Influence of Doping-Ion-Type on the Characteristics of Al2O3-Based Nanocomposites and Their Capabilities of Removing Indigo Carmine from Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Adsorption of IGC
2.3. Kinetics of IGC Adsorption
2.4. Adsorption Isotherms
2.5. Thermodynamic
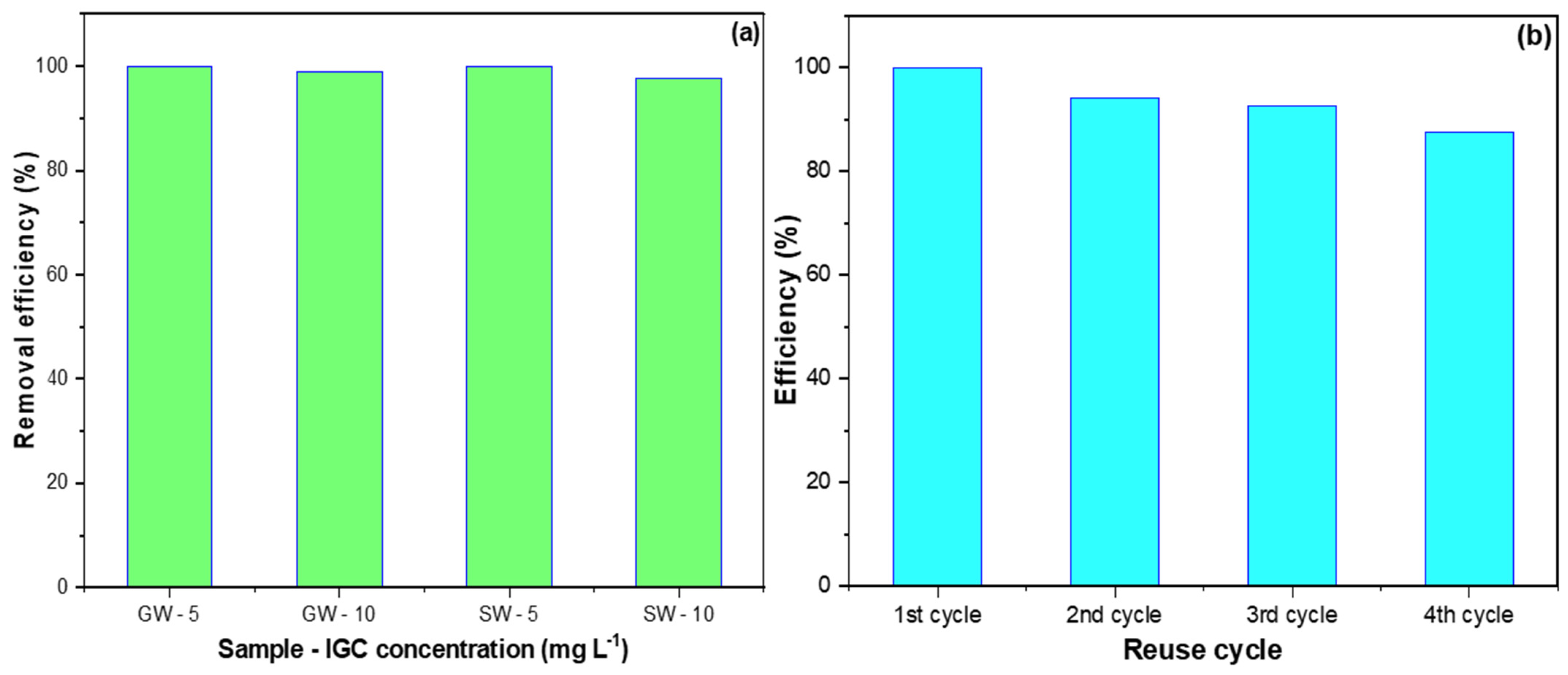
2.6. Application to Natural Water Samples and Regeneration of Al2O3-NiO Nanocomposite
3. Experimental
3.1. Materials
3.2. Synthesis of Al2O3 Nanoparticle and Its Composites
3.3. Characterization of Al2O3 Nanoparticle and Its Composites
3.4. Adsorption of IGC on Prepared Nanomaterials
3.5. Adsorption Equilibria
3.6. Application to Natural Water Samples
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elamin, M.R.; Ibnaouf, K.H.; Elamin, N.Y.; Adam, F.A.; Alolayan, A.H.; Abdulkhair, B.Y. Spontaneous Adsorption and Efficient Photodegradation of Indigo Carmine under Visible Light by Bismuth Oxyiodide Nanoparticles Fabricated Entirely at Room Temperature. Inorganics 2022, 10, 65. [Google Scholar] [CrossRef]
- Rahbar-Shamskar, K.; Azar, P.A.; Rashidi, A.; Baniyaghoob, S.; Yousefi, M. Synthesis of micro/mesoporous carbon adsorbents by in-situ fast pyrolysis of reed for recovering gasoline vapor. J. Clean. Prod. 2020, 259, 120832. [Google Scholar] [CrossRef]
- Silva, A.R.; Cavaleiro, A.J.; Soares, O.S.G.; Braga, C.S.; Salvador, A.F.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Detoxification of ciprofloxacin in an anaerobic bioprocess supplemented with magnetic carbon nanotubes: Contribution of adsorption and biodegradation mechanisms. Int. J. Mol. Sci. 2021, 22, 2932. [Google Scholar] [CrossRef] [PubMed]
- Bożęcka, A.; Orlof-Naturalna, M.; Kopeć, M. Methods of Dyes Removal from Aqueous Environment. J. Ecol. Eng. 2021, 22, 111–118. [Google Scholar] [CrossRef]
- Ho, S.; Protection, E. Removal of Dyes from Wastewater by Adsorption onto Activated Carbon: Mini Review. J. Geosci. Environ. Prot. 2020, 8, 120. [Google Scholar] [CrossRef]
- Sivaprakash, S.; Kumar, P.S.; Krishna, S. Adsorption study of various dyes on Activated Carbon Fe3O4 Magnetic Nano Composite. Int. J. Appl. Chem. 2017, 13, 255–266. [Google Scholar]
- Dastgerdi, Z.H.; Meshkat, S.S.; Esrafili, M.D. Enhanced adsorptive removal of Indigo carmine dye performance by functionalized carbon nanotubes based adsorbents from aqueous solution: Equilibrium, kinetic, and DFT study. J. Nanostruct. Chem. 2019, 9, 323–334. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Labiadh, L.; Barbucci, A.; Carpanese, M.P.; Gadri, A.; Ammar, S.; Panizza, M. Direct and indirect electrochemical oxidation of Indigo Carmine using PbO2 and TiRuSnO2. J. Solid State Electrochem. 2017, 21, 2167–2175. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Gemeay, A.H.; Aboelfetoh, E.F.; El-Sharkawy, R.G. Immobilization of green synthesized silver nanoparticles onto amino-functionalized silica and their application for indigo carmine dye removal. Water Air Soil Pollut. 2018, 229, 16. [Google Scholar] [CrossRef]
- Emik, S.; Işık, S.; Yıldırım, E. Simultaneous removal of cationic and anionic dyes from binary solutions using carboxymethyl chitosan based IPN Type resin. J. Polym. Environ. 2021, 29, 1963–1977. [Google Scholar] [CrossRef]
- Zein, R.; Hevira, L.; Fauzia, S.; Ighalo, J.O. The improvement of indigo carmine dye adsorption by Terminalia catappa shell modified with broiler egg white. Biomass Convers. Biorefin. 2022, 1–18. [Google Scholar] [CrossRef]
- Topare, N.S.; Bokil, S.A. Adsorption of textile industry effluent in a fixed bed column using activated carbon prepared from agro-waste materials. Mater. Today Proc. 2021, 43, 530–534. [Google Scholar] [CrossRef]
- Yurtsever, A.; Basaran, E.; Ucar, D.; Sahinkaya, E. Self-forming dynamic membrane bioreactor for textile industry wastewater treatment. Sci. Total Environ. 2021, 751, 141572. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, B.; Yue, Q.; Guo, K. Flocculation performance of papermaking sludge-based flocculants in different dye wastewater treatment: Comparison with commercial lignin and coagulants. Chemosphere 2021, 262, 128416. [Google Scholar] [CrossRef]
- Othman, M.H.D.; Adam, M.R.; Kamaludin, R.; Ismail, N.J.; Rahman, M.A.; Jaafar, J. Advanced Membrane Technology for Textile Wastewater Treatment. In Membrane Technology Enhancement for Environmental Protection and Sustainable Industrial Growth; Springer: Cham, Switzerland, 2021; pp. 91–108. [Google Scholar]
- Ahsan, M.A.; Fernandez-Delgado, O.; Deemer, E.; Wang, H.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Carbonization of Co-BDC MOF results in magnetic C@ Co nanoparticles that catalyze the reduction of methyl orange and 4-nitrophenol in water. J. Mol. Liq. 2019, 290, 111059. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Deemer, E.; Fernandez-Delgado, O.; Wang, H.; Curry, M.L.; El-Gendy, A.A.; Noveron, J.C. Fe nanoparticles encapsulated in MOF-derived carbon for the reduction of 4-nitrophenol and methyl orange in water. Catal. Commun. 2019, 130, 105753. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Ultrafast catalytic reduction of environmental pollutants in water via MOF-derived magnetic Ni and Cu nanoparticles encapsulated in porous carbon. Appl. Surf. Sci. 2019, 497, 143608. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Elzupir, A.O. Removal of ciprofloxacin and indigo carmine from water by carbon nanotubes fabricated from a low-cost precursor: Solution parameters and recyclability. Ain Shams Eng. J. 2022, 101844. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Harrache, Z.; Abbas, M.; Aksil, T.; Trari, M. Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem. J. 2019, 144, 180–189. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the fate and removal of antibiotics in engineered biological treatment systems: A critical review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Kassim, M.A. Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Energies 2021, 14, 8421. [Google Scholar] [CrossRef]
- Almufarij, R.S.; Abdulkhair, B.Y.; Salih, M.; Aldosari, H.; Aldayel, N.W. Optimization, Nature, and Mechanism Investigations for the Adsorption of Ciprofloxacin and Malachite Green onto Carbon Nanoparticles Derived from Low-Cost Precursor via a Green Route. Molecules 2022, 27, 4577. [Google Scholar] [CrossRef]
- Ghoniem, M.G.; Ali, F.A.M.; Abdulkhair, B.Y.; Elamin, M.R.A.; Alqahtani, A.M.; Rahali, S.; Ben Aissa, M.A. Highly selective removal of cationic dyes from wastewater by MgO nanorods. Nanomaterials 2022, 12, 1023. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Algethami, F.K.; Khezami, L. Linear and nonlinear investigations for the adsorption of paracetamol and metformin from water on acid-treated clay. Sci. Rep. 2021, 11, 13606. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Elzupir, A.O. Insight to aspirin sorption behavior on carbon nanotubes from aqueous solution: Thermodynamics, kinetics, influence of functionalization and solution parameters. Sci. Rep. 2019, 9, 12795. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Piskunov, S.; Lisovski, O.; Bocharov, D.; Evarestov, R.A. Doped 1D Nanostructures of Transition-metal Oxides: First-principles Evaluation of Photocatalytic Suitability. Isr. J. Chem. 2017, 57, 461–476. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Gao, X.; Ge, Z.; Zhu, G.; Wang, Z.; Ashok, J.; Kawi, S. Anti-coking and anti-sintering Ni/Al2O3 catalysts in the dry reforming of methane: Recent progress and prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y.; Fu, T.; Hussain, S.; Saad Algarni, T.; Wang, J.; Zhang, X.; Ali, S. Effects of Cr2O3 content on microstructure and mechanical properties of Al2O3 matrix composites. Coatings 2021, 11, 234. [Google Scholar] [CrossRef]
- Khan, M.; Janjua, N.K.; Khan, S.; Qazi, I.; Ali, S.; Saad Algarni, T. Electro-oxidation of ammonia at novel Ag2O−PrO2/γ-Al2O3 catalysts. Coatings 2021, 11, 257. [Google Scholar] [CrossRef]
- Khan, S.; Shah, S.S.; Anjum, M.A.R.; Khan, M.R.; Janjua, N.K. Electro-oxidation of ammonia over copper oxide impregnated γ-Al2O3 nanocatalysts. Coatings 2021, 11, 313. [Google Scholar] [CrossRef]
- Yeşiltepe Özcelik, D.; Ebin, B.; Stopic, S.; Gürmen, S.; Friedrich, B. Mixed oxides NiO/ZnO/Al2O3 synthesized in a single step via ultrasonic spray pyrolysis (USP) method. Metals 2022, 12, 73. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Horiguchi, J.; Kobayashi, S.; Yamazaki, Y.; Omata, K.; Nagao, D.; Konno, M.; Yamada, M. Effect of NiO content in mesoporous NiO–Al2O3 catalysts for high pressure partial oxidation of methane to syngas. Appl. Catal. A Gen. 2011, 395, 129–137. [Google Scholar] [CrossRef]
- Liu, H.; Ning, G.; Gan, Z.; Lin, Y. A simple procedure to prepare spherical α-alumina powders. Mater. Res. Bull. 2009, 44, 785–788. [Google Scholar] [CrossRef]
- Di, S.; Gong, L.; Zhou, B. Physics. Precipitated synthesis of Al2O3-ZnO nanorod for high-performance symmetrical supercapacitors. Mater. Chem. Phys. 2020, 253, 123289. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889. [Google Scholar]
- Rajeswari, V.D.; Khalifa, A.S.; Elfasakhany, A.; Badruddin, I.A.; Kamangar, S.; Brindhadevi, K.J.A.N. Green and ecofriendly synthesis of cobalt oxide nanoparticles using Phoenix dactylifera L: Antimicrobial and photocatalytic activity. Appl. Nanosci. 2021, 1–9. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Prabu, P.; Nagarajan, S.; Kim, C.; Park, J.; Kim, H.Y. Synthesis of nickel oxide nanoparticles using nickel acetate and poly (vinyl acetate) precursor. Mater. Sci. Eng. B 2006, 128, 111–114. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2, 4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, R.; Samadder, S.R. Low cost and easy synthesis of aluminium oxide nanoparticles for arsenite removal from groundwater: A complete batch study. J. Mol. Liq. 2018, 250, 192–201. [Google Scholar] [CrossRef]
- Nallusamy, S.; Sujatha, K. Experimental analysis of nanoparticles with cobalt oxide synthesized by coprecipitation method on electrochemical biosensor using FTIR and TEM. Mater. Today Proc. 2021, 37, 728–732. [Google Scholar] [CrossRef]
- Sun, S.; Liang, F.; Tang, L.; Wu, J.; Ma, C. Exploitation. Microstructural investigation of gas shale in Longmaxi formation, Lower Silurian, NE Sichuan basin, China. Energy Explor. Exploit. 2017, 35, 406–429. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Huang, X.-L. Effect of temperature and salinity on phosphate sorption on marine sediments. Environ. Sci. Technol. 2011, 45, 6831–6837. [Google Scholar] [CrossRef]
- Flower, H.; Rains, M.; Lewis, D.; Zhang, J.-Z.; Price, R. Saltwater intrusion as potential driver of phosphorus release from limestone bedrock in a coastal aquifer. Estuar. Coast. Shelf Sci. 2017, 184, 166–176. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem. Eng. Res. Des. 2015, 97, 135–144. [Google Scholar] [CrossRef]
- An, B.J.P. Cu (II) and As (V) adsorption kinetic characteristic of the multifunctional amino groups in chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Hameed, B.; El-Khaiary, M.I. Malachite green adsorption by rattan sawdust: Isotherm, kinetic and mechanism modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single-and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Jain, S.N.; Shaikh, Z.; Mane, V.S.; Vishnoi, S.; Mawal, V.N.; Patel, O.R.; Bhandari, P.S.; Gaikwad, M.S. Nonlinear regression approach for acid dye remediation using activated adsorbent: Kinetic, isotherm, thermodynamic and reusability studies. Microchem. J. 2019, 148, 605–615. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; Volume 2. [Google Scholar]
- Darryle, C.M.; Acayanka, E.; Takam, B.; Line, L.N.; Kamgang, G.Y.; Laminsi, S.; Sellaoui, L.; Bonilla-Petriciolet, A. Influence of plasma-based surface functionalization of palm fibers on the adsorption of diclofenac from water: Experiments, thermodynamics and removal mechanism. J. Water Process Eng. 2021, 43, 102254. [Google Scholar] [CrossRef]
- Wang, N.; Han, Y.; Li, S. Adsorption characteristic of Cr (VI) onto different activated coal fly ashes: Kinetics, thermodynamic, application feasibility, and error analysis. Water Air Soil Pollut. 2019, 230, 1–13. [Google Scholar] [CrossRef]

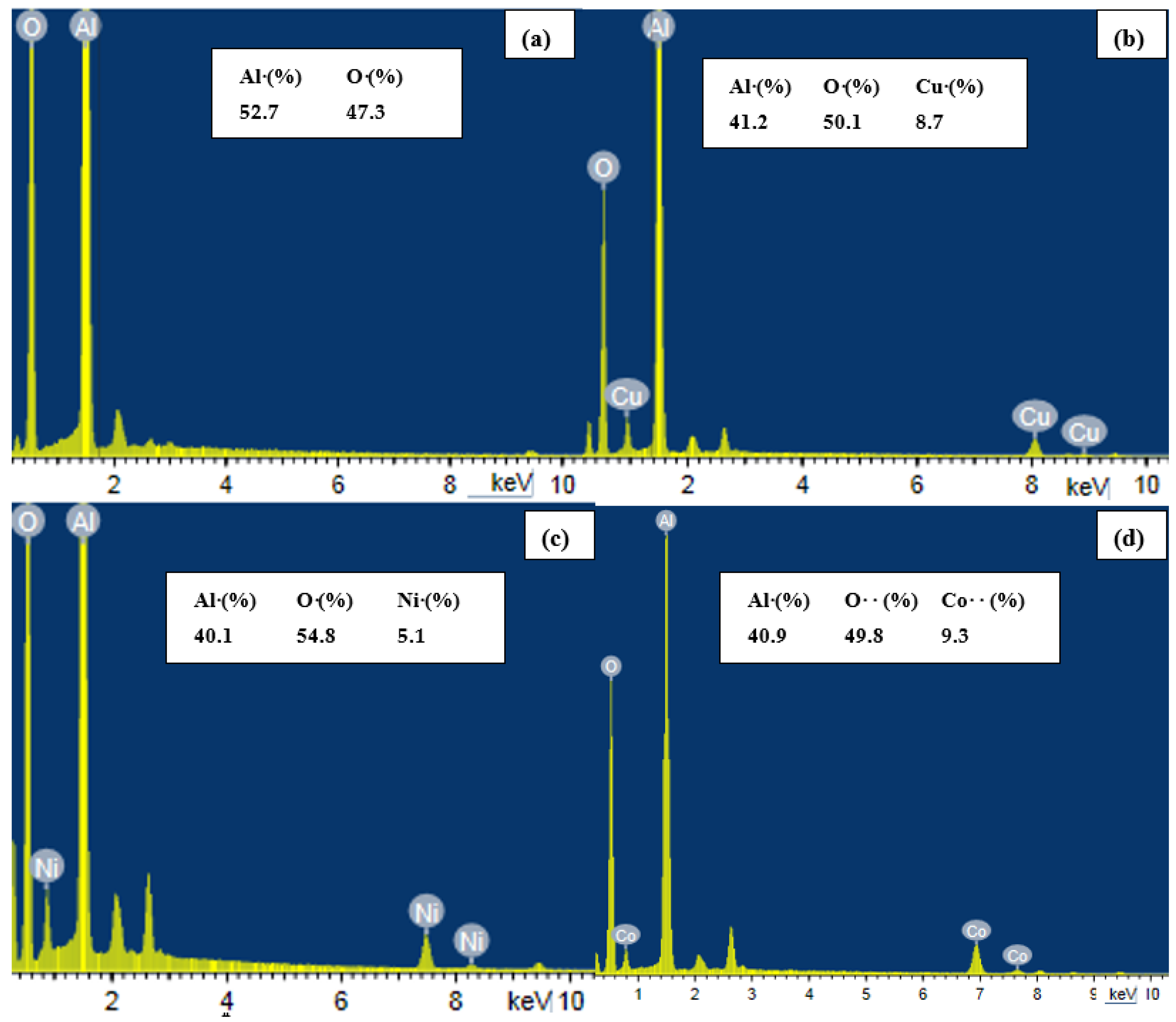
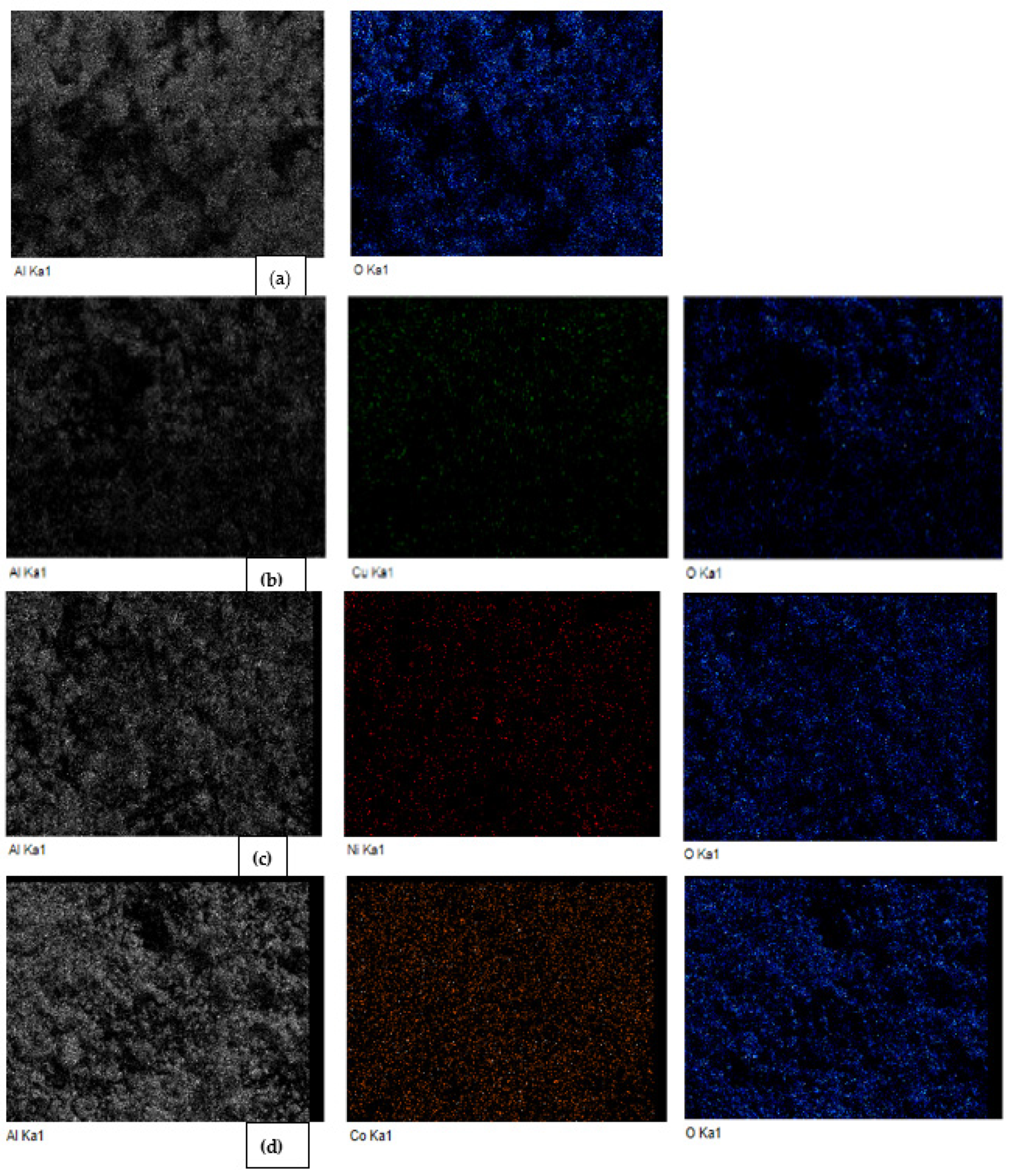

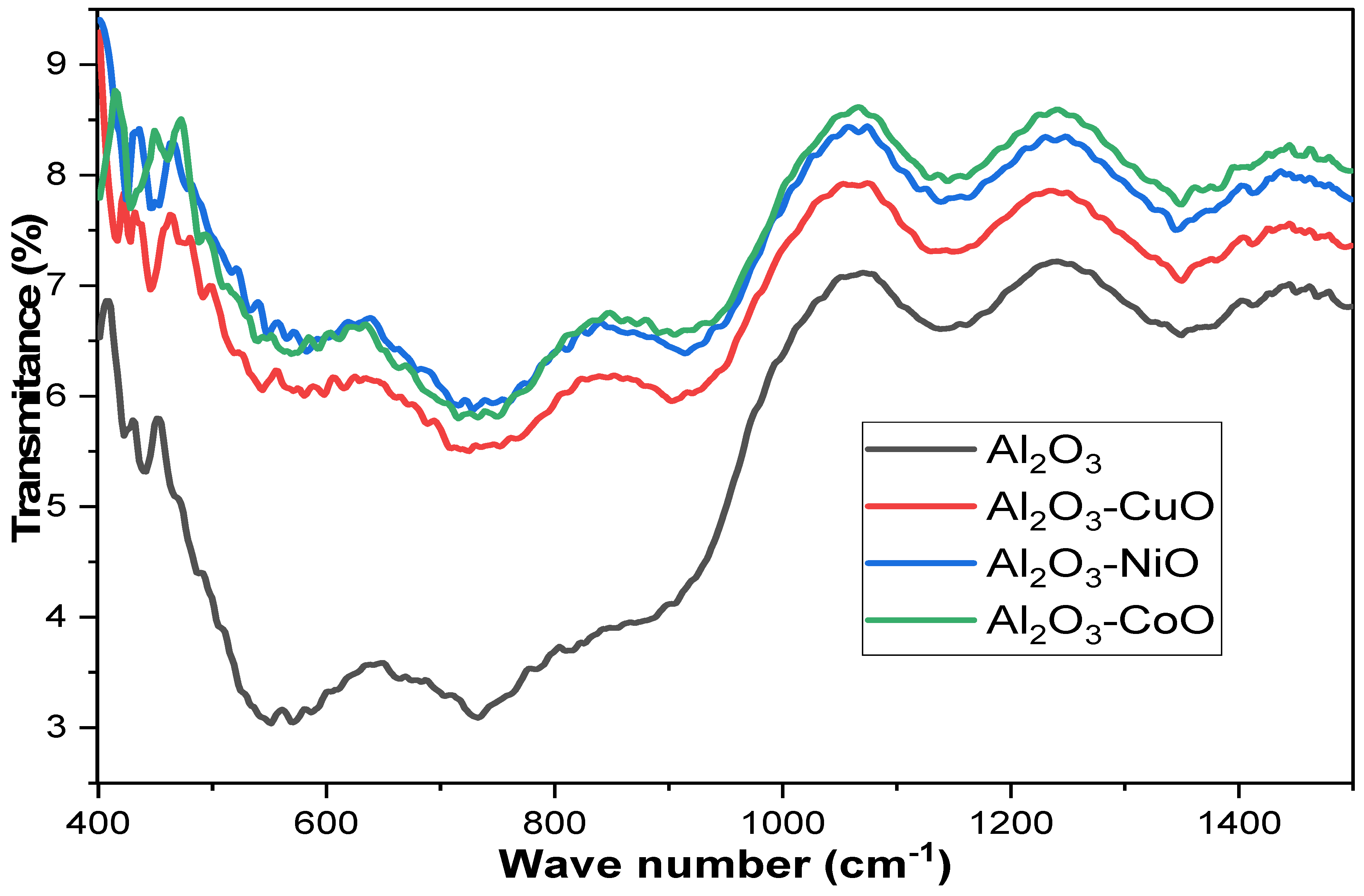
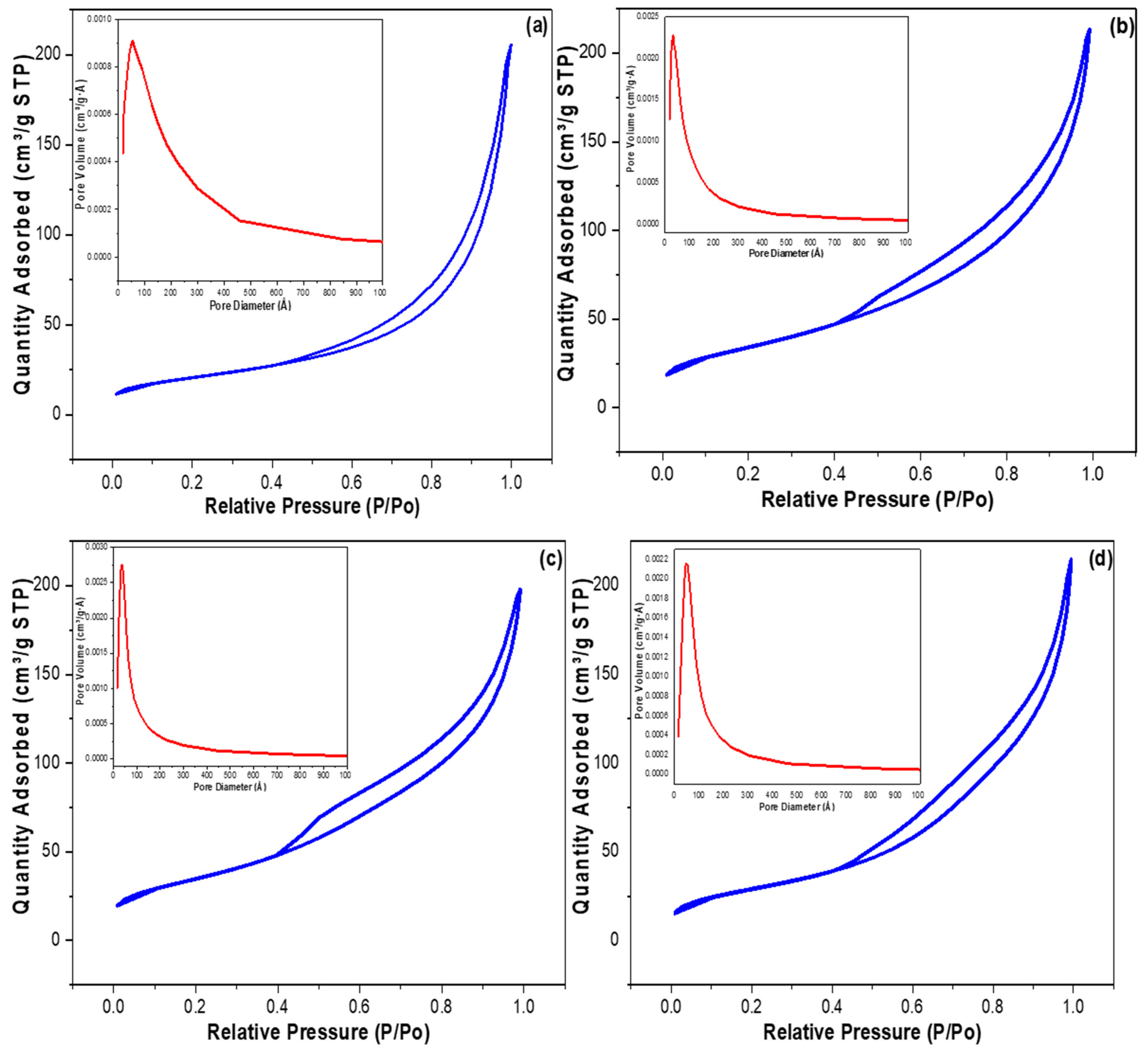

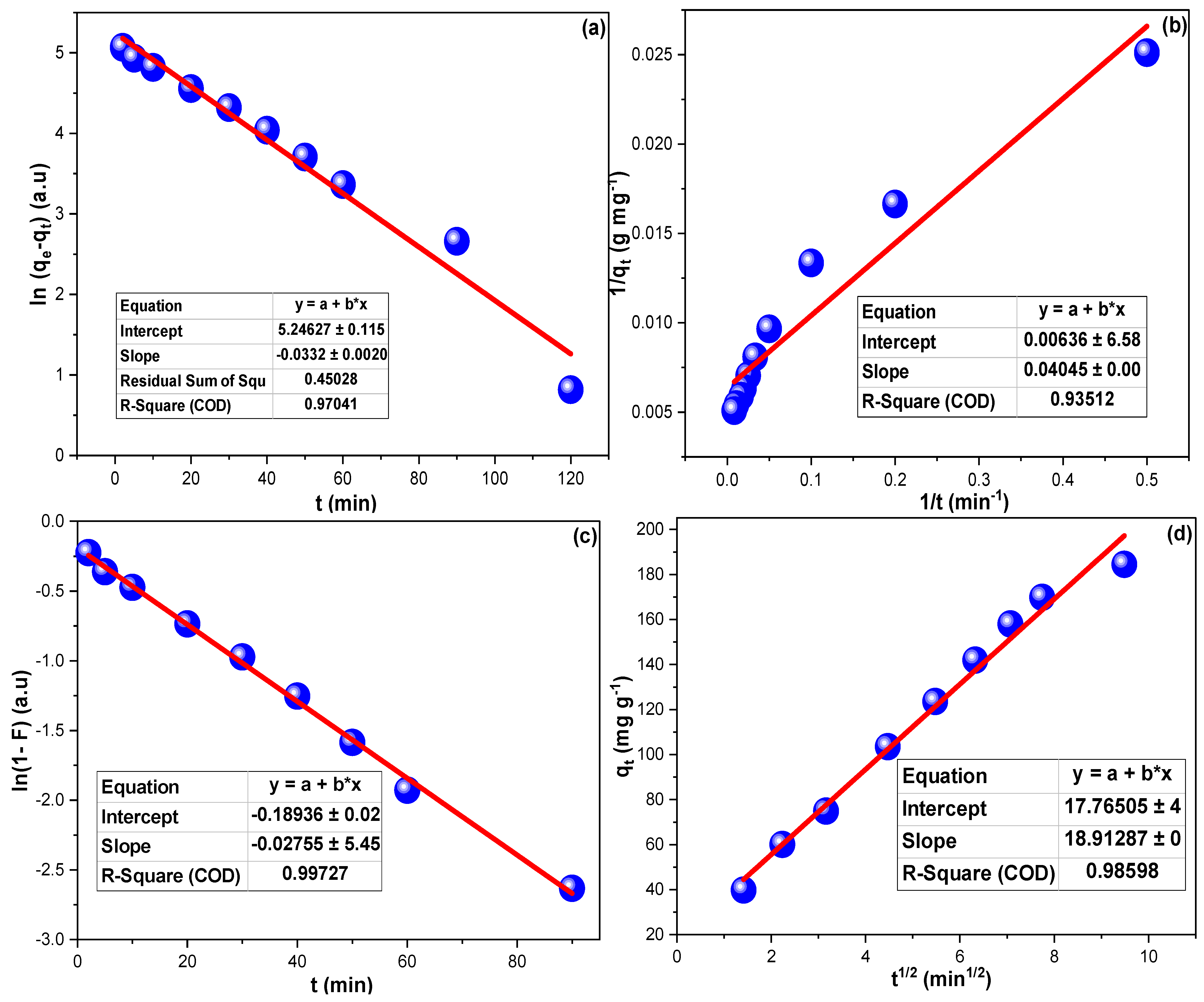


| Parameter | Al2O3 | Al2O3-CuO | Al2O3-NiO | Al2O3-CoO |
|---|---|---|---|---|
| BET Surface Area (m2 g−1) | 74.709 | 123.984 | 125.636 | 105.653 |
| average pore diameter (Å) | 75.301 | 95.088 | 137.949 | 84.152 |
| average pore volume (cm3 g−1) | 0.310 | 0.331 | 0.335 | 0.319 |
| Adsorption Isotherms | |||||
|---|---|---|---|---|---|
| Langmuir | Freundlich | ||||
| R2 (a.u.) | KL (L mg−1) | qm (mg g−1) | R2(a.u.) | Kf (L mg−1) | n−1 (a.u.) |
| 0.932 | 265.024 | 0.178 | 0.910 | 54.307 | 0.365 |
| Thermodynamic parameters | |||||
| Fed conc. (mg L−1) | ΔH° (kJmol−1) | ΔS° (kJmol−1) | ΔG° (kJmol−1) 298 K | ΔG° (kJmol−1) 308 K | ΔG° (kJmol−1) 318 K |
| 25 | 84.369 | 0.304 | −6.372 | −10.940 | −15.507 |
| 50 | 55.616 | 0.211 | −7.214 | −10.377 | −13.539 |
| 100 | 98.857 | 0.342 | −2.919 | −8.041 | −13.164 |
| 200 | 66.112 | 0.225 | −0.816 | −4.184 | −7.553 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, F.A. Influence of Doping-Ion-Type on the Characteristics of Al2O3-Based Nanocomposites and Their Capabilities of Removing Indigo Carmine from Water. Inorganics 2022, 10, 144. https://doi.org/10.3390/inorganics10090144
Adam FA. Influence of Doping-Ion-Type on the Characteristics of Al2O3-Based Nanocomposites and Their Capabilities of Removing Indigo Carmine from Water. Inorganics. 2022; 10(9):144. https://doi.org/10.3390/inorganics10090144
Chicago/Turabian StyleAdam, Fatima A. 2022. "Influence of Doping-Ion-Type on the Characteristics of Al2O3-Based Nanocomposites and Their Capabilities of Removing Indigo Carmine from Water" Inorganics 10, no. 9: 144. https://doi.org/10.3390/inorganics10090144
APA StyleAdam, F. A. (2022). Influence of Doping-Ion-Type on the Characteristics of Al2O3-Based Nanocomposites and Their Capabilities of Removing Indigo Carmine from Water. Inorganics, 10(9), 144. https://doi.org/10.3390/inorganics10090144




