Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats
Abstract
1. Introduction
2. Results
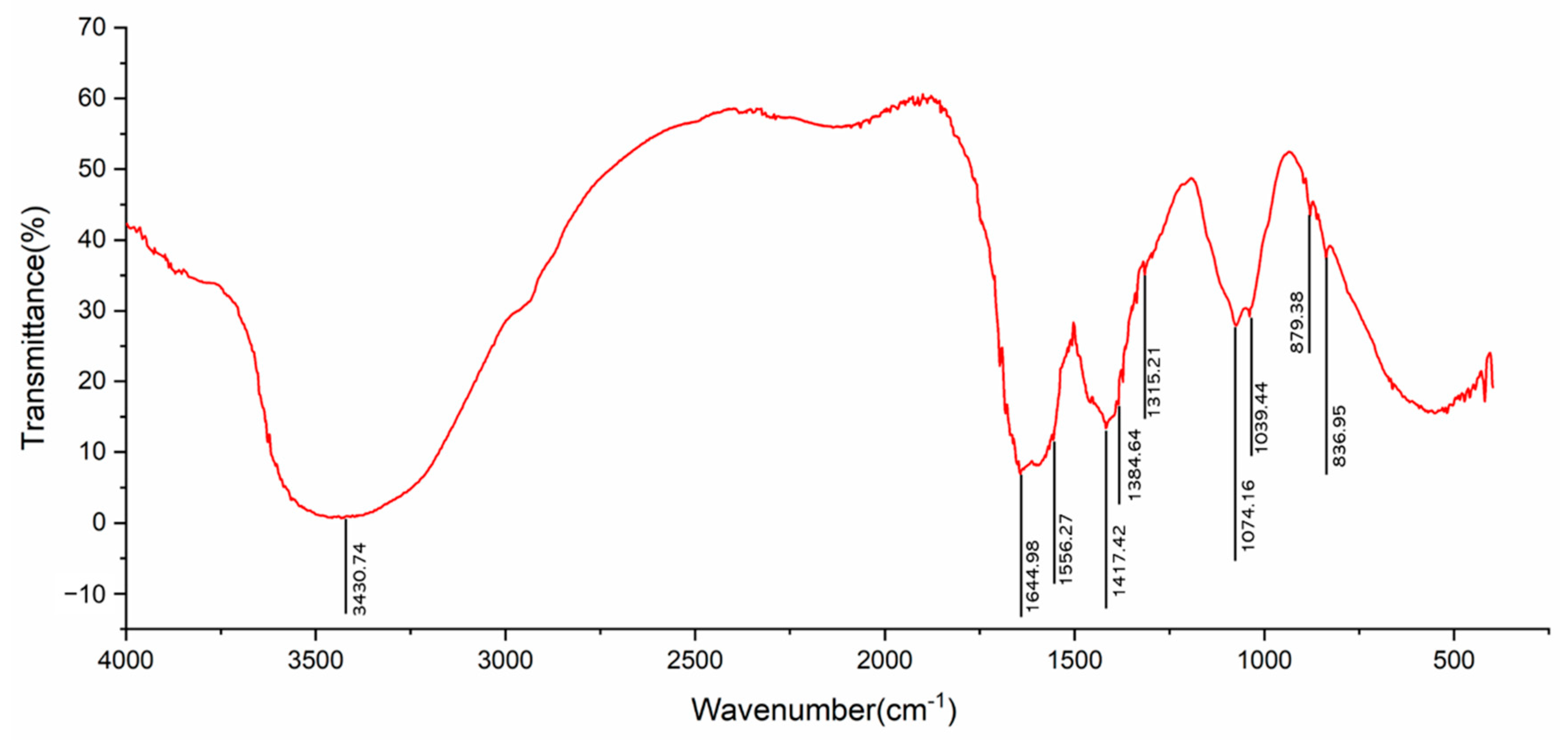
2.1. FT-IR Spectrum
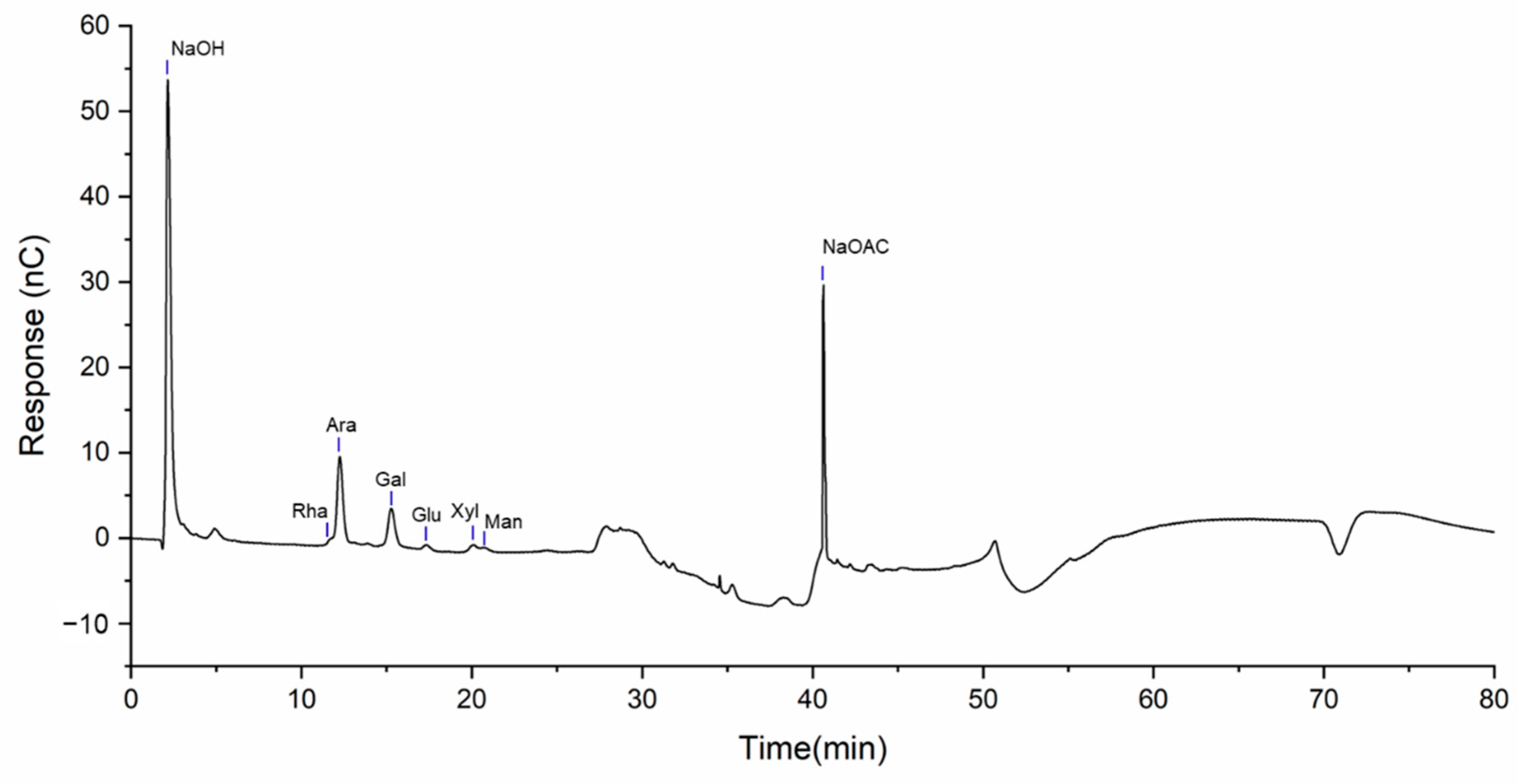
2.2. Monosaccharide Composition Analysis
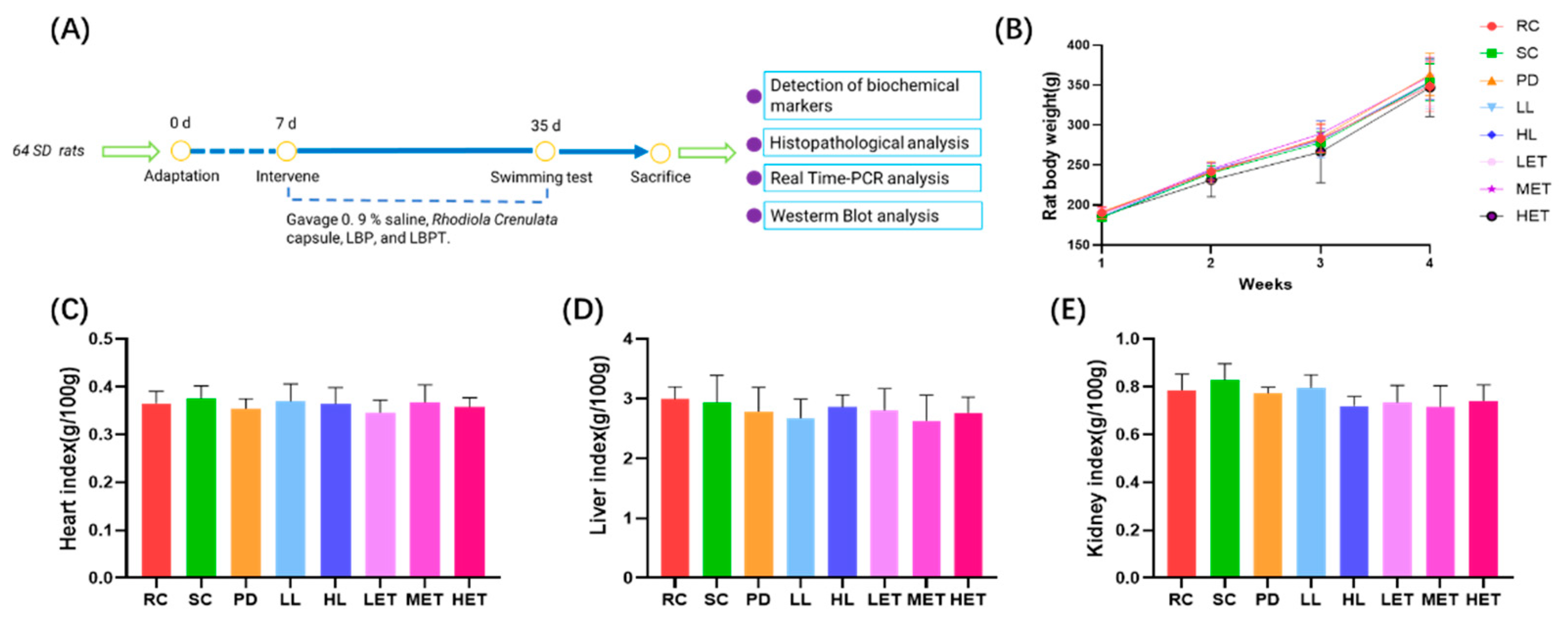
2.3. Effects of LBP and LBPT on Body Weight and Organ Index in Rats
2.4. Effects of LBP and LBPT on Body Weight and Organ Index in Rats
2.5. Biochemical Parameters of LBP and LBPT in Improving Exercise Fatigue
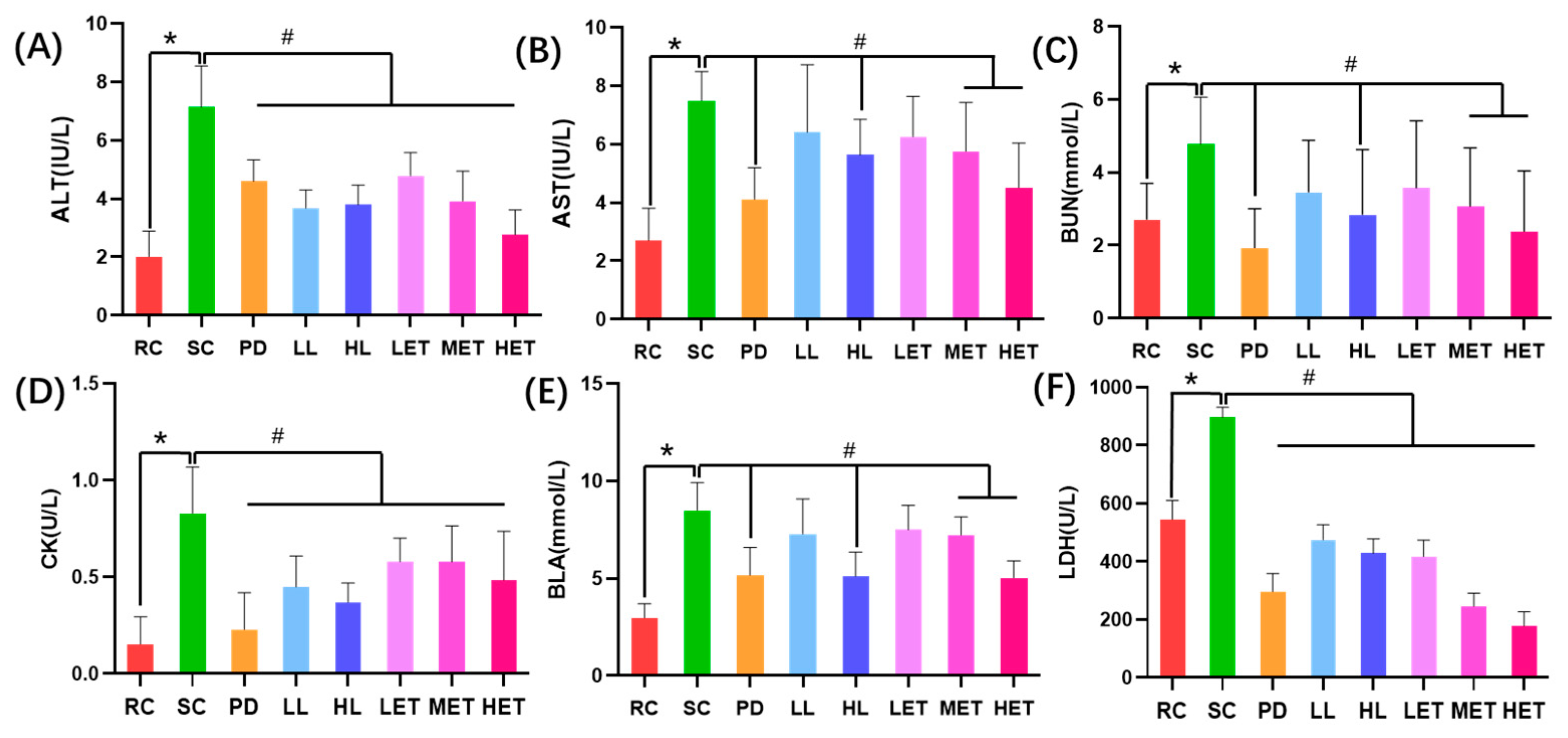
2.5.1. The Effects of LBP and LBPT on Serum Biochemical Parameters in Fatigue Rats
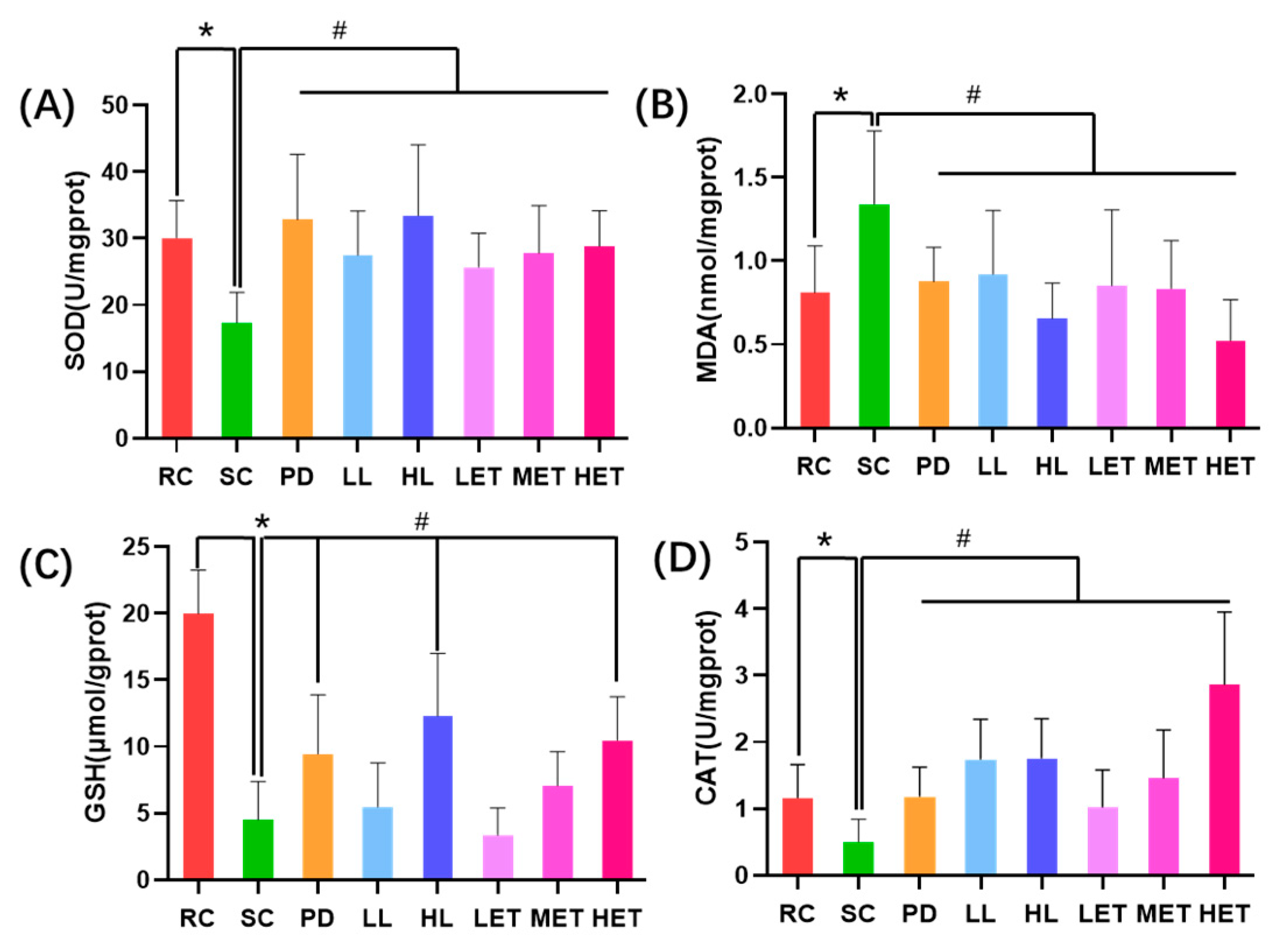
2.5.2. Regulation of LBP and LBPT on Muscle Tissue Oxidative Stress in Fatigue Rats
2.5.3. Regulation of LBP and LBPT on Energy Metabolism in Fatigue Rats
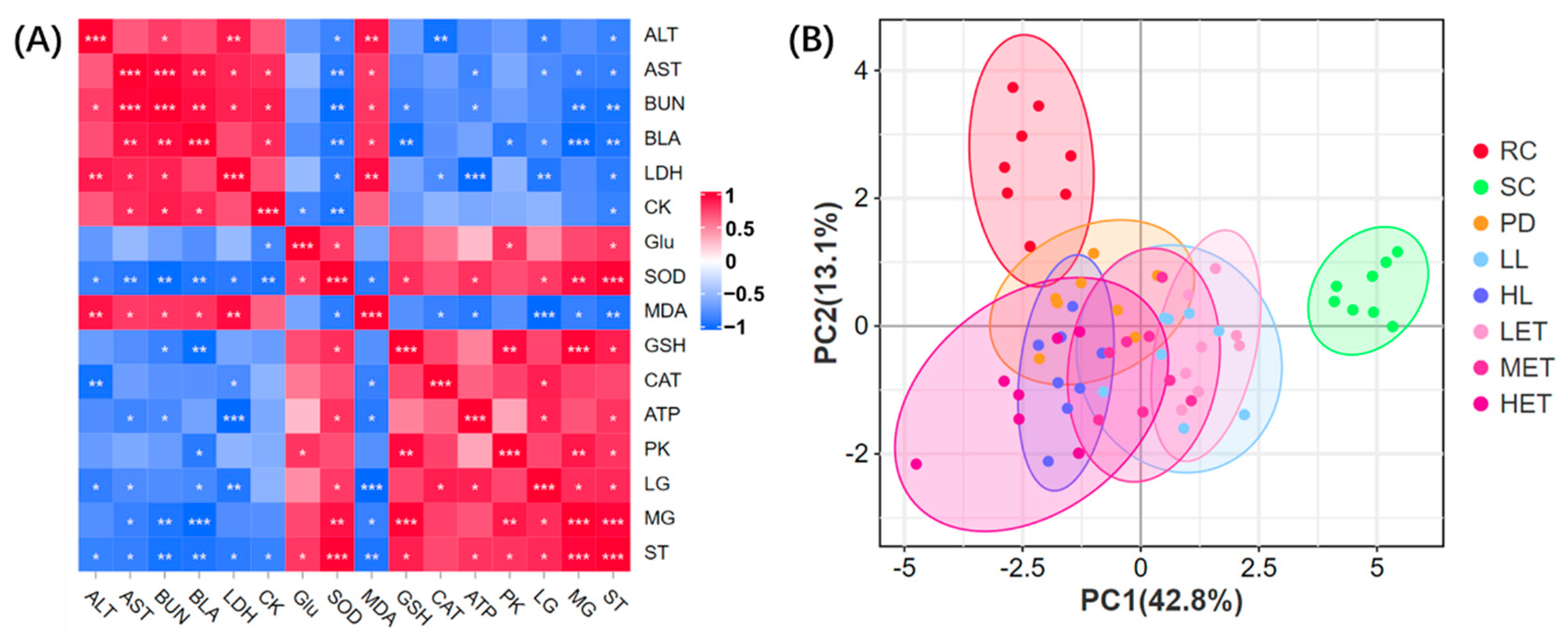
2.5.4. Correlation Analysis and PCA Analysis of Biochemical Parameters
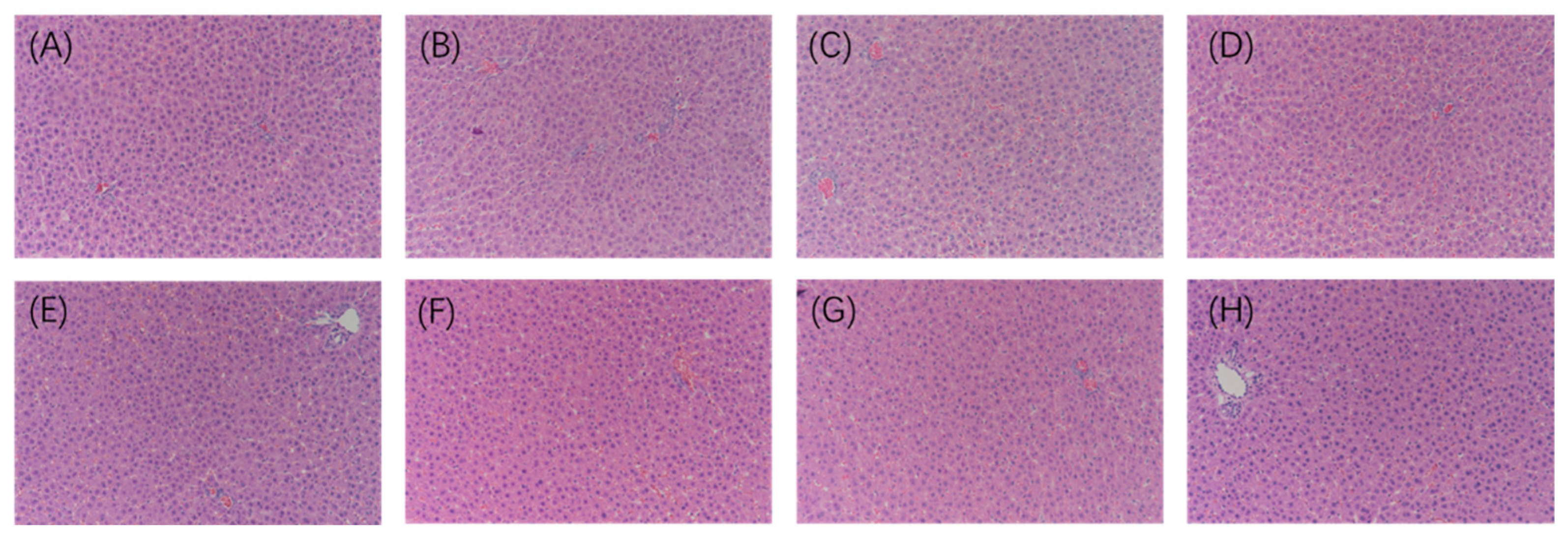
2.6. Histopathological Analysis
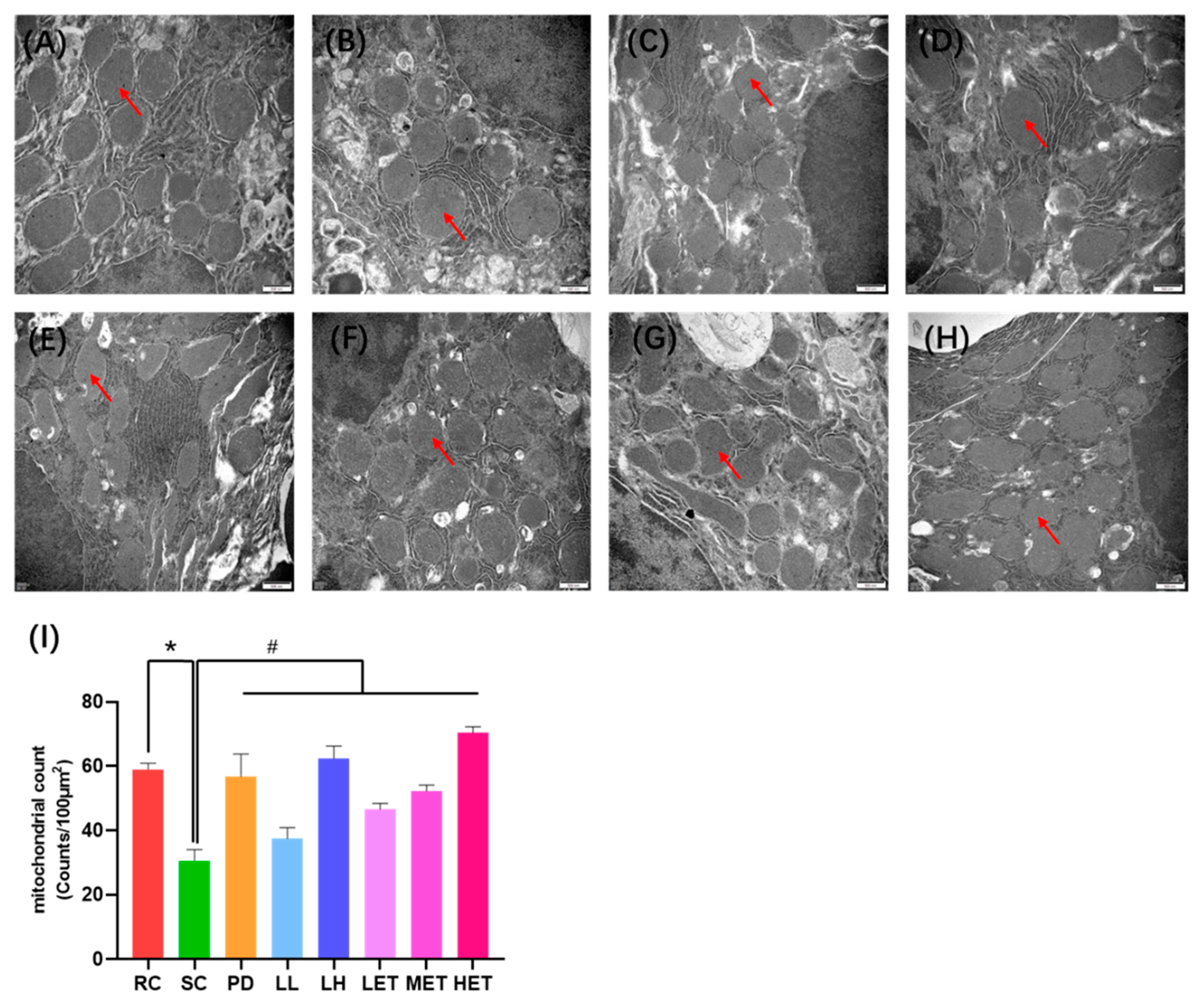
2.7. Transmission Electron Microscopy Analysis
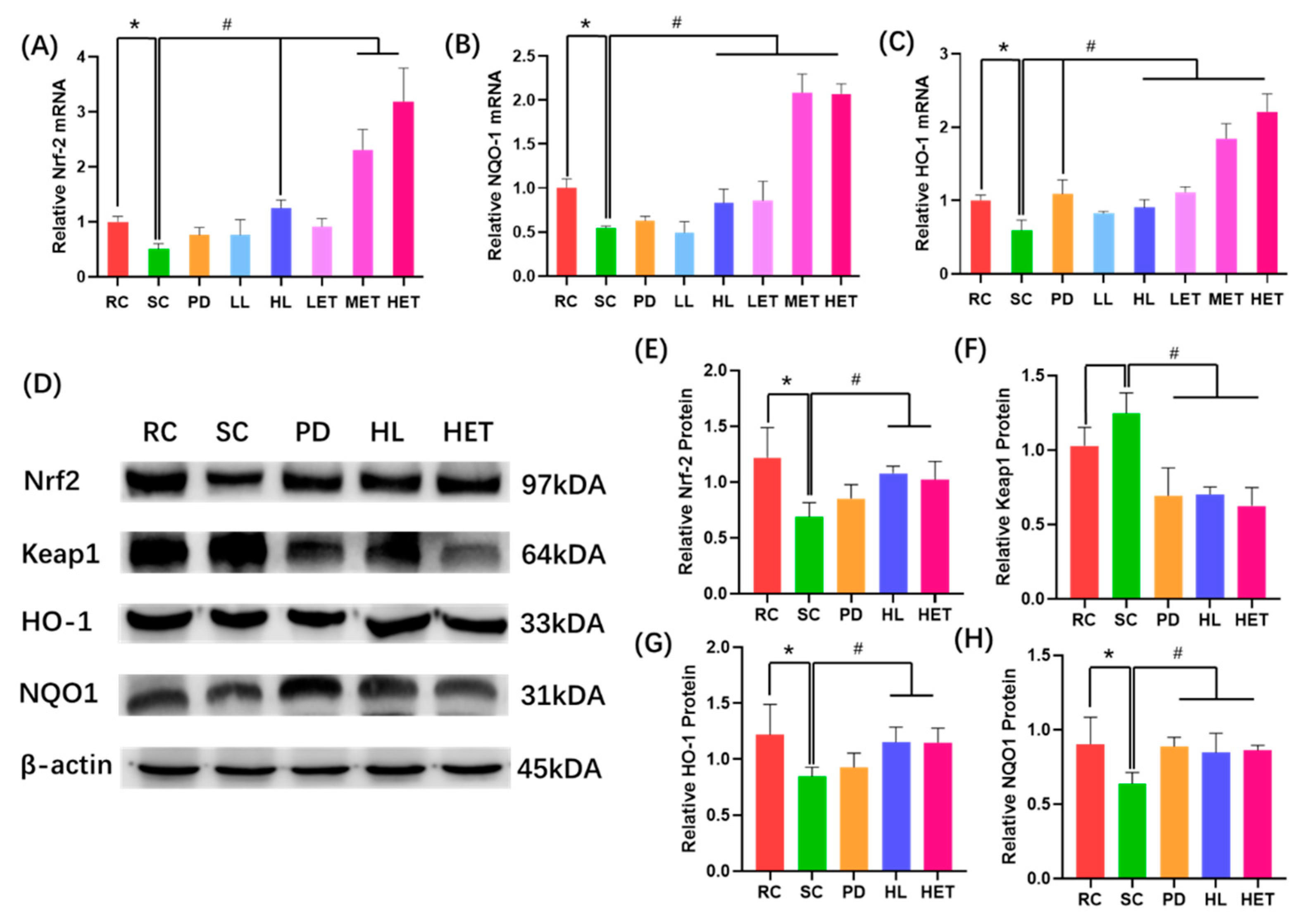
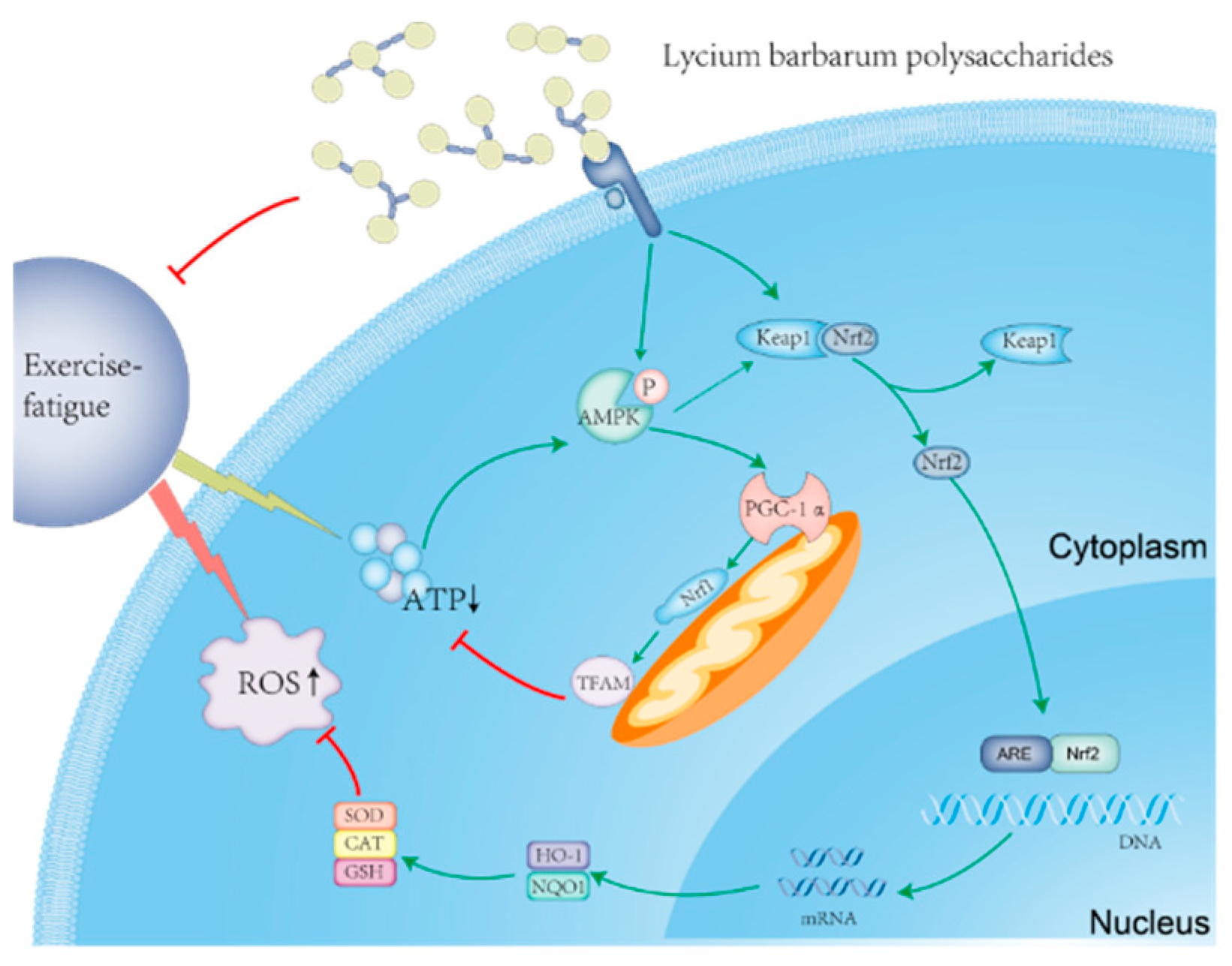
2.8. Molecular Mechanism of LBP and LBPT Regulating Oxidative Stress in Muscle Tissue of Fatigue Rats
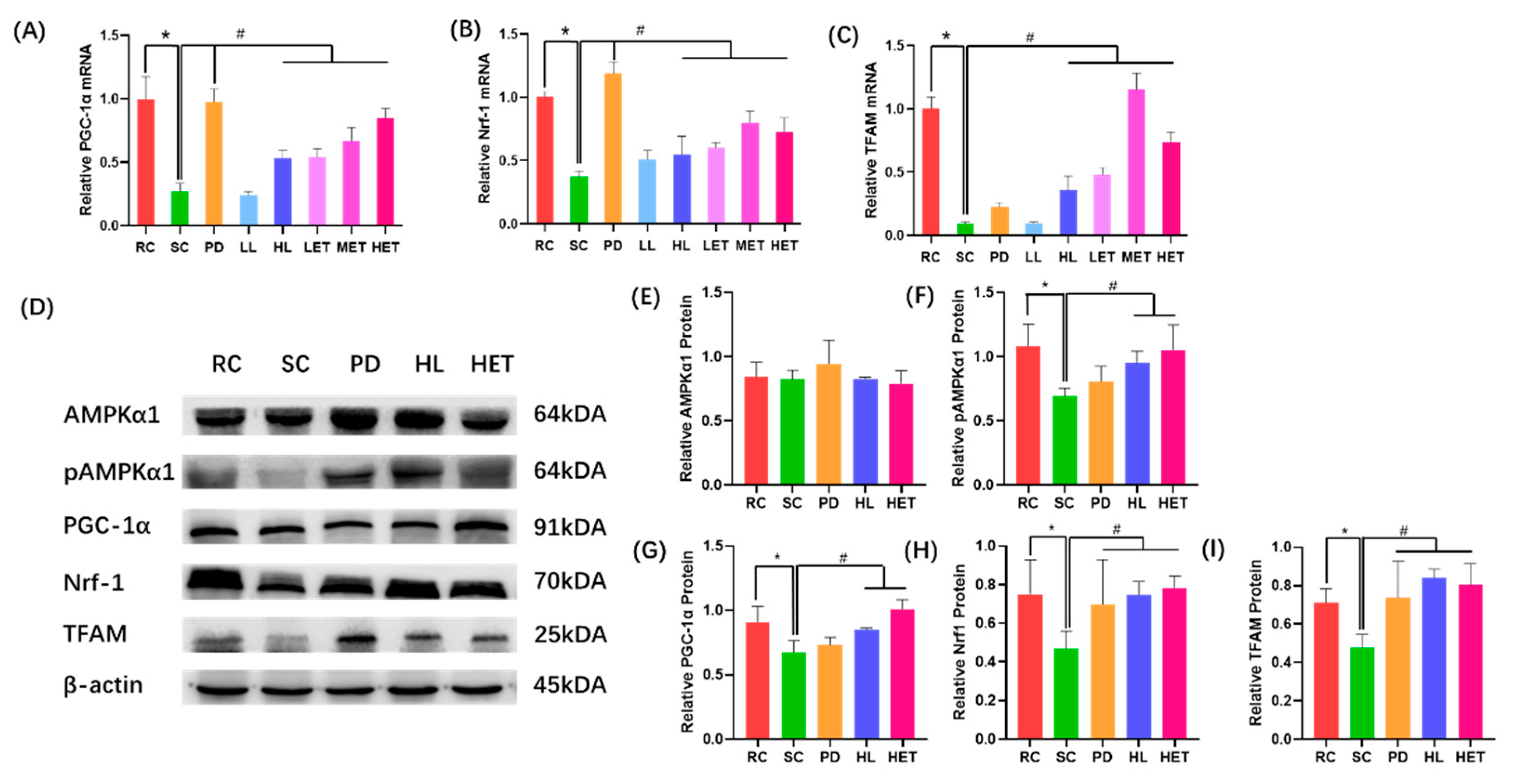
2.9. Molecular Mechanism of LBP and LBPT Regulating Energy Metabolism in Muscle Tissue of Fatigue Rats
3. Discussion
4. Materials and Methods
4.1. Rats and Reagents
4.2. Method
4.2.1. Preparation of LBP
4.2.2. Determination of LBP Content
4.2.3. Purification of LBP
4.2.4. FT-IR Spectroscopy
4.2.5. Monosaccharide Composition Analysis
4.2.6. Preparation of LBPT
4.2.7. Animal Experiment
- Rest control group (RC, N = 8) received 0.9% saline by gavage;
- The swimming control group (SC, N = 8) received 0.9% normal saline by gavage;
- Positive drug group (PD, N = 8) received 54 mg/kg/d Rhodiola Crenulata capsules by gavage;
- LBP low-dose group (LL, N = 8) received 120 mg/kg/d LBP by gavage;
- LBP high-dose group (HL, N = 8) received 360 mg/kg/d LBP by gavage;
- LBPT low-dose group (LET, N = 8) received 600 mg/kg/d LBPT by gavage;
- LBPT middle-dose group (MET, N = 8) received 1200 mg/kg/d LBPT by gavage;
- LBPT high-dose group (HET, N = 8) received 1800 mg/kg/d LBPT by gavage;
4.2.8. Forced Swimming Test
4.2.9. Detection of Biochemical Markers in Rats
4.2.10. Histopathological Analysis of Rat Liver Tissue
4.2.11. RT-PCR Analysis of Rat Muscle Tissue
4.2.12. Western Blot Analysis of Rat Muscle Tissue
4.2.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.; Newham, D.J. Fatigue and functional performance of human biceps muscle following concentric or eccentric contractions. J. Appl. Physiol. 2007, 102, 207–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.L.; Ren, F.; Huang, W.; Ding, R.T.; Zhou, Q.S.; Liu, X.W. Anti-fatigue activity of extracts of stem bark from Acanthopanax senticosus. Molecules 2010, 16, 28–37. [Google Scholar] [CrossRef]
- Matura, L.A.; Malone, S.; Jaime-Lara, R.; Riegel, B. A Systematic Review of Biological Mechanisms of Fatigue in Chronic Illness. Biol. Res. Nurs. 2018, 20, 410–421. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Li, Y.; Wang, J. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr. Res. 2017, 61, 1334485. [Google Scholar] [CrossRef]
- Narkhede, A.N.; Jagtap, S.D.; Nirmal, P.S.; Giramkar, S.A.; Nagarkar, B.E.; Kulkarni, O.P.; Harsulkar, A.M. Anti-fatigue effect of Amarkand on endurance exercise capacity in rats. BMC Complement. Altern. Med. 2016, 16, 23. [Google Scholar] [CrossRef]
- Guest, A.J.; Clemes, S.A.; King, J.A.; Chen, Y.L.; Ruettger, K.; Sayyah, M.; Sherry, A.; Varela-Mato, V.; Paine, N.J. Attenuated cardiovascular reactivity is related to higher anxiety and fatigue symptoms in truck drivers. Psychophysiology 2021, 58, e13872. [Google Scholar] [CrossRef]
- Penner, I.K.; Paul, F. Fatigue as a symptom or comorbidity of neurological diseases. Nat. Rev. Neurol. 2017, 13, 662–675. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Z.; Wang, X.; Wang, C. Recent Advances in Panax ginseng C.A. Meyer as a Herb for Anti-Fatigue: An Effects and Mechanisms Review. Foods 2021, 10, 1030. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, S.; Lin, H.; Sun, W.; Jiang, W.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice. Food Funct. 2019, 10, 7755–7766. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef] [PubMed]
- Hureau, T.J.; Ducrocq, G.P.; Blain, G.M. Peripheral and Central Fatigue Development during All-Out Repeated Cycling Sprints. Med. Sci. Sports Exerc. 2016, 48, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Gao, T.; Wang, H.; Du, Y.; Li, J.; Li, C.; Wei, L.; Bi, H. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J. Ethnopharmacol. 2013, 150, 529–535. [Google Scholar] [CrossRef]
- Ma, R.H.; Zhang, X.X.; Ni, Z.J.; Thakur, K.; Wang, W.; Yan, Y.M.; Cao, Y.L.; Zhang, J.G.; Rengasamy, K.R.R.; Wei, Z.J. Lycium barbarum (Goji) as functional food: A review of its nutrition, phytochemical structure, biological features, and food industry prospects. Crit. Rev. Food Sci. Nutr. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, F.; Yang, M.; Tan, B.; Yin, Y.; Chen, J.; Yang, Z. Lycium barbarum Polysaccharides as Antibiotic Substitutes Improve Growth Performance, Serum Immunity, Antioxidant Status, and Intestinal Health for Weaned Piglets. Front. Microbiol. 2022, 12, 819993. [Google Scholar] [CrossRef]
- Han, J.; Liu, B.; Liu, Q.M.; Zhang, Y.F.; Liu, Y.X.; Liu, H.; Cao, M.J.; Liu, G.M. Red Algae Sulfated Polysaccharides Effervescent Tablets Attenuated Ovalbumin-Induced Anaphylaxis by Upregulating Regulatory T cells in Mouse Models. J. Agric. Food Chem. 2019, 67, 11911–11921. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, Y.; Wang, X.; Ai, X.; Wang, H.; Li, X.; Chen, X.; Zhang, Y.; Hu, Y.; Meng, X.; et al. Rhodiola Crenulata ameliorates exhaustive exercise-induced fatigue in mice by suppressing mitophagy in skeletal muscle. Exp. Ther. Med. 2020, 20, 3161–3173. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lin, F.; Huang, Y.; Ye, J.; Xiao, M. Separation, purification, structural analysis and immune-enhancing activity of sulfated polysaccharide isolated from sea cucumber viscera. Int. J. Biol. Macromol. 2020, 155, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Hu, W.; Zheng, X.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Structure-activity relationship of Citrus segment membrane RG-I pectin against Galectin-3: The galactan is not the only important factor. Carbohydr. Polym. 2020, 245, 116526. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ahmed, M.I.; Ma, M.; Wang, H. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from Medemia argun fruit. Int. J. Biol. Macromol. 2020, 155, 919–926. [Google Scholar] [CrossRef] [PubMed]
- da Silva Chagas, F.D.; Lima, G.C.; Dos Santos, V.I.N.; Costa, L.E.C.; de Sousa, W.M.; Sombra, V.G.; de Araújo, D.F.; Barros, F.C.N.; Marinho-Soriano, E.; de Andrade Feitosa, J.P.; et al. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Vanavil, B.; Selvaraj, K.; Aanandhalakshmi, R.; Usha, S.K.; Arumugam, M. Bioactive and thermostable sulphated polysaccharide from Sargassum swartzii with drug deliv-ery applications. Int. J. Biol. Macromol. 2020, 153, 190–200. [Google Scholar]
- Guo, L.; Dai, H.; Ma, J.; Wang, J.; Hua, Y.; Zhou, L. Isolation, structure characteristics and antioxidant activity of two water-soluble polysaccharides from Lenzites betulina. BMC Chem. 2021, 15, 19. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.; Jin, S.; Wang, D.; Wu, K.; Yang, Q.; Han, R.; Chen, S.; Liang, Z.; Jia, Q. Structure characterization and bioactivity of neutral polysaccharides from different sources of Polygonatum Mill. Biopolymers 2022, 113, e23490. [Google Scholar] [CrossRef]
- Alvarez, V.M.; Jurelevicius, D.; Serrato, R.V.; Barreto-Bergter, E.; Seldin, L. Chemical characterization and potential application of exopolysaccharides produced by Ensifer adhaerens JHT2 as a bioemulsifier of edible oils. Int. J. Biol. Macromol. 2018, 114, 18–25. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, J.; Kang, Q.; Huang, J.; Cui, Y.; Zhang, G.; Wang, Z.; Zhang, L.; Zheng, Z.; Lu, J.; et al. Anti-fatigue activity of hemp leaves water extract and the related biochemical changes in mice. Food Chem. Toxicol. 2021, 150, 112054. [Google Scholar] [CrossRef]
- Yang, Z.; Sunil, C.; Jayachandran, M.; Zheng, X.; Cui, K.; Su, Y.; Xu, B. Anti-fatigue effect of aqueous extract of Hechong (Tylorrhynchus heterochaetus) via AMPK linked pathway. Food Chem. Toxicol. 2020, 135, 111043. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Baskaran, R.; Shibu, M.A.; Lin, W.T. Anti-Fatigue and Exercise Performance Improvement Effect of Glossogyne tenuifolia Extract in Mice. Nutrients 2022, 14, 1011. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef]
- Orhan, C.; Gencoglu, H.; Tuzcu, M.; Sahin, N.; Ojalvo, S.P.; Sylla, S.; Komorowski, J.R.; Sahin, K. Maca could improve endurance capacity possibly by increasing mitochondrial biogenesis pathways and antioxidant response in exercised rats. J. Food Biochem. 2022, 46, e14159. [Google Scholar] [CrossRef]
- Ortenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, W.; Wang, B.; Hui, A.; Du, B.; Wang, T.; Meng, L.; Bian, H.; Wu, Z. Purification, characterization and anti-fatigue activity of polysaccharide fractions from okra (Abelmoschus esculentus (L.) Moench). Food Funct. 2018, 9, 1088–1101. [Google Scholar] [CrossRef]
- Kuwahara, H.; Horie, T.; Ishikawa, S.; Tsuda, C.; Kawakami, S.; Noda, Y.; Kaneko, T.; Tahara, S.; Tachibana, T.; Okabe, M.; et al. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic. Biol. Med. 2010, 48, 1252–1262. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Zheng, X.; Zhang, X.; Wang, Z. Magnesium Trisilicate Coated Fe3O4 Nanoparticles as Prompt and Efficient Lactic Acid Removers Potential for Exercise-Induce Fatigue Prevention. J. Biomed. Nanotechnol. 2020, 16, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Fang, C.C.; Lee, Y.H.; Yang, M.T.; Chan, K.H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chaki, B.; Chattopadhyay, S.; Bandyopadhyay, A. High-Intensity Exercise Induced Oxidative Stress and Skeletal Muscle Damage in Postpubertal Boys and Girls: A Comparative Study. J. Strength Cond. Res. 2018, 32, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Chang, X.; Chen, X.; Gong, P.; Yang, W.; Wang, L.; Liu, N.; Su, Y.; Zhao, Y. Anti-oxidant and anti-fatigue properties of apple pomace polysaccharides by acid or alkali extraction. Int. J. Food Sci. Technol. 2022, 57, 78–91. [Google Scholar] [CrossRef]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Narasimhan, M.; Rajasekaran, N.S. Exercise, Nrf2 and Antioxidant Signaling in Cardiac Aging. Front. Physiol. 2016, 7, 241. [Google Scholar] [CrossRef]
- Sun, L.; Shen, W.; Liu, Z.; Guan, S.; Liu, J.; Ding, S. Endurance exercise causes mitochondrial and oxidative stress in rat liver: Effects of a combination of mitochondrial targeting nutrients. Life Sci. 2010, 86, 39–44. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Lee, Y.B.; Min, J.K.; Kim, J.G.; Cap, K.C.; Islam, R.; Hossain, A.J.; Dogsom, O.; Hamza, A.; Mahmud, S.; Choi, D.R.; et al. Multiple functions of pyruvate kinase M2 in various cell types. J. Cell Physiol. 2022, 237, 128–148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, C.; Zhan, X.; Li, B.; Zhang, Z.; Li, S.; Xie, Y.; Song, X.; Shen, Y.; Liu, J.; et al. R13 preserves motor performance in SOD1G93A mice by improving mitochondrial function. Theranostics 2021, 11, 7294–7307. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Chen, K.; Liu, P.; Chang, H.; Zhu, J.; Mi, M. Dihydromyricetin Improves Physical Performance under Simulated High Altitude. Med. Sci. Sports Exerc. 2014, 46, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Huang, H.; Chen, F.; Long, R.; Huang, G. The antioxidant activities in vivo of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2020, 145, 141–144. [Google Scholar] [CrossRef]
- Wei, H.; Shi, Y.; Yuan, Z.; Huang, Z.; Cai, F.; Zhu, J.; Zhang, W.; Li, J.; Xiong, Q.; Wang, Y.; et al. Isolation, Identification, and Anti-Inflammatory Activity of Polysaccharides of Typha angustifolia. Biomacromolecules 2021, 22, 2451–2459. [Google Scholar] [CrossRef]
- Chen, F.; Huang, S.; Huang, G. Preparation, activity, and antioxidant mechanism of rice bran polysaccharide. Food Funct. 2021, 12, 834–839. [Google Scholar] [CrossRef]
- Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Analysis of the chemical composition of natural carbohydrates—An overview of methods. Food Chem. 2022, 394, 133466. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Yang, Y.; Gao, Y.; Sun, Q.; Liu, J.; Yang, X.; Wang, J.; Zhang, J. β-glucan Salecan Improves Exercise Performance and Displays Anti-Fatigue Effects through Regulating Energy Metabolism and Oxidative Stress in Mice. Nutrients 2018, 10, 858. [Google Scholar] [CrossRef]


| Project | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Nrf2 | CCTTCCTCTGCTGCCATTAGTC | GAACTCCACCGTGCCTTCAG |
| NQO1 | GCCTACACGTATGCCACCAT | TGGACACCCTGCAGAGAGTA |
| HO-1 | CTAAGACCGCCTTCCTGCTC | GCCTCTGGCGAAGAAACTCT |
| PGC1-1α | CGGTGGATGAAGACGGATTGCC | ATTGTAGCTGAGCTGAGTGTTGGC |
| Nrf1 | TCTGCTGTGGCTGATGGAGAGG | GATGCTTGCGTCGTCTGGATGG |
| TFAM | CCGGCAGAAACGCCTAAAGA | ATCCTTAGCCCCCTGGAAGC |
| β-actin | GCGCAAGTACTCTGTGTGGA | CATCGTACTCCTGCTTGCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Zhao, L.; Hu, K.; Yang, Y.; Ma, J.; Zhai, Y.; Jiang, Y.; Zhang, D. Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats. Int. J. Mol. Sci. 2022, 23, 10920. https://doi.org/10.3390/ijms231810920
Peng Y, Zhao L, Hu K, Yang Y, Ma J, Zhai Y, Jiang Y, Zhang D. Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats. International Journal of Molecular Sciences. 2022; 23(18):10920. https://doi.org/10.3390/ijms231810920
Chicago/Turabian StylePeng, Yanfeng, Linlin Zhao, Ke Hu, Yongjing Yang, Jin Ma, Yuqing Zhai, Yan Jiang, and Dejun Zhang. 2022. "Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats" International Journal of Molecular Sciences 23, no. 18: 10920. https://doi.org/10.3390/ijms231810920
APA StylePeng, Y., Zhao, L., Hu, K., Yang, Y., Ma, J., Zhai, Y., Jiang, Y., & Zhang, D. (2022). Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats. International Journal of Molecular Sciences, 23(18), 10920. https://doi.org/10.3390/ijms231810920







