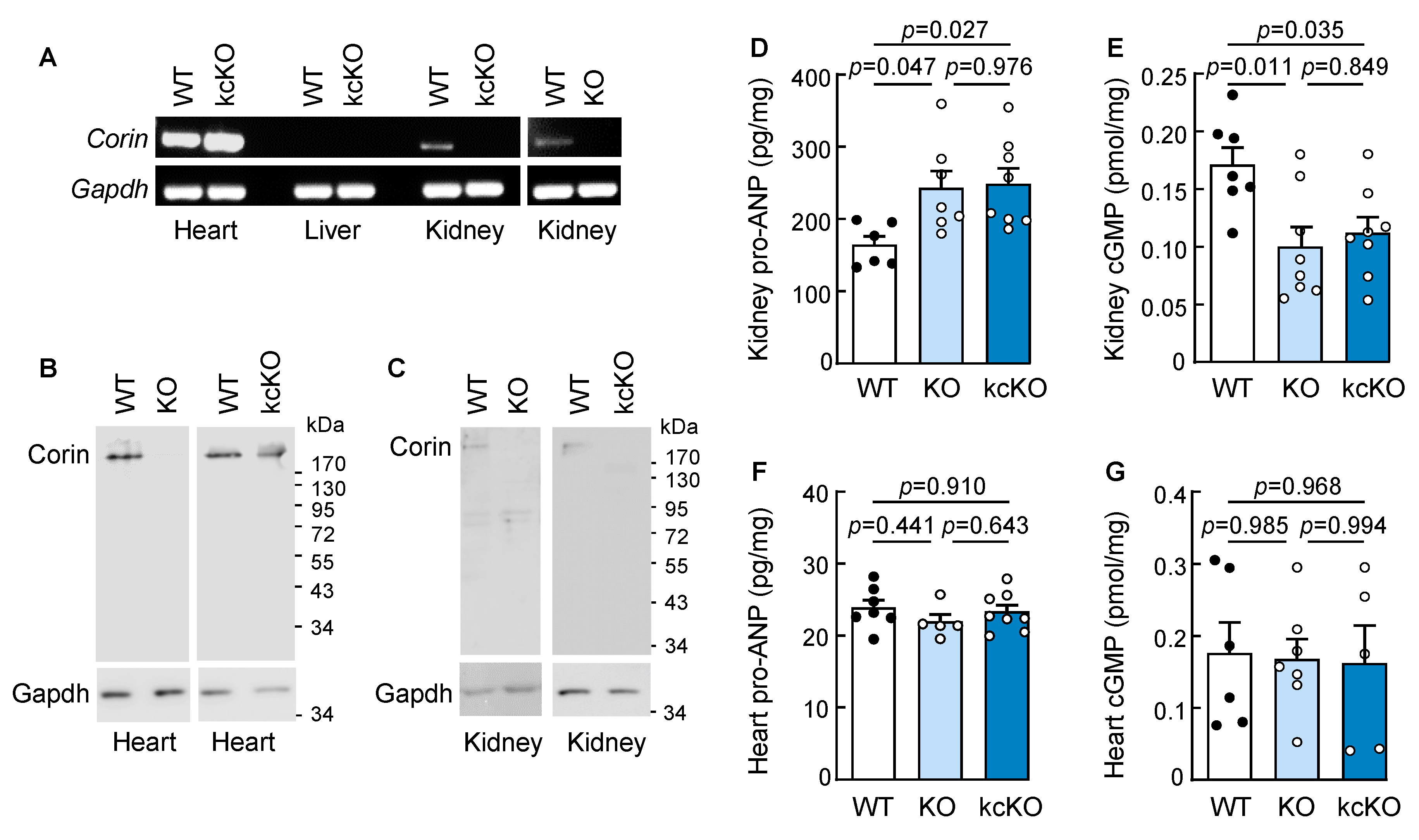
2.1. Generation of Corin KcKO Mice
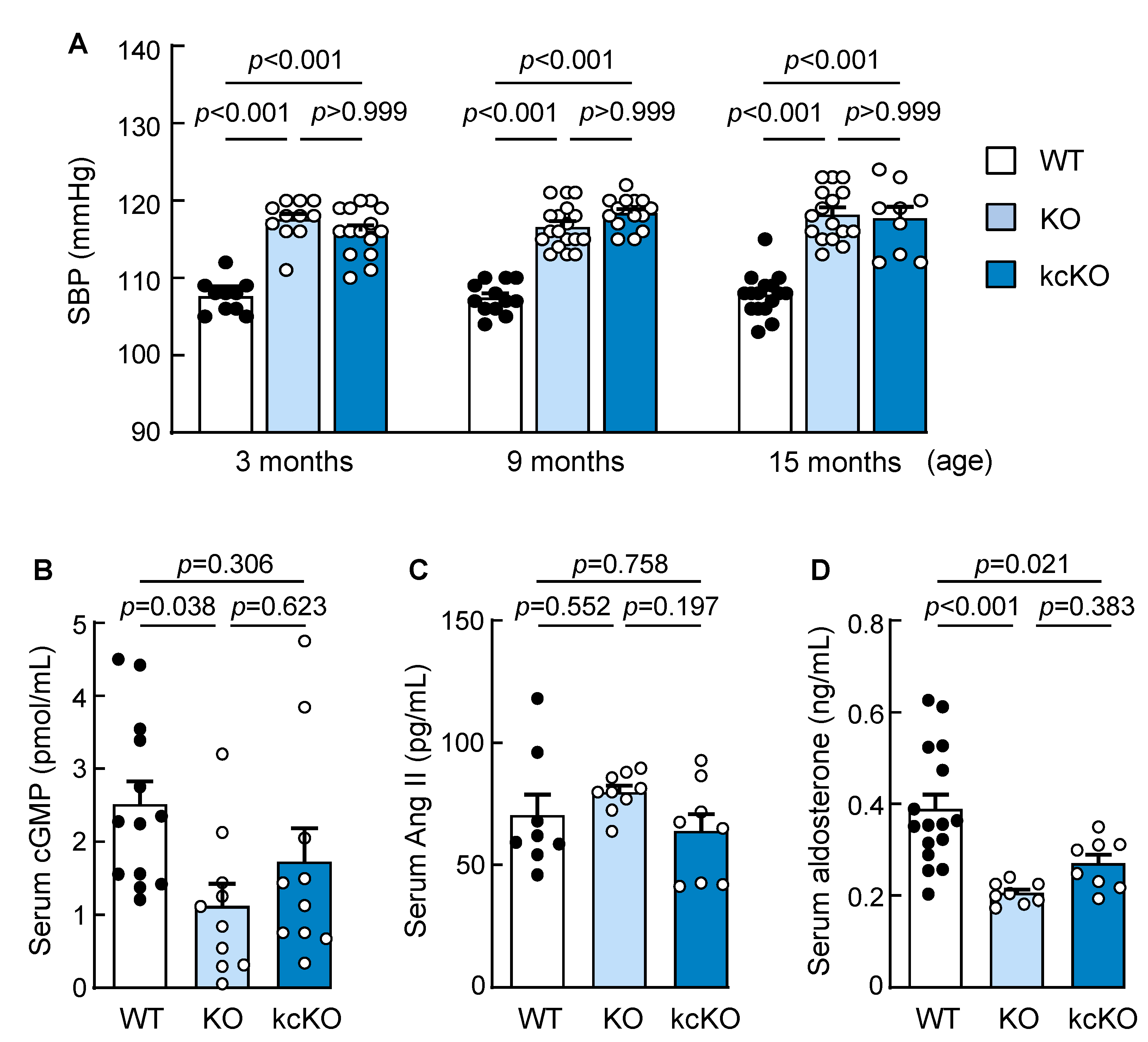
2.2. Increased Blood Pressure in Corin KcKO Mice
2.3. Renal Histology in Corin KcKO Mice
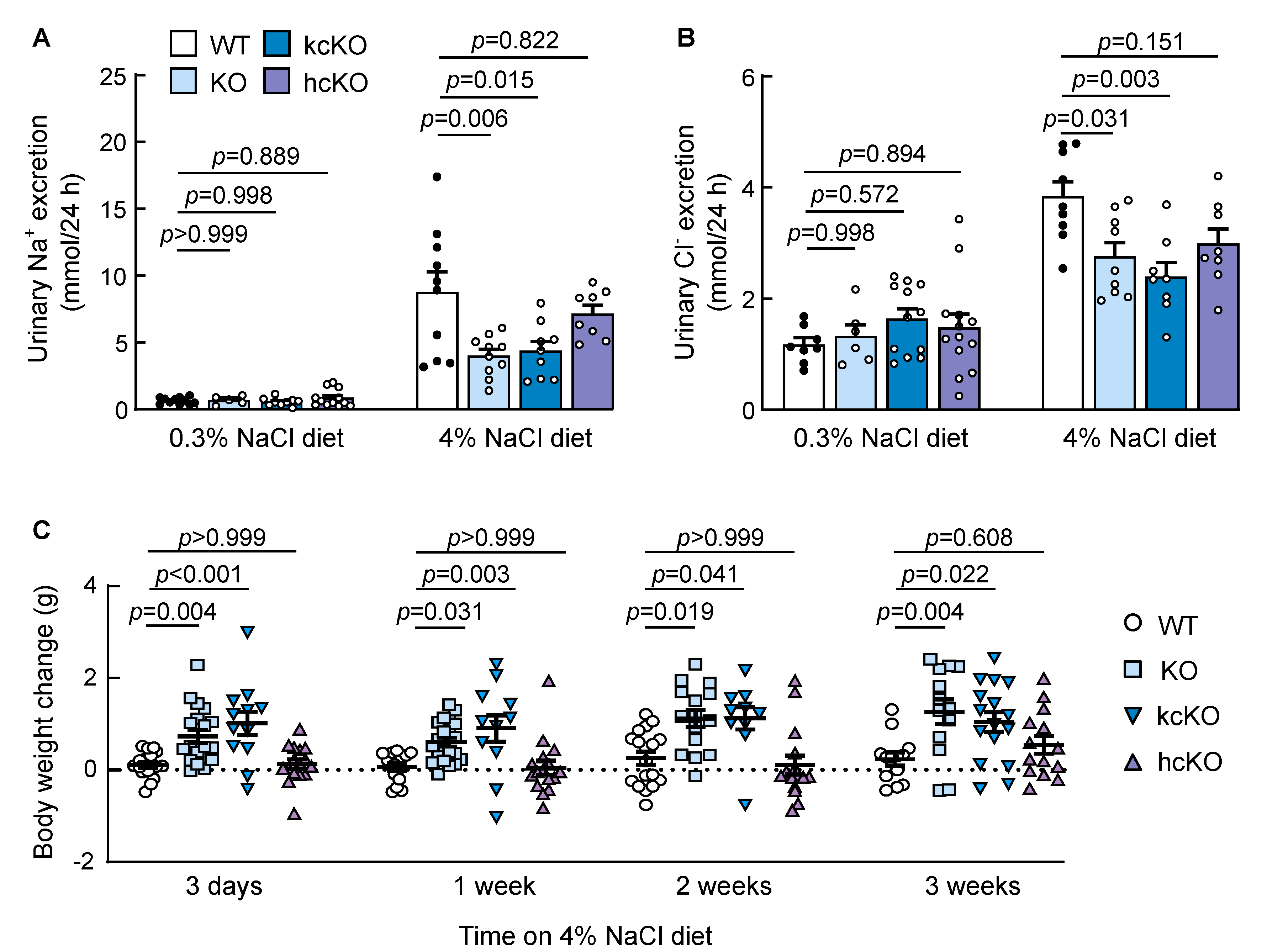
2.4. Reduced Urinary Salt Excretion in Corin KcKO Mice
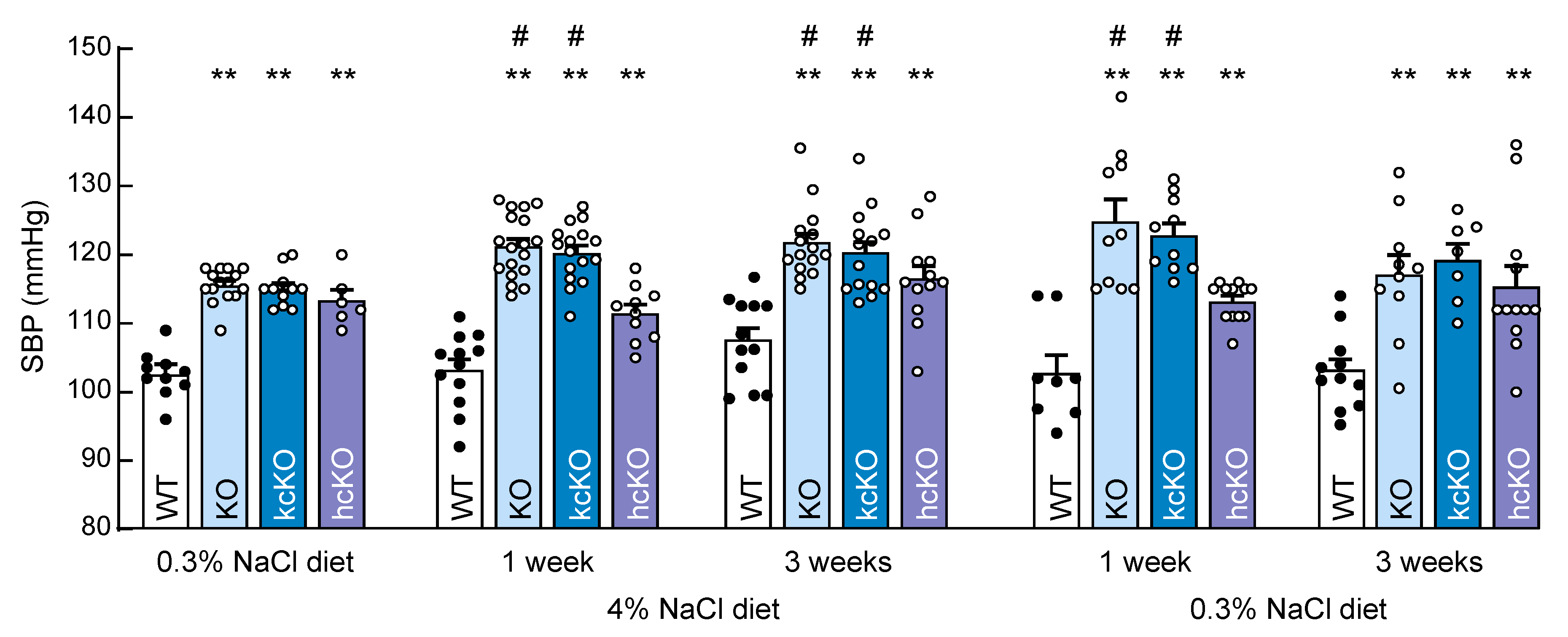
2.5. Salt-Exacerbated Hypertension in Corin KcKO Mice
2.6. Water and Food Intakes and Urine Volume in Corin KcKO Mice
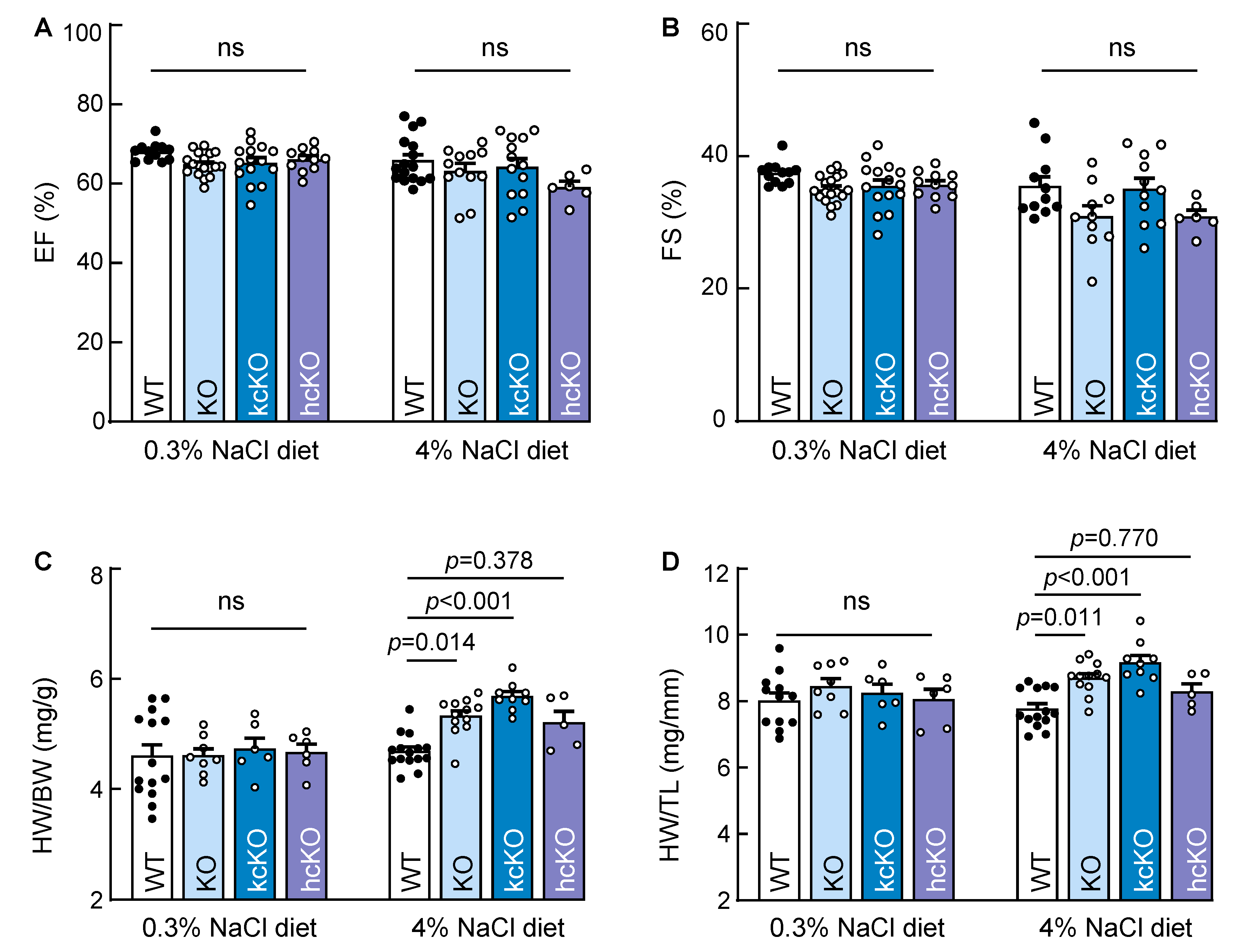
2.7. Cardiac Hypertrophy in Corin KcKO Mice
3. Discussion
4. Materials and Methods
4.1. Mouse Models
4.2. PCR and RT-PCR
4.3. Western Blotting
4.4. Pro-ANP, cGMP, Angiotensin, and Aldosterone Measurements
4.5. Urinary Proteins and Electrolytes
4.6. Serum Creatinine and Urea
4.7. Renal Histology
4.8. Blood Pressure
4.9. Water and Food Intakes and Urine Volume
4.10. Echocardiography and Heart Weights
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCK Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Guyton, A.C. Blood pressure control—Special role of the kidneys and body fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulou, E.; Braconnier, P.; Burnier, M. New Insights on the Role of Sodium in the Physiological Regulation of Blood Pressure and Development of Hypertension. Front. Cardiovasc. Med. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- McGrath, M.F.; de Bold, M.L.; de Bold, A.J. The endocrine function of the heart. Trends Endocrinol. Metab. 2005, 16, 469–477. [Google Scholar] [CrossRef]
- Theilig, F.; Wu, Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Ren. Physiol. 2015, 308, F1047–F1055. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef]
- Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Zoccai, G.B.; Frati, G.; Sciarretta, S. Cardiovascular Pleiotropic Effects of Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 3874. [Google Scholar] [CrossRef]
- Cannone, V.; Cabassi, A.; Volpi, R.; Burnett, J.C. Atrial Natriuretic Peptide: A Molecular Target of Novel Therapeutic Approaches to Cardio-Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 3265. [Google Scholar] [CrossRef]
- Rubattu, S.; Forte, M.; Marchitti, S.; Volpe, M. Molecular Implications of Natriuretic Peptides in the Protection from Hypertension and Target Organ Damage Development. Int. J. Mol. Sci. 2019, 20, 798. [Google Scholar] [CrossRef] [PubMed]
- Newton-Cheh, C.; Larson, M.G.; Vasan, R.S.; Levy, D.; Bloch, K.D.; Surti, A.; Guiducci, C.; Kathiresan, S.; Benjamin, E.J.; Struck, J.; et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat. Genet. 2009, 41, 348–353. [Google Scholar] [CrossRef]
- Rubattu, S.; Bigatti, G.; Evangelista, A.; Lanzani, C.; Stanzione, R.; Zagato, L.; Manunta, P.; Marchitti, S.; Venturelli, V.; Bianchi, G.; et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J. Am. Coll. Cardiol. 2006, 48, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Marchitti, S.; Bianchi, F.; Forte, M.; Volpe, M. The T2238C Human Atrial Natriuretic Peptide Molecular Variant and the Risk of Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 540. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Sheng, N.; Seto, M.; Morser, J.; Wu, Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J. Biol. Chem. 1999, 274, 14926–14935. [Google Scholar] [CrossRef]
- Bugge, T.H.; Antalis, T.M.; Wu, Q. Type II transmembrane serine proteases. J. Biol. Chem. 2009, 284, 23177–23181. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Zhang, Y.; Dong, N.; Wu, Q. Corin: A Key Mediator in Sodium Homeostasis, Vascular Remodeling, and Heart Failure. Biology 2022, 11, 717. [Google Scholar] [CrossRef]
- Dong, N.; Niu, Y.; Chen, Y.; Sun, S.; Wu, Q. Function and regulation of corin in physiology and disease. Biochem. Soc. Trans. 2020, 48, 1905–1916. [Google Scholar] [CrossRef]
- Yan, W.; Wu, F.; Morser, J.; Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 8525–8529. [Google Scholar] [CrossRef]
- Chan, J.C.; Knudson, O.; Wu, F.; Morser, J.; Dole, W.P.; Wu, Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. USA 2005, 102, 785–790. [Google Scholar] [CrossRef]
- Wang, W.; Shen, J.Z.; Cui, Y.J.; Jiang, J.J.; Chen, S.H.; Peng, J.H.; Wu, Q.Y. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012, 82, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liao, X.; Fukuda, K.; Knappe, S.; Wu, F.; Dries, D.L.; Qin, J.; Wu, Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ. Res. 2008, 103, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.G.; Tamm, N.N.; Seferian, K.R.; Postnikov, A.B.; Karpova, N.S.; Serebryanaya, D.V.; Koshkina, E.V.; Krasnoselsky, M.I.; Katrukha, A.G. Processing of pro-B-type natriuretic peptide: Furin and corin as candidate convertases. Clin. Chem. 2010, 56, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Huntley, B.K.; Heublein, D.M.; Sandberg, S.M.; McKie, P.M.; Martin, F.L.; Jougasaki, M.; Burnett, J.C., Jr. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin. Chem. 2011, 57, 40–47. [Google Scholar] [CrossRef]
- Nishikimi, T.; Nakagawa, Y.; Minamino, N.; Ikeda, M.; Tabei, K.; Fujishima, A.; Takayama, K.; Akimoto, K.; Yamada, C.; Nakao, K.; et al. Pro-B-type natriuretic peptide is cleaved intracellularly: Impact of distance between O-glycosylation and cleavage sites. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R639–R649. [Google Scholar] [CrossRef]
- Chen, S.; Cao, P.; Dong, N.; Peng, J.; Zhang, C.; Wang, H.; Zhou, T.; Yang, J.; Zhang, Y.; Martelli, E.E.; et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat. Med. 2015, 21, 1048–1053. [Google Scholar] [CrossRef]
- Rame, J.E.; Drazner, M.H.; Post, W.; Peshock, R.; Lima, J.; Cooper, R.S.; Dries, D.L. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension 2007, 49, 857–864. [Google Scholar] [CrossRef]
- Rame, J.E.; Tam, S.W.; McNamara, D.; Worcel, M.; Sabolinski, M.L.; Wu, A.H.; Dries, D.L. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: Results from the Genetic Risk Assessment in Heart Failure substudy. Circ. Heart Fail. 2009, 2, 541–548. [Google Scholar] [CrossRef]
- Dong, N.; Fang, C.; Jiang, Y.; Zhou, T.; Liu, M.; Zhou, J.; Shen, J.; Fukuda, K.; Qin, J.; Wu, Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J. Biol. Chem. 2013, 288, 7867–7874. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Niu, Y.; He, M.; Wang, C.; Liu, M.; Yang, J.; Zhang, Y.; Zhou, J.; Fukuda, K.; et al. Identification and functional analysis of CORIN variants in hypertensive patients. Hum. Mutat. 2017, 38, 1700–1710. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Zhong, Y.; Zhang, Y.; Zhang, S.; Li, S.; Zhao, Y.; Zheng, W.; Liu, J.; Xia, Y.; et al. Single-Nucleotide Polymorphisms in the 3’ Untranslated Region of CORIN Associated With Cardiovascular Diseases in a Chinese Han Population: A Case-Control Study. Front. Cardiovasc. Med. 2021, 8, 625072. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, W.; Dong, N.; Lou, J.; Srinivasan, D.K.; Cheng, W.; Huang, X.; Liu, M.; Fang, C.; Peng, J.; et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012, 484, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Abbey-Hosch, S.; Dickey, D.M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 2006, 27, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.D.; Scarman, A.L.; Clarke, B.E.; Normyle, J.F.; Antalis, T.M. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur. J. Biochem. 2000, 267, 6931–6937. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar]
- Polzin, D.; Kaminski, H.J.; Kastner, C.; Wang, W.; Krämer, S.; Gambaryan, S.; Russwurm, M.; Peters, H.; Wu, Q.; Vandewalle, A.; et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010, 78, 650–659. [Google Scholar] [CrossRef]
- Lai, F.J.; Hsieh, M.C.; Hsin, S.C.; Lin, S.R.; Guh, J.Y.; Chen, H.C.; Shin, S.J. The cellular localization of increased atrial natriuretic peptide mRNA and immunoreactivity in diabetic rat kidneys. J. Histochem. Cytochem. 2002, 50, 1501–1508. [Google Scholar]
- Ramirez, G.; Saba, S.R.; Dietz, J.R.; Vesely, D.L. Immunocytochemical localization of proANF 1-30, proANF 31-67 and atrial natriuretic factor in the kidney. Kidney Int. 1992, 41, 334–341. [Google Scholar] [CrossRef][Green Version]
- Figueroa, C.D.; Lewis, H.M.; MacIver, A.G.; Mackenzie, J.C.; Bhoola, K.D. Cellular localisation of atrial natriuretic factor in the human kidney. Nephrol. Dial. Transplant. 1990, 5, 25–31. [Google Scholar] [CrossRef]
- McKenzie, J.C.; Scott, J.N.; Inagami, T. Immunohistochemical localization of atrial natriuretic peptide in the developing and adult mammalian kidney. Am. J. Anat. 1991, 190, 182–191. [Google Scholar] [CrossRef]
- Fang, C.; Shen, L.; Dong, L.; Liu, M.; Shi, S.; Dong, N.; Wu, Q. Reduced urinary corin levels in patients with chronic kidney disease. Clin. Sci. 2013, 124, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, H.; Dong, N.; Zhang, C.; Xue, B.; Wu, Q. Localization of corin and atrial natriuretic peptide expression in human renal segments. Clin. Sci. 2016, 130, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Sun, S.; Zhang, Y.; Wang, L.; Luo, Z.; Liu, M.; Dong, L.; Dong, N.; Wu, Q. A conserved LDL-receptor motif regulates corin and CD320 membrane targeting in polarized renal epithelial cells. eLife 2020, 9, e56059. [Google Scholar] [CrossRef]
- van der Wijst, J.; Belge, H.; Bindels, R.J.M.; Devuyst, O. Learning Physiology From Inherited Kidney Disorders. Physiol. Rev. 2019, 99, 1575–1653. [Google Scholar] [CrossRef] [PubMed]
- Nawata, C.M.; Pannabecker, T.L. Mammalian urine concentration: A review of renal medullary architecture and membrane transporters. J. Comp. Physiol. B 2018, 188, 899–918. [Google Scholar] [CrossRef]
- Rossier, B.C.; Pradervand, S.; Schild, L.; Hummler, E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu. Rev. Physiol. 2002, 64, 877–897. [Google Scholar] [CrossRef]
- Stoops, E.H.; Caplan, M.J. Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 2014, 25, 1375–1386. [Google Scholar] [CrossRef]
- He, M.; Zhou, T.; Niu, Y.; Feng, W.; Gu, X.; Xu, W.; Zhang, S.; Wang, Z.; Zhang, Y.; Wang, C.; et al. The protease corin regulates electrolyte homeostasis in eccrine sweat glands. PLoS Biol. 2021, 19, e3001090. [Google Scholar] [CrossRef]
- Gladysheva, I.P.; Robinson, B.R.; Houng, A.K.; Kováts, T.; King, S.M. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J. Mol. Cell. Cardiol. 2008, 44, 131–142. [Google Scholar] [CrossRef]
- de Bold, A.J.; Borenstein, H.B.; Veress, A.T.; Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981, 28, 89–94. [Google Scholar] [CrossRef]
- Matsuo, A.; Nagai-Okatani, C.; Nishigori, M.; Kangawa, K.; Minamino, N. Natriuretic peptides in human heart: Novel insight into their molecular forms, functions, and diagnostic use. Peptides 2019, 111, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Knappe, S.; Wu, F.; Masikat, M.R.; Morser, J.; Wu, Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: Design and characterization of a soluble corin. J. Biol. Chem. 2003, 278, 52363–52370. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.; Jaffe, A.S.; Hasin, Y. Enzyme-linked immunoabsorbent assay for detection of human serine protease corin in blood. Clin. Chim. Acta 2009, 409, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Chen, S.; Wang, W.; Zhou, Y.; Wu, Q. Corin in clinical laboratory diagnostics. Clin. Chim. Acta 2012, 413, 378–383. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Q.; Cai, X.; Liu, Y.; Ding, J.; Tian, H.; Chao, X.; Sheng, H.; Jiang, L.; Jin, J.; et al. Association Between High Serum Soluble Corin and Hypertension: A Cross-Sectional Study in a General Population of China. Am. J. Hypertens. 2015, 28, 1141–1149. [Google Scholar] [CrossRef]
- Yu, R.; Han, X.; Zhang, X.; Wang, Y.; Wang, T. Circulating soluble corin as a potential biomarker for cardiovascular diseases: A translational review. Clin. Chim. Acta 2018, 485, 106–112. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, S.; Wang, W.; Chen, S.; Peng, J.; Zhang, X.; Wu, Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J. Biol. Chem. 2011, 286, 10066–10072. [Google Scholar] [CrossRef]
- Kurtz, A.; Della Bruna, R.; Pfeilschifter, J.; Taugner, R.; Bauer, C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc. Natl. Acad. Sci. USA 1986, 83, 4769–4773. [Google Scholar] [CrossRef]
- Kudo, T.; Baird, A. Inhibition of aldosterone production in the adrenal glomerulosa by atrial natriuretic factor. Nature 1984, 312, 756–757. [Google Scholar] [CrossRef]
- Burnett, J.C., Jr.; Granger, J.P.; Opgenorth, T.J. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am. J. Physiol. 1984, 247, F863–F866. [Google Scholar] [CrossRef]
- Inoue, K.; Sakamoto, T.; Yuge, S.; Iwatani, H.; Yamagami, S.; Tsutsumi, M.; Hori, H.; Cerra, M.C.; Tota, B.; Suzuki, N.; et al. Structural and functional evolution of three cardiac natriuretic peptides. Mol. Biol. Evol. 2005, 22, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Hiroi, J.; Takahashi, H.; Sakamoto, T. Diverse mechanisms for body fluid regulation in teleost fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R778–R792. [Google Scholar] [CrossRef] [PubMed]
- Loretz, C.A.; Pollina, C. Natriuretic peptides in fish physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 169–187. [Google Scholar] [CrossRef]
- Sabrane, K.; Kruse, M.N.; Fabritz, L.; Zetsche, B.; Mitko, D.; Skryabin, B.V.; Zwiener, M.; Baba, H.A.; Yanagisawa, M.; Kuhn, M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J. Clin. Investig. 2005, 115, 1666–1674. [Google Scholar] [CrossRef]
- Kuhn, M. Endothelial actions of atrial and B-type natriuretic peptides. Br. J. Pharmacol. 2012, 166, 522–531. [Google Scholar] [CrossRef]
- Brismar, H.; Holtbäck, U.; Aperia, A. Mechanisms by which intrarenal dopamine and ANP interact to regulate sodium metabolism. Clin. Exp. Hypertens. 2000, 22, 303–307. [Google Scholar] [CrossRef]
- Winaver, J.; Burnett, J.C.; Tyce, G.M.; Dousa, T.P. ANP inhibits Na(+)-H+ antiport in proximal tubular brush border membrane: Role of dopamine. Kidney Int. 1990, 38, 1133–1140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bacic, D.; Hernando, N.; Traebert, M.; Lederer, E.; Völkl, H.; Biber, J.; Kaissling, B.; Murer, H. Regulation of the renal type IIa Na/Pi cotransporter by cGMP. Pflug. Arch. 2001, 443, 306–313. [Google Scholar] [CrossRef]
- Light, D.B.; Schwiebert, E.M.; Karlson, K.H.; Stanton, B.A. Atrial natriuretic peptide inhibits a cation channel in renal inner medullary collecting duct cells. Science 1989, 243, 383–385. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Chen, L.; Chu, C.; Wang, Y.; Lv, Y.; He, M.; Martin, M.; Huang, P.H.; Mu, J.J.; et al. Short-Term High-Salt Diet Increases Corin Level to Regulate the Salt-Water Balance in Humans and Rodents. Am. J. Hypertens. 2018, 31, 253–260. [Google Scholar] [CrossRef]
- Zou, T.; Yao, S.; Du, M.F.; Mu, J.J.; Chu, C.; Hu, G.L.; Liao, Y.Y.; Chen, C.; Wang, D.; Ma, Q.; et al. Associations of corin genetic polymorphisms with salt sensitivity, blood pressure changes, and hypertension incidence in Chinese adults. J. Clin. Hypertens. 2021, 23, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.E.; Fokra, A.; Kinaneh, S.; Knaney, Y.; Aronson, D.; Abassi, Z. Distribution of Cardiac and Renal Corin and Proprotein Convertase Subtilisin/Kexin-6 in the Experimental Model of Cardio-Renal Syndrome of Various Severities. Front. Physiol. 2021, 12, 673497. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Schreiber, C.A.; Harris, P.C.; LaRusso, N.F.; Ramirez-Alvarado, M.; Cataliotti, A.; Torres, V.E.; Ikeda, Y. B-type natriuretic peptide overexpression ameliorates hepatorenal fibrocystic disease in a rat model of polycystic kidney disease. Kidney Int. 2017, 92, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Tonne, J.M.; Holditch, S.J.; Oehler, E.A.; Schreiber, C.A.; Ikeda, Y.; Cataliotti, A. Cardiac BNP gene delivery prolongs survival in aged spontaneously hypertensive rats with overt hypertensive heart disease. Aging 2014, 6, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Cataliotti, A.; Tonne, J.M.; Bellavia, D.; Martin, F.L.; Oehler, E.A.; Harders, G.E.; Campbell, J.M.; Peng, K.W.; Russell, S.J.; Malatino, L.S.; et al. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation 2011, 123, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Belluardo, P.; Cataliotti, A.; Bonaiuto, L.; Giuffrè, E.; Maugeri, E.; Noto, P.; Orlando, G.; Raspa, G.; Piazza, B.; Babuin, L.; et al. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1529–H1535. [Google Scholar] [CrossRef] [PubMed]
- Macheret, F.; Heublein, D.; Costello-Boerrigter, L.C.; Boerrigter, G.; McKie, P.; Bellavia, D.; Mangiafico, S.; Ikeda, Y.; Bailey, K.; Scott, C.G.; et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J. Am. Coll. Cardiol. 2012, 60, 1558–1565. [Google Scholar] [CrossRef]
- Yang, S.F.; Li, S.Y.; Lin, F.Y.; Hsieh, T.H.; Huang, P.H.; Lin, S.J. Chronic Kidney Disease Is Associated With Increased Cardiac Corin Expression But Decreased Proatrial Natriuretic Peptide Conversion Activity. J. Am. Heart Assoc. 2022, 11, e025208. [Google Scholar] [CrossRef]
- Kuhn, M. Cardiac and intestinal natriuretic peptides: Insights from genetically modified mice. Peptides 2005, 26, 1078–1085. [Google Scholar] [CrossRef]
- Chen, W.; Spitzl, A.; Mathes, D.; Nikolaev, V.O.; Werner, F.; Weirather, J.; Špiranec, K.; Röck, K.; Fischer, J.W.; Kämmerer, U.; et al. Endothelial Actions of ANP Enhance Myocardial Inflammatory Infiltration in the Early Phase After Acute Infarction. Circ. Res. 2016, 119, 237–248. [Google Scholar] [CrossRef]
- Buckley, C.L.; Stokes, A.J. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul. Pept. 2011, 172, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, F.; Baron, U.; Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995, 23, 5080–5081. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.S.; Nghiem, M.; Crackower, M.A.; Witt, S.A.; Kimball, T.R.; Tymitz, K.M.; Penninger, J.M.; Molkentin, J.D. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 2001, 89, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Chobert, M.N.; Lahuna, O.; Lebargy, F.; Kurauchi, O.; Darbouy, M.; Bernaudin, J.F.; Guellaen, G.; Barouki, R.; Laperche, Y. Tissue-specific expression of two gamma-glutamyl transpeptidase mRNAs with alternative 5’ ends encoded by a single copy gene in the rat. J. Biol. Chem. 1990, 265, 2352–2357. [Google Scholar] [CrossRef]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002, 110, 341–350. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; He, M.; Zhou, T.; Niu, Y.; Sun, S.; Li, H.; Zhang, C.; Zhang, S.; Liu, M.; et al. Krüppel-like factor 17 upregulates uterine corin expression and promotes spiral artery remodeling in pregnancy. Proc. Natl. Acad. Sci. USA 2020, 117, 19425–19434. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Zhang, S.; Du, C.; Wang, K.; Gu, X.; Sun, S.; Zhang, X.; Niu, Y.; Wang, C.; Liu, M.; et al. Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis. Int. J. Mol. Sci. 2022, 23, 11251. https://doi.org/10.3390/ijms231911251
Zhou T, Zhang S, Du C, Wang K, Gu X, Sun S, Zhang X, Niu Y, Wang C, Liu M, et al. Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis. International Journal of Molecular Sciences. 2022; 23(19):11251. https://doi.org/10.3390/ijms231911251
Chicago/Turabian StyleZhou, Tiantian, Shengnan Zhang, Chunyu Du, Kun Wang, Xiabing Gu, Shijin Sun, Xianrui Zhang, Yayan Niu, Can Wang, Meng Liu, and et al. 2022. "Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis" International Journal of Molecular Sciences 23, no. 19: 11251. https://doi.org/10.3390/ijms231911251
APA StyleZhou, T., Zhang, S., Du, C., Wang, K., Gu, X., Sun, S., Zhang, X., Niu, Y., Wang, C., Liu, M., Dong, N., & Wu, Q. (2022). Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis. International Journal of Molecular Sciences, 23(19), 11251. https://doi.org/10.3390/ijms231911251




