Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model
Abstract
1. Introduction
2. Results
2.1. PEG-bis-AA/HA-DXM Hydrogels Improve Motor Function after TBI
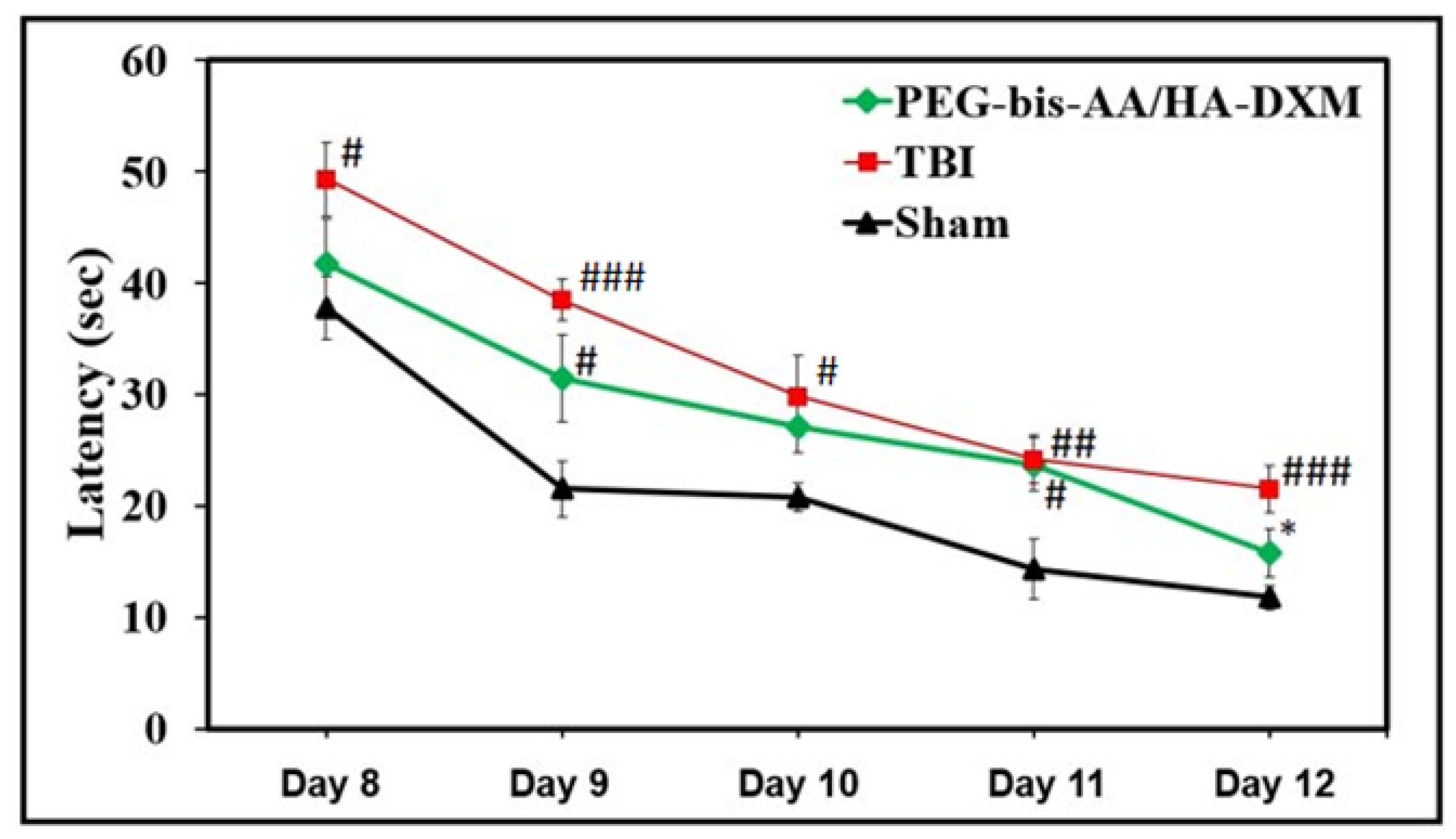
2.2. PEG-bis-AA/HA-DXM Hydrogels Improve Cognitive Function by Morris Water Maze Test
2.3. Effect of PEG-bis-AA/HA-DXM Hydrogels on Lesion Volume
2.4. Effect of PEG-bis-AA/HA-DXM Hydrogels on Neuroinflammatory Response
2.4.1. Expression of Inflammatory Cytokine Genes
2.4.2. Histological Analysis of Activated Microglia/Infiltrated Macrophages
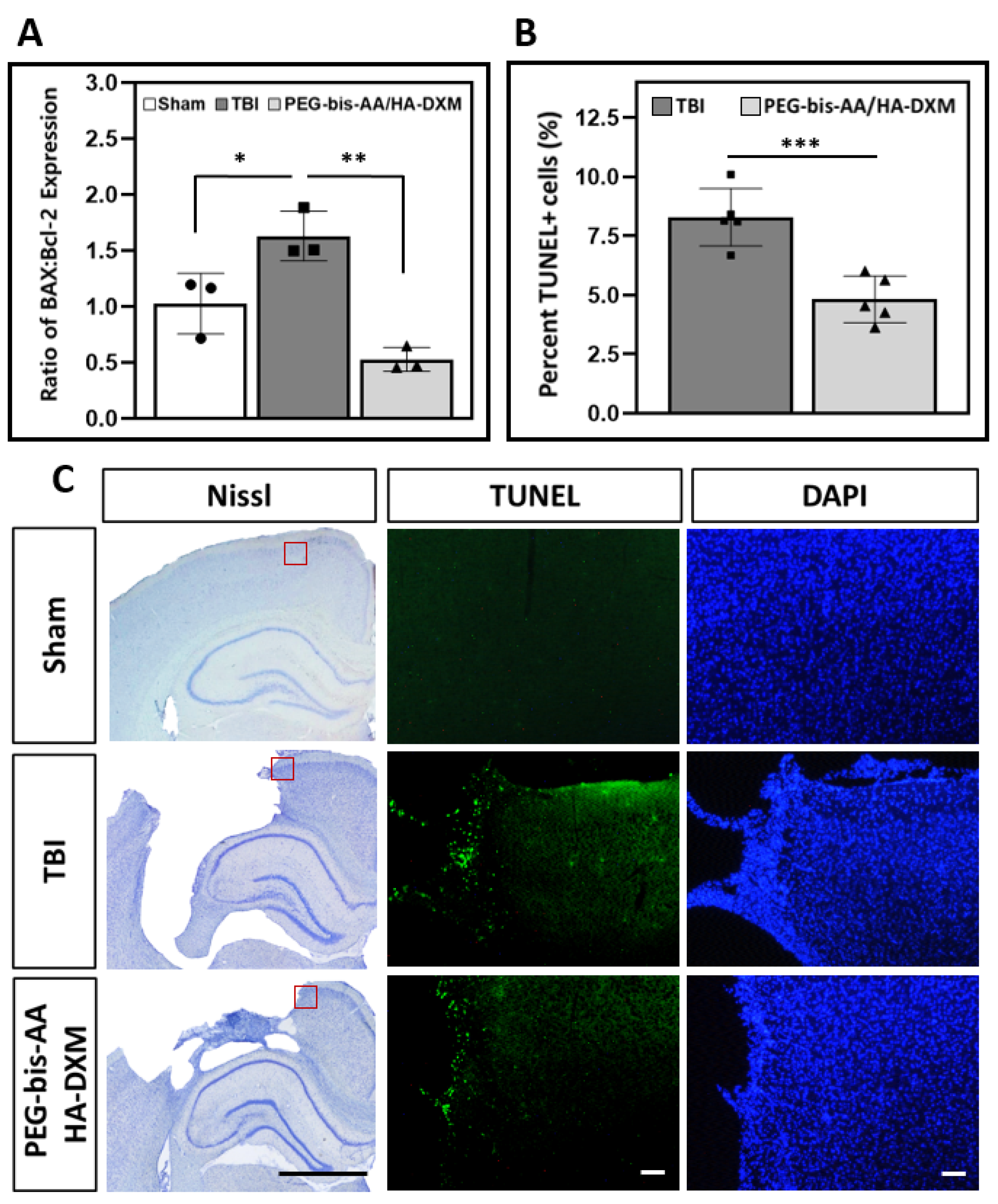
2.5. Effect of PEG-bis-AA/HA-DXM on Apoptosis in the Ipsilateral Cortex
2.5.1. Gene Expression of BAX and Bcl-2
2.5.2. Apoptotic Response by TUNEL Assay
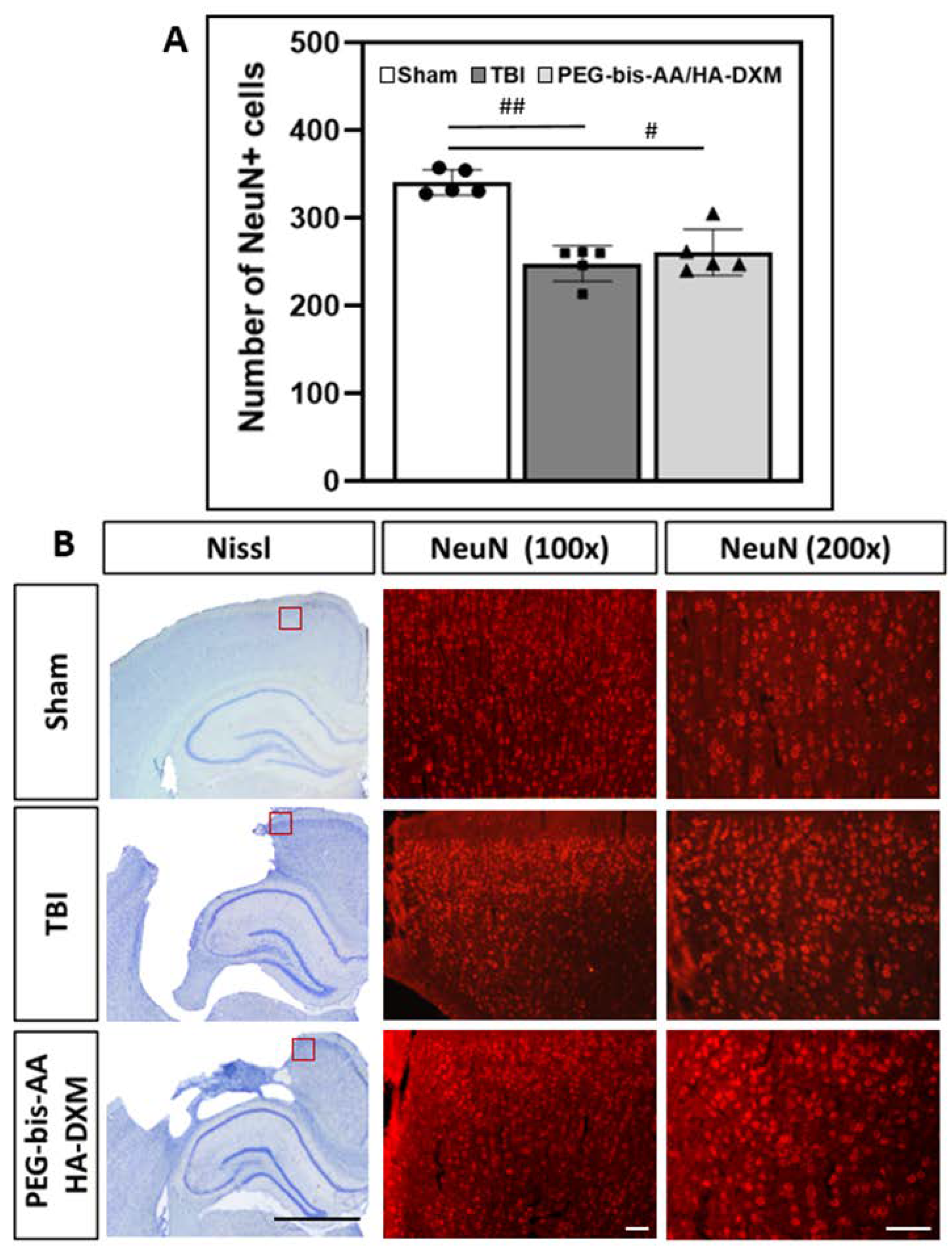
2.6. Effect of PEG-bis-AA/HA-DXM Hydrogels on Neuronal Cell Survival in the Ipsilateral Cortex
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Preparation of PEG-bis-AA/HA-DXM Hydrogels
4.3. Generation of a Rat Mild Traumatic Brain Injury Model and Hydrogel Treatment
4.4. Rotarod Test
4.5. Morris Water Maze Test
4.6. RT-PCR for Gene Expression
4.7. Histological Analysis
4.8. Lesion Volume Measurement
4.9. Immunohistochemical Staining for Neuroinflammatory Response, Neuronal Cell Survival, and Apoptosis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating global incidence of traumatic brain injury. J. Neurosurg. 2018; online ahead of print. [Google Scholar] [CrossRef]
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef]
- Frieden, T.R.; Houry, D.; Baldwin, G. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation; National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention: Atlanta, GA, USA, 2015; pp. 1–65. [CrossRef]
- Hill, C.S.; Coleman, M.P.; Menon, D.K. Traumatic axonal injury: Mechanisms and translational opportunities. Trends Neurosci. 2016, 39, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal models of traumatic brain injury and assessment of injury severity. Mol. Neurobiol. 2019, 56, 5332–5345. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain-barrier breakdown as a therapeutic target in trauamatic brain injury. Nat. Rev. Neurol. 2013, 6, 393–403. [Google Scholar] [CrossRef]
- Ahmad, K.; Alrais, Z.F.; Elkholy, H.M.; Elkhouly, A.E.; Beniamein, M.M.; Had, A.A.; Majeed, S.; Shoaib, A. Effect of early correction of hyponatremia on neurological outcome in traumatic brain injury patients. J. Intensive Crit. Care 2017, 3, 1–6. [Google Scholar] [CrossRef][Green Version]
- Faden, A.I.; Wu, J.; Stoica, B.A.; Loane, D.J. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br. J. Pharmacol. 2016, 173, 681–691. [Google Scholar] [CrossRef]
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology associated with traumatic brain injury; Current treatments and potential novel therapeutics. Cell Mol. Neurobiol. 2017, 37, 571–585. [Google Scholar] [CrossRef]
- Hall, E.D. High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J. Neurosurg. 1985, 62, 882–887. [Google Scholar] [CrossRef]
- Marklund, N. Pharmacological Neuroprotection. In Management of Severe Traumatic Brain Injury, 2nd ed.; Sundstrom, T., Grande, P., Luoto, T., Rosenlund, C., Unden, J., Wester, K.G., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 409–420. [Google Scholar] [CrossRef]
- Newton, R. Molecular mechanisms of glucocorticoid action: What is important? Thorax 2000, 55, 603–613. [Google Scholar] [CrossRef]
- CRASH Trial Collaborators. Final results of MRC CRASH, a randomized placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005, 265, 1957–1959. [Google Scholar] [CrossRef]
- Din, B.; Wang, X.; Shi, Y.; Li, Y. Long-term effect of high-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 2015, 133, 124–128. [Google Scholar] [CrossRef]
- Dearden, N.M.; Gibson, J.S.; McDowall, D.G.; Gibson, R.M.; Cameron, M.M. Effect of high-dose dexamethasone on outcome from severe head injury. J. Neurosurg. 1986, 64, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.L.; Chestnut, R.M.; Ghajar, J.; Hammond, F.F.M.; Harris, O.A.; Hartl, R.; Manley, G.T.; Nemecek, A.; Newell, D.W.; Rosenthal, G.; et al. XV. Steroids. J. Neurotrauma 2007, 24, S91–S95. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2016, 80, 6–15. [Google Scholar] [CrossRef]
- Holmin, S.; Mathiesen, T. Dexamethasone and colchicine reduce inflammation and delayed oedema following experimental brain contusion. Acta Neurochi. 1996, 138, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Artelt, M.; Burnet, M.; Schluesener, H.J. Dexamethasone attenuates early expression of three molecules associated with microglia/macrophages activation following rat traumatic brain injury. Acta Neuropathol. 2007, 113, 675–682. [Google Scholar] [CrossRef]
- Campolo, M.; Ahmad, A.; Crupi, R.; Impellizzeri, D.; Morabito, R.; Esposito, E.; Cuzzocrea, S. Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J. Endocrinol. 2013, 217, 291–301. [Google Scholar] [CrossRef]
- Bae, S.; Lee, H.J.; Lee, J.S.; Webb, K. Cell-mediated dexamethasone release from semi-IPNs stimulates osteogenic differentiation of encapsulated mesenchymal stem cells. Biomacromolecules 2015, 16, 2757–2765. [Google Scholar] [CrossRef]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 035002. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Alluri, H.; Wiggins-Dohlvik, K.; Davis, M.L.; Huang, J.; Tharakan, B. Blood-brain barrier dysfunction following traumatic brain injury. Metab. Brain Dis. 2015, 30, 1093–1104. [Google Scholar] [CrossRef]
- Thal, S.; Neuhaus, W. The Blood-Brain Barrier as a Target in Traumatic Brain Injury Treatment. Arch. Med. Res. 2014, 45, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Zarruk, J.G.; Greenhalgh, A.D.; David, S. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp. Neurol. 2018, 301, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Moll, A.; Lara, M.; Pomar, J.; Orozco, M.; Frontera, G.; Llompart-Pou, J.A.; Moratinos, L.; González, V.; Ibáñez, J.; Pérez-Bárcena, J. Effects of dexamethasone in traumatic brain injury patients with pericontusional vasogenic edema. Medicine 2020, 99, e22879. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, X.; Wang, L.; Hao, S.; Xu, X.; Niu, F.; He, W.; Liu, B. Dexamethasone impairs neurofunctional recovery in rats following traumatic brain injury by reducing circulating endothelial progenitor cells and angiogenesis. Brain Res. 2019, 1725, 146469. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Y.-P.; Wang, N.; Zhang, J.-N. Dexamethasone exacerbates spatial acquisition deficits after traumatic brain injury in rats. Neurol. Res. 2010, 32, 1097–1102. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, X.; Niu, F.; Mao, X.; Dong, J.; Yang, M.; Gao, F.; Liu, B. Corticosterone Replacement Alleviates Hippocampal Neuronal Apoptosis and Spatial Memory Impairment Induced by Dexamethasone via Promoting Brain Corticosteroid Receptor Rebalance after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 262–272. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Lin, Y.; Tan, T.; Yang, Z.; Dayao, C.; Liu, L.; Jiang, R.; Zhang, J. Impairment of synaptic plasticity in hippocampus is exacerbated by methylprednisolone in a rat model of traumatic brain injury. Brain Res. 2011, 1382, 165–172. [Google Scholar] [CrossRef]
- Agoston, D.V.; Vink, R.; Helmy, A.; Risling, M.; Nelson, D.W.; Prins, M. How to Translate Time: The Temporal Aspects of Rodent and Human Pathobiological Processes in Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1724–1737. [Google Scholar] [CrossRef]
- Rodney, T.; Osier, N.; Gill, J. Pro- and anti-inflammatory biomarkers and traumatic brain injury outcomes: A review. Cytokine 2018, 110, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, J.; Xu, X.; Li, W.; Zhong, R.; Qi, L.; Chen, J.; Cui, G.; Wang, S.; Zheng, Y.; et al. M-CSF, IL-6, and TGF-β promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci. Adv. 2021, 7, eabb6260. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Chang, C.-F.; Goods, B.A.; Hammond, M.D.; Mac Grory, B.; Ai, Y.; Steinschneider, A.F.; Renfroe, S.C.; Askenase, M.H.; McCullough, L.D.; et al. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Investig. 2016, 127, 280–292. [Google Scholar] [CrossRef]
- Lindholm, D.; Castrén, E.; Kiefer, R.; Zafra, F.; Thoenen, H. Transforming growth factor-beta 1 in the rat brain: Increase after injury and inhibition of astrocyte proliferation. J. Cell Biol. 1992, 117, 395–400. [Google Scholar] [CrossRef]
- Patel, R.K.; Prasad, N.; Kuwar, R.; Haldar, D.; Abdul-Muneer, P. Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 2017, 64, 244–258. [Google Scholar] [CrossRef]
- Sulhan, S.; Lyon, K.A.; Shapiro, L.A.; Huang, J.H. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets. J. Neurosci. Res. 2018, 98, 19–28. [Google Scholar] [CrossRef]
- Cao, T.; Thomas, T.; Ziebell, J.; Pauly, J.; Lifshitz, J. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience 2012, 225, 65–75. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Woodcock, T.; Morganti-Kossmann, M.C. The Role of Markers of Inflammation in Traumatic Brain Injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Lee, I.-N.; Cheng, W.-C.; Chung, C.-Y.; Lee, M.-H.; Lin, M.H.-C.; Kuo, C.-H.; Weng, H.-H.; Yang, J.-T. Dexamethasone reduces brain cell apoptosis and inhibits inflammatory response in rats with intracerebral hemorrhage. J. Neurosci. Res. 2014, 93, 178–188. [Google Scholar] [CrossRef]
- Drakulić, D.; Velickovic, N.; Stanojlovic, M.; Grkovic, I.; Mitrovic, N.; Lavrnja, I.; Horvat, A. Low-Dose Dexamethasone Treatment Promotes the Pro-Survival Signalling Pathway in the Adult Rat Prefrontal Cortex. J. Neuroendocr. 2013, 25, 605–616. [Google Scholar] [CrossRef]
- Hoang, K.N.; Dinh, C.T.; Bas, E.; Chen, S.; Eshraghi, A.A.; Van De Water, T.R. Dexamethasone treatment of naïve organ of Corti explants alters the expression pattern of apoptosis-related genes. Brain Res. 2009, 1301, 1–8. [Google Scholar] [CrossRef]
- Sur, P.; Sribnick, E.A.; Patel, S.J.; Ray, S.K.; Banik, N.L. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia 2005, 50, 160–167. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Liang, G.-B.; Xue, Y.-X.; Liu, Y.-H. Effects of combination treatment of dexamethasone and melatonin on brain injury in intracerebral hemorrhage model in rats. Brain Res. 2009, 1264, 98–103. [Google Scholar] [CrossRef]
- Wada, T.; Pippin, J.W.; Marshall, C.B.; Griffin, S.V.; Shankland, S.J. Dexamethasone Prevents Podocyte Apoptosis Induced by Puromycin Aminonucleoside: Role of p53 and Bcl-2–Related Family Proteins. J. Am. Soc. Nephrol. 2005, 16, 2615–2625. [Google Scholar] [CrossRef]
- Sun, W.-H.; He, F.; Zhang, N.-N.; Zhao, Z.-A.; Chen, H.-S. Time dependent neuroprotection of dexamethasone in experimental focal cerebral ischemia: The involvement of NF-κB pathways. Brain Res. 2018, 1701, 237–245. [Google Scholar] [CrossRef]
- Cho, E.; Kutty, J.K.; Datar, K.; Lee, J.S.; Vyavahare, N.R.; Webb, K. A novel synthetic route for the preparation of hydrolytically degradable synthetic hydrogels. J. Biomed. Mater. Res. Part A 2008, 90A, 1073–1082. [Google Scholar] [CrossRef]



| Assessment | 7 Days | 14 Days |
|---|---|---|
| Lesion volume |  |  |
| Macrophage/microglia infiltration |  |  |
| Reactive gliosis (GFAP) |  | n/a |
| Apoptosis |  |  |
| Inflammatory gene expression |  |  |
| Neuronal cell preservation |  |  |
| Motor function |  | n/a |
| Cognitive Function | n/a |  |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | GeneBank No. | Product Size (bp) |
|---|---|---|---|---|
| IL1β | AGCTCTCCACCTCAATGGAC | GCCGTCTTTCATCACACAGG | NM_031512.2 | 148 |
| TGFβ1 | GTCAACTGTGGAGCAACACG | AGCCACTCAGGCGTATCAG | NM_021578.2 | 105 |
| TNFα | AAGCATGATCCGAGATGTGG | AGGAATGAGAAGAGGTGAGG | NM_012675.3 | 105 |
| IFN | TTGAAAGACAACCAGGCCATC | GCTTTGTGCTGGATCTGTGG | NM_138880.2 | 149 |
| IL10 | CAATAACTGCACCCACTTCCC | GTCAGCAGTATGTTGTCCAGC | NM_012854.2 | 120 |
| BAX | GGATCGAGCAGAGAGGATGG | CAATTCGCCTGAGACACTCG | NM_017059.2 | 104 |
| BCL2 | GGAGGATTGTGGCCTTCTTTG | TCAGGTACTCAGTCATCCACAG | NM_016993.1 | 111 |
| GAPDH | ATGGCCTTCCGTGTTCCTAC | AACTTTGGCATCGTGGAAGG | NM_107008.4 | 111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macks, C.; Jeong, D.; Bae, S.; Webb, K.; Lee, J.S. Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. Int. J. Mol. Sci. 2022, 23, 11153. https://doi.org/10.3390/ijms231911153
Macks C, Jeong D, Bae S, Webb K, Lee JS. Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. International Journal of Molecular Sciences. 2022; 23(19):11153. https://doi.org/10.3390/ijms231911153
Chicago/Turabian StyleMacks, Christian, Daun Jeong, Sooneon Bae, Ken Webb, and Jeoung Soo Lee. 2022. "Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model" International Journal of Molecular Sciences 23, no. 19: 11153. https://doi.org/10.3390/ijms231911153
APA StyleMacks, C., Jeong, D., Bae, S., Webb, K., & Lee, J. S. (2022). Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. International Journal of Molecular Sciences, 23(19), 11153. https://doi.org/10.3390/ijms231911153








