Protective Effect of Portulaca oleracea on Streptozotocin-Induced Type I Diabetes-Associated Reproductive System Dysfunction and Inflammation
Abstract
1. Introduction
2. Results
2.1. The Effects of PO Extract on the Water and Food Consumption of Diabetic Rats
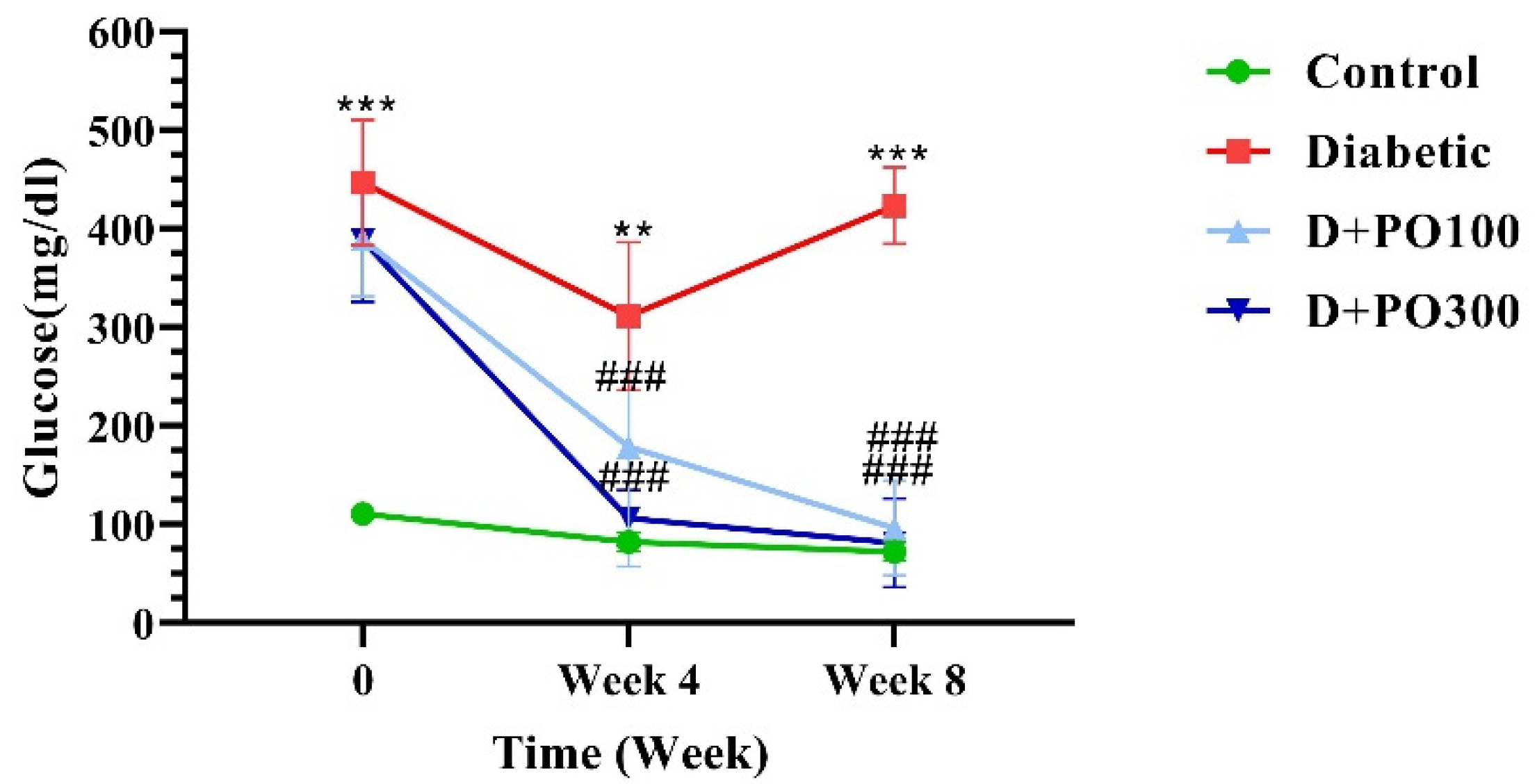
2.2. The Effect of PO Extract on Blood Glucose Level
2.3. The Effect of PO Extract on Body Weight
2.4. The Effect of PO Extract on the Testicular Weight and Testicular/Body Weight Index
2.5. The Effect of PO Extract on the Epididymis Weight and Epididymis/Body Weight
2.6. The Histopathological Evaluations
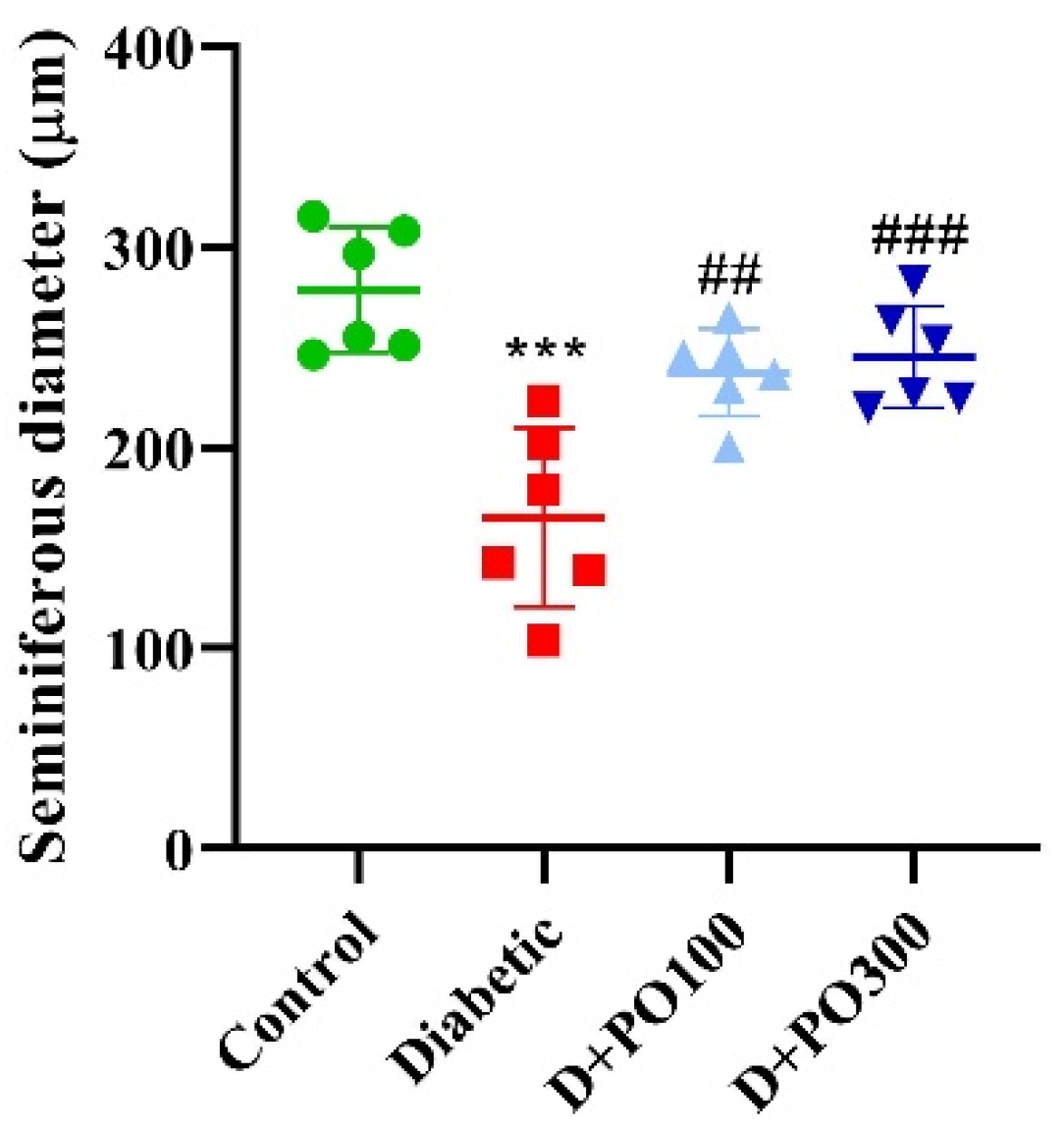
2.7. The Effect of PO Extract on the Diameter of the Seminiferous Tubules
2.8. The Effect of PO Extract on the Count and Motility of Sperm
2.9. The Effect of PO Extract on the Levels of LH, FSH, and Testosterone
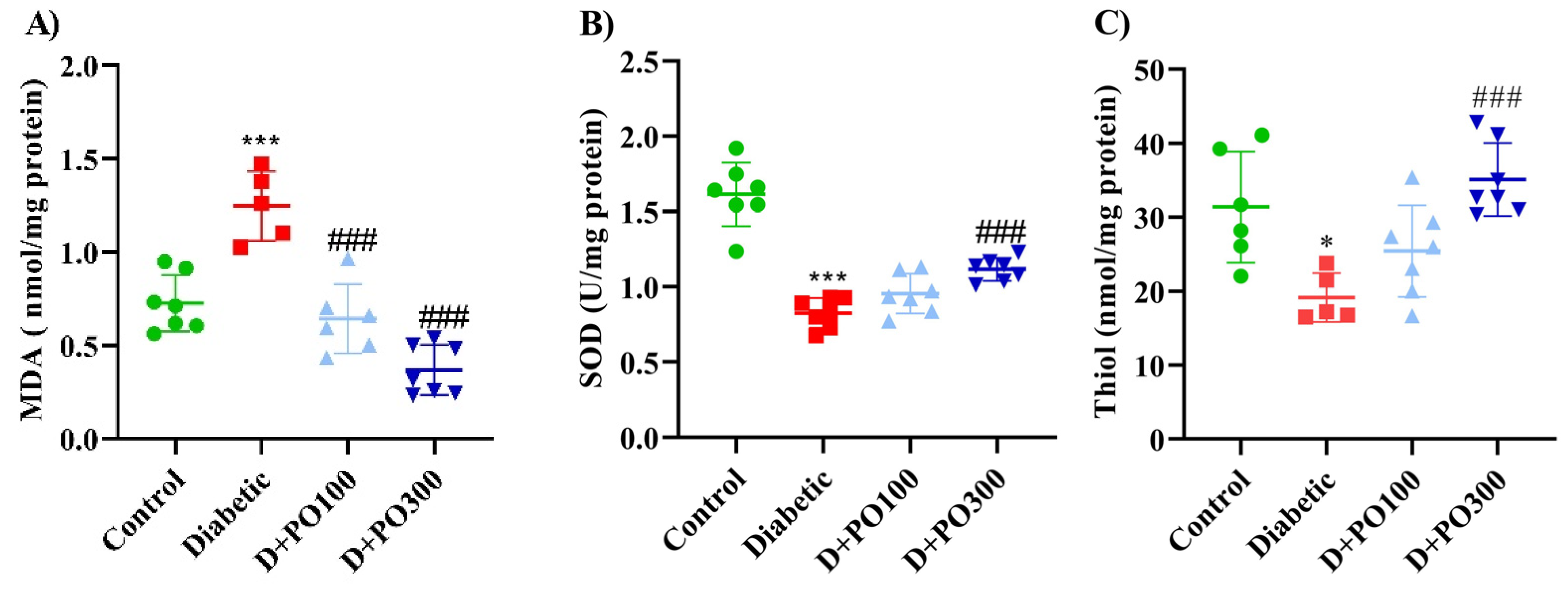
2.10. The Effect of PO Extract on the Oxidative and Antioxidative Factors
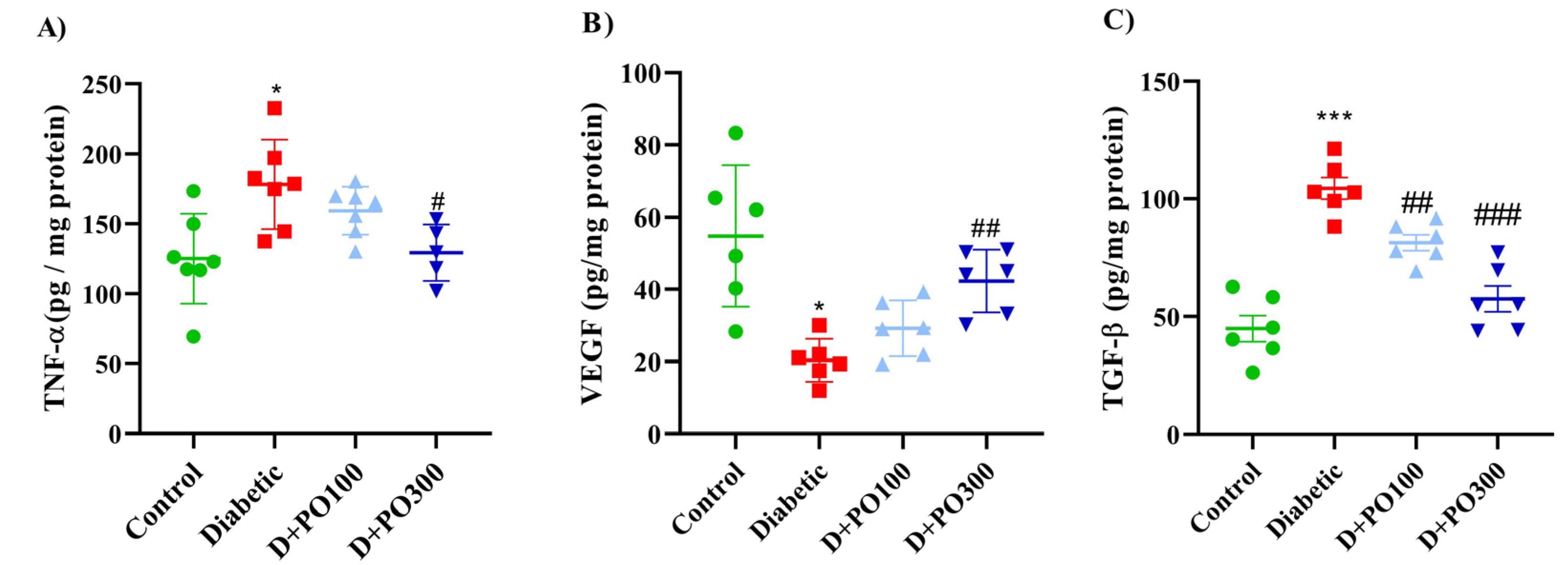
2.11. The Effect of PO Extract on TNF-α, VEGF, and TGF-β Levels
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Preparation of Portulaca oleracea Extract and Liquid Chromatography-Mass Spectrometers (LC-MS) Characterisation
4.3. Animals’ Husbandry and Ethics
4.4. Experimental Diabetes Induction
4.5. Study Design
- Control group: receiving a single dose of streptozotocin carrier (with a volume equal to 50 mg/kg; i.p.) + PO extract vehicle (oral gavage; for eight weeks).
- Diabetic group: receiving a single dose of streptozotocin (50 mg/kg; i.p.) + PO extract vehicle (daily oral gavage; for eight weeks).
- Treatment group-1: receiving a single dose of streptozotocin (50 mg/kg; i.p.) + PO extract (100 mg/kg; daily oral gavage for eight weeks).
- Treatment group-2: receiving a single dose of streptozotocin (50 mg/kg; i.p.) + PO extract (300 mg/kg; daily oral gavage for eight weeks).
4.6. Measurement of Blood Glucose Levels
4.7. Sample Preparation
4.8. Assessment of Hormonal Factors
4.9. Assessment of Oxidative and Anti-Oxidative, Inflammatory, Fibrosis and Angiogenesis Biomarkers
4.10. Histological Evaluations and Measurement of the Seminiferous Diameter, Sperm Count and Motility
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
References
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. Adv. Exp. Med. Biol. 2012, 771, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.L.; Liu, Y.; Liu, M.E.; Pan, J.X.; Guo, M.X.; Sheng, J.Z.; Huang, H.F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Giorda, C.B.; Cucinotta, D.; Guida, P.; Nada, E. Sexual dysfunction at the onset of type 2 diabetes: The interplay of depression, hormonal and cardiovascular factors. J. Sex. Med. 2014, 11, 2065–2073. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Mousavi, S.H.; Haghighi, S.; Soheili-Far, S.; Askari, V.R. Cytotoxicity and apoptogenic properties of the standardized extract of Portulaca oleracea on glioblastoma multiforme cancer cell line (U-87): A mechanistic study. EXCLI J. 2019, 18, 165–186. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Ajam, F.; Rakhshandeh, H.; Askari, V.R. A Pharmacological Review on Portulaca oleracea L.: Focusing on Anti-Inflammatory, Anti-Oxidant, Immuno-Modulatory and Antitumor Activities. J. Pharmacopunct. 2019, 22, 7–15. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R.; Shirazinia, R.; Soheili-Far, S.; Askari, N.; Rahmanian-Devin, P.; Sanei-Far, Z.; Mousavi, S.H.; Ghodsi, R. Protective effects of hydro-ethanolic extract of Terminalia chebula on primary microglia cells and their polarization (M1/M2 balance). Mult. Scler. Relat. Disord. 2018, 25, 5–13. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R. Promising anti-melanogenic impacts of Portulaca oleracea on B16F1 murine melanoma cell line: An in-vitro vision. S. Afr. J. Bot. 2021, 142, 477–485. [Google Scholar] [CrossRef]
- Jaafari, A.; Baradaran Rahimi, V.; Vahdati-Mashhadian, N.; Yahyazadeh, R.; Ebrahimzadeh-Bideskan, A.; Hasanpour, M.; Iranshahi, M.; Ehtiati, S.; Rajabi, H.; Mahdinezhad, M.; et al. Evaluation of the Therapeutic Effects of the Hydroethanolic Extract of Portulaca oleracea on Surgical-Induced Peritoneal Adhesion. Mediat. Inflamm. 2021, 2021, 8437753. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zang, X.; Ma, J.; Xu, G. Anti-Diabetic Effect of Portulaca oleracea L. Polysaccharideandits Mechanism in Diabetic Rats. Int. J. Mol. Sci. 2016, 17, 1201. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Hashemzehi, M.; Khazdair, M.R.; Askari, V.R. Hydro-ethanolic Extract of Portulaca oleracea Affects Beta-adrenoceptors of Guinea Pig Tracheal Smooth Muscle. Iran. J. Pharm. Res. 2016, 15, 867–874. [Google Scholar]
- Yan, J.; Sun, L.R.; Zhou, Z.Y.; Chen, Y.C.; Zhang, W.M.; Dai, H.F.; Tan, J.W. Homoisoflavonoids from the medicinal plant Portulaca oleracea. Phytochemistry 2012, 80, 37–41. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Y.P.; Liu, J.Y. Determination of noradrenaline and dopamine in Chinese herbal extracts from Portulaca oleracea L. by high-performance liquid chromatography. J. Chromatogr. A 2003, 1003, 127–132. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef]
- Tian, J.L.; Liang, X.; Gao, P.Y.; Li, D.Q.; Sun, Q.; Li, L.Z.; Song, S.J. Two new alkaloids from Portulaca oleracea and their cytotoxic activities. J. Asian Nat. Prod. Res. 2014, 16, 259–264. [Google Scholar] [CrossRef]
- Liang, X.; Tian, J.; Li, L.; Gao, J.; Zhang, Q.; Gao, P.; Song, S. Rapid determination of eight bioactive alkaloids in Portulaca oleracea L. by the optimal microwave extraction combined with positive-negative conversion multiple reaction monitor (+/−MRM) technology. Talanta 2014, 120, 167–172. [Google Scholar] [CrossRef]
- Sakai, N.; Inada, K.; Okamoto, M.; Shizuri, Y.; Fukuyama, Y. Portuloside A, a monoterpene glucoside, from Portulaca oleracea. Phytochemistry 1996, 42, 1625–1628. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.; Aziz, M.A. Portulene, a new diterpene from Portulaca oleracea L. J. Asian Nat. Prod. Res. 2008, 10, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Shin, J.; Cha, H.J.; Kim, Y.-A.; Ahn, J.-W.; Lee, B.-J.; Lee, D.S. A new monoterpene glucoside from Portulaca oleracea. Bull. Korean Chem. Soc. 2003, 24, 1475–1477. [Google Scholar] [CrossRef]
- Xin, H.-L.; Xu, Y.-F.; Hou, Y.-H.; Zhang, Y.-N.; Yue, X.-Q.; Lu, J.-C.; Ling, C.-Q. Two Novel Triterpenoids from Portulaca oleracea L. Helv. Chim. Acta 2008, 91, 2075–2080. [Google Scholar] [CrossRef]
- Xu, X.; Yu, L.; Chen, G. Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2006, 41, 493–499. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Liu, Y.; Xia, Y.; Tang, T. Analysis of Flavonoids in Portulaca oleracea L. by UV–Vis Spectrophotometry with Comparative Study on Different Extraction Technologies. Food Anal. Methods 2010, 3, 90–97. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; McAvoy, R.J.; Bible, B.B. Stage of harvest and polyunsaturated essential fatty acid concentrations in purslane (Portulaca oleraceae) leaves. J. Agric. Food Chem. 2001, 49, 3490–3493. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Xiang, L.; Zheng, Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytother. Res. 2009, 23, 1032–1035. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.; Ali, M.E.; Rahman, M.M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, D.; Zhang, W.; Du, Y.; Wang, Y.; Zhai, Y.; Ying, X.; Kang, T. LC determination and pharmacokinetic study of the main phenolic components of Portulaca oleracea L. extract in rat plasma after oral administration. Nat. Prod. Res. 2012, 26, 2247–2250. [Google Scholar] [CrossRef]
- Elçioğlu, H.K.; Kabasakal, L.; Özkan, N.; Çelikel, Ç.; Ayanoğlu-Dülger, G. A study comparing the effects of rosiglitazone and/or insulin treatments on streptozotocin induced diabetic (type I diabetes) rat aorta and cavernous tissues. Eur. J. Pharmacol. 2011, 660, 476–484. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.F.; Maranhão, H.M.; Batista, T.M.; Carneiro, E.M.; Ferreira, F.; Costa, J.; Soares, L.A.; Sá, M.D.; Souza, T.P.; Wanderley, A.G. Hypoglycaemic activity and molecular mechanisms of Caesalpinia ferrea Martius bark extract on streptozotocin-induced diabetes in Wistar rats. J. Ethnopharmacol. 2011, 137, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Lee, Y.J.; Lee, S.M.; Yoon, J.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Portulaca oleracea Ameliorates Diabetic Vascular Inflammation and Endothelial Dysfunction in db/db Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 741824. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, B.K.; Schaalan, M.F.; Tolba, A.M. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Barakat, L.A.; Mahmoud, R.H. The antiatherogenic, renal protective and immunomodulatory effects of purslane, pumpkin and flax seeds on hypercholesterolemic rats. N. Am. J. Med. Sci. 2011, 3, 411–417. [Google Scholar] [CrossRef]
- Ricci, G.; Catizone, A.; Esposito, R.; Pisanti, F.A.; Vietri, M.T.; Galdieri, M. Diabetic rat testes: Morphological and functional alterations. Andrologia 2009, 41, 361–368. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Karabulut, D.; Kilic, E.; Akalin, H.; Sakalar, C.; Gunduz, Y.; Kara, A.; Dundar, M. The effects of streptozotocin-induced diabetes on ghrelin expression in rat testis: Biochemical and immunohistochemical study. Folia Histochem. Cytobiol. 2015, 53, 26–34. [Google Scholar] [CrossRef]
- Kotian, S.R.; Kumar, A.; Mallik, S.B.; Bhat, N.P.; Souza, A.D.; Pandey, A.K. Effect of Diabetes on the Male Reproductive System—A Histomorphological Study. J. Morphol. Sci. 2019, 36, 017–023. [Google Scholar] [CrossRef]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.; Maleki, N.A.; Khamnei, H.J.; Ahmadi, P. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother. Res. 2010, 24, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Afifi, M.; Almaghrabi, O.A.; Kadasa, N.M. Ameliorative Effect of Zinc Oxide Nanoparticles on Antioxidants and Sperm Characteristics in Streptozotocin-Induced Diabetic Rat Testes. BioMed Res. Int. 2015, 2015, 153573. [Google Scholar] [CrossRef] [PubMed]
- Obinna, V.; Kagbo, H.; Agu, G. Effects of Lipophilic and Hydrophilic leaf extracts of Portulaca oleracea Linn. (Purslane) on male reproductive parameters in albino rats. Am. J. Physiol. Biochem. Pharmacol. 2019, 9, 21–32. [Google Scholar] [CrossRef]
- Xu, Y.; Lei, H.; Guan, R.; Gao, Z.; Li, H.; Wang, L.; Song, W.; Gao, B.; Xin, Z. Studies on the mechanism of testicular dysfunction in the early stage of a streptozotocin induced diabetic rat model. Biochem. Biophys. Res. Commun. 2014, 450, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ghaheri, M.; Miraghaee, S.; Babaei, A.; Mohammadi, B.; Kahrizi, D.; Saivosh Haghighi, Z.M.; Bahrami, G. Effect of Stevia rebaudiana Bertoni extract on sexual dysfunction in Streptozotocin-induced diabetic male rats. Cell. Mol. Biol. 2018, 64, 6–10. [Google Scholar] [CrossRef]
- Rastrelli, G.; Maggi, M.; Corona, G. Pharmacological management of late-onset hypogonadism. Expert Rev. Clin. Pharmacol. 2018, 11, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Aladamat, N.; Tadi, P. Histology, Leydig Cells. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Acién, P.; Acién, M. Disorders of Sex Development: Classification, Review, and Impact on Fertility. J. Clin. Med. 2020, 9, 3555. [Google Scholar] [CrossRef]
- Ballester, J.; Muñoz, M.C.; Domínguez, J.; Rigau, T.; Guinovart, J.J.; Rodríguez-Gil, J.E. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J. Androl. 2004, 25, 706–719. [Google Scholar] [CrossRef]
- Farag, O.M.; Abd-Elsalam, R.M.; El Badawy, S.A.; Ogaly, H.A.; Alsherbiny, M.A.; Ahmed, K.A. Portulaca oleracea seeds’ extract alleviates acrylamide-induced testicular dysfunction by promoting oxidative status and steroidogenic pathway in rats. BMC Complement. Med. Ther. 2021, 21, 122. [Google Scholar] [CrossRef]
- Hozayen, W.G.; Ahmed, O.M.; Abo Sree, H.T.; Ahmed, M.B. Effects of ethanolic purslane shoot and seed extracts on doxorubicin-induced testicular toxicity in albino rats. Life Sci. J. 2013, 10, 2550–2558. [Google Scholar]
- Abdel-Aziz, A.M.; Abozaid, S.M.M.; Yousef, R.K.M.; Mohammed, M.M.; Khalaf, H.M. Fenofibrate ameliorates testicular damage in rats with streptozotocin-induced type 1 diabetes: Role of HO-1 and p38 MAPK. Pharmacol. Rep. 2020, 72, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Shrilatha, B.; Muralidhara. Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: Its progression and genotoxic consequences. Reprod. Toxicol. 2007, 23, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Shrilatha, B. Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int. J. Androl. 2007, 30, 508–518. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Farkhondeh, T. Attenuation of Oxidative Stress and Inflammation by Portulaca oleracea in Streptozotocin-Induced Diabetic Rats. J. Evid. Based Complement. Altern. Med. 2017, 22, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, Y.; Li, J.; Tang, Z.; Sun, J.; Zhang, H.; Hao, S.; Wen, A.; Liu, L. Protective effects of ethanol extract from Portulaca oleracea L on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am. J. Transl. Res. 2016, 8, 2138–2148. [Google Scholar]
- Ahangarpour, A.; Lamoochi, Z.; Fathi Moghaddam, H.; Mansouri, S.M. Effects of Portulaca oleracea ethanolic extract on reproductive system of aging female mice. Int. J. Reprod. Biomed. 2016, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh Mashhadi, S.N.; Askari, V.R.; Ghorani, V.; Jelodar, G.A.; Boskabady, M.H. The effect of Portulaca oleracea and α-linolenic acid on oxidant/antioxidant biomarkers of human peripheral blood mononuclear cells. Indian J. Pharmacol. 2018, 50, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Coştur, P.; Filiz, S.; Gonca, S.; Çulha, M.; Gülecen, T.; Solakoğlu, S.; Canberk, Y.; Çalışkan, E. Êxpression of inducible nitric oxide synthase (iNOS) in the azoospermic human testis. Andrologia 2012, 44 (Suppl. S1), 654–660. [Google Scholar] [CrossRef]
- Calvo, P.M.; Pastor, A.M.; de la Cruz, R.R. Vascular endothelial growth factor: An essential neurotrophic factor for motoneurons? Neural Regen. Res. 2018, 13, 1181–1182. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar] [PubMed]
- Sarkar, O.; Bahrainwala, J.; Chandrasekaran, S.; Kothari, S.; Mathur, P.P.; Agarwal, A. Impact of inflammation on male fertility. Front. Biosci. 2011, 3, 89–95. [Google Scholar] [CrossRef]
- Sanocka, D.; Jedrzejczak, P.; Szumała-Kaekol, A.; Fraczek, M.; Kurpisz, M. Male genital tract inflammation: The role of selected interleukins in regulation of pro-oxidant and antioxidant enzymatic substances in seminal plasma. J. Androl. 2003, 24, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Albasher, G. Modulation of reproductive dysfunctions associated with streptozocin-induced diabetes by Artemisia judaica extract in rats fed a high-fat diet. Mol. Biol. Rep. 2020, 47, 7517–7527. [Google Scholar] [CrossRef] [PubMed]
- Yigitturk, G.; Acara, A.C.; Erbas, O.; Oltulu, F.; Yavasoglu, N.U.K.; Uysal, A.; Yavasoglu, A. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed. Pharmacother. 2017, 87, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tabecka-Lonczynska, A.; Mytych, J.; Solek, P.; Kulpa-Greszta, M.; Sowa-Kucma, M.; Koziorowski, M. Vascular endothelial growth factor (VEGF-A) and fibroblast growth factor (FGF-2) as potential regulators of seasonal reproductive processes in male European bison (Bison bonasus, Linnaeus 1758). Gen. Comp. Endocrinol. 2018, 263, 72–79. [Google Scholar] [CrossRef]
- Anand, R.J.; Paust, H.J.; Altenpohl, K.; Mukhopadhyay, A.K. Regulation of vascular endothelial growth factor production by Leydig cells in vitro: The role of protein kinase A and mitogen-activated protein kinase cascade. Biol. Reprod. 2003, 68, 1663–1673. [Google Scholar] [CrossRef]
- Sisman, A.R.; Kiray, M.; Camsari, U.M.; Evren, M.; Ates, M.; Baykara, B.; Aksu, I.; Guvendi, G.; Uysal, N. Potential novel biomarkers for diabetic testicular damage in streptozotocin-induced diabetic rats: Nerve growth factor Beta and vascular endothelial growth factor. Dis. Markers 2014, 2014, 108106. [Google Scholar] [CrossRef]
- Long, L.; Qiu, H.; Cai, B.; Chen, N.; Lu, X.; Zheng, S.; Ye, X.; Li, Y. Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget 2018, 9, 5321–5336. [Google Scholar] [CrossRef]
- Tunçkiran, A.; Cayan, S.; Bozlu, M.; Yilmaz, N.; Acar, D.; Akbay, E. Protective effect of vascular endothelial growth factor on histologic changes in testicular ischemia-reperfusion injury. Fertil. Steril. 2005, 84, 468–473. [Google Scholar] [CrossRef]
- He, Y.; Long, H.; Zou, C.; Yang, W.; Jiang, L.; Xiao, Z.; Li, Q.; Long, S. Anti-nociceptive effect of Portulaca oleracea L. ethanol extracts attenuated zymosan-induced mouse joint inflammation via inhibition of Nrf2 expression. Innate Immun. 2021, 27, 230–239. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Mousavi, S.H. Ellagic acid dose and time-dependently abrogates d-galactose-induced animal model of aging: Investigating the role of PPAR-gamma. Life Sci. 2019, 232, 116595. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.; Zor, T. Linearization of the bradford protein assay. JoVE J. Vis. Exp. 2010, 38, e1918. [Google Scholar] [CrossRef]
- Saviano, A.; Casillo, G.M.; Raucci, F.; Pernice, A.; Santarcangelo, C.; Piccolo, M.; Ferraro, M.G.; Ciccone, M.; Sgherbini, A.; Pedretti, N.; et al. Supplementation with ribonucleotide-based ingredient (Ribodiet®) lessens oxidative stress, brain inflammation, and amyloid pathology in a murine model of Alzheimer. Biomed. Pharmacother. 2021, 139, 111579. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Rahimi, V.B.; Zargarani, R.; Ghodsi, R.; Boskabady, M.; Boskabady, M.H. Anti-oxidant and anti-inflammatory effects of auraptene on phytohemagglutinin (PHA)-induced inflammation in human lymphocytes. Pharmacol. Rep. 2021, 73, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Baradaran Rahimi, V.; Moradi, E.; Hasanpour, M.; Clark, C.C.T.; Iranshahi, M.; Rakhshandeh, H.; Askari, V.R. Standardised pomegranate peel extract lavage prevents postoperative peritoneal adhesion by regulating TGF-β and VEGF levels. Inflammopharmacology 2021, 29, 855–868. [Google Scholar] [CrossRef]
- Roshankhah, S.; Jalili, C.; Salahshoor, M.R. Effects of Crocin on Sperm Parameters and Seminiferous Tubules in Diabetic Rats. Adv. Biomed. Res. 2019, 8, 4. [Google Scholar] [CrossRef] [PubMed]
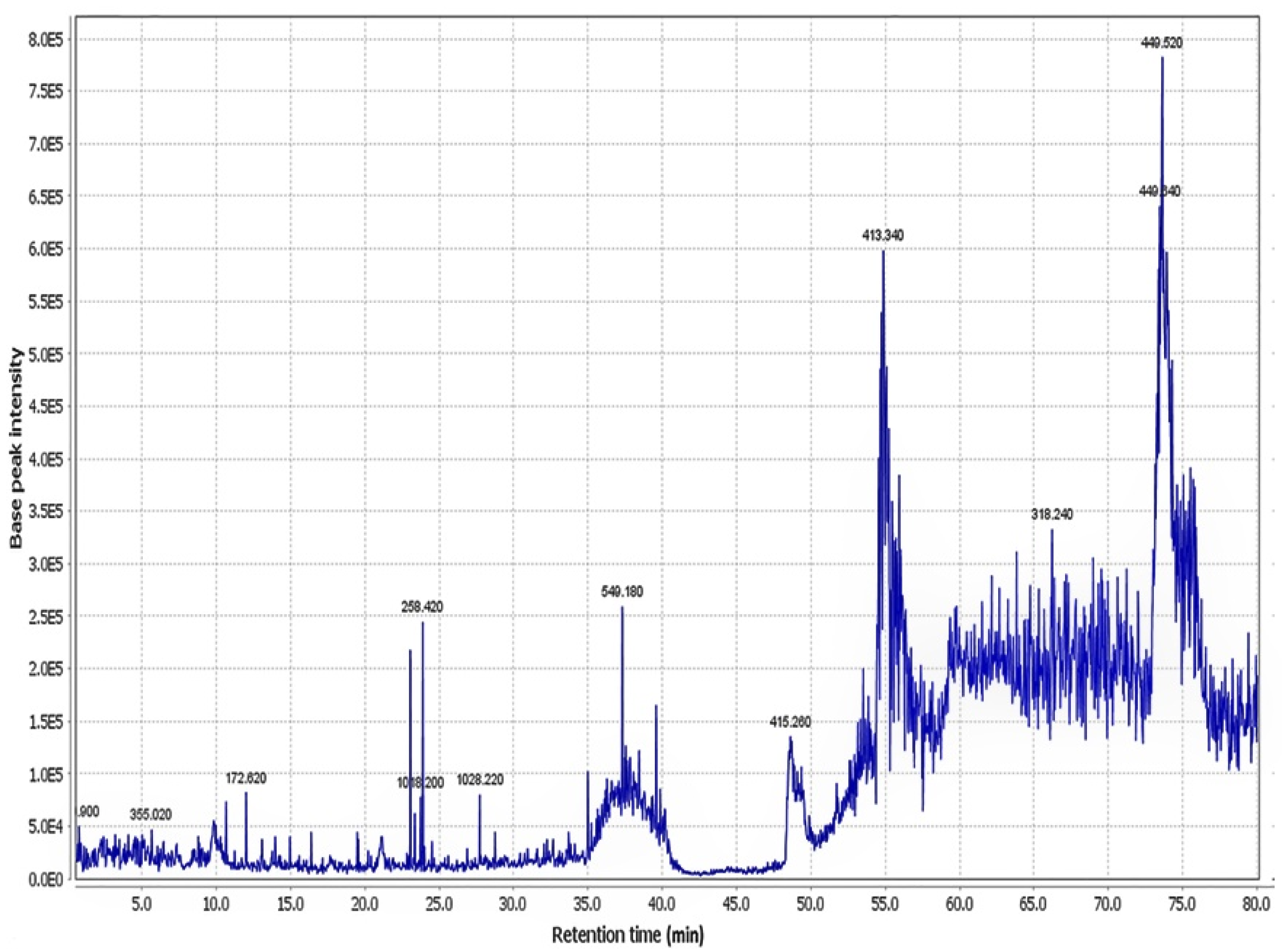








| Peak No. | Compound Identification | tR (min) | M+H (m/z) | Reference |
|---|---|---|---|---|
| 1 | Portulacanone D | 26.9 | 299.76 | [16] |
| 2 | Noradrenaline | 37.0 | 170.7 | [17] |
| 3 | Dopa | 15.0 | 198.12 | [18] |
| 4 | Oleraceins A | 62.5 | 504.66 | [18] |
| 5 | Oleraceins B | 9.5 | 533.76 | [18] |
| 6 | Oleraceins C | 64.1 | 666.06 | [18] |
| 7 | Oleraceins D | 13.1 | 696.84 | [18] |
| 8 | Adenosine | 19.8 | 268.8 | [18] |
| 9 | (3R)-3,5-Bis(3-methoxy-4-hydroxyphenyl)-2,3-dihydro-2(1H)-pyridinone | 89.3 | 342.36 | [19] |
| 10 | Aurantiamide acetate | 36.4 | 445.8 | [20] |
| 11 | Cyclo(L-tyrosinyl-L-tyrosinyl) | 67.7 | 327.24 | [20] |
| 12 | Portuloside A | 72.2 | 332.22 | [21] |
| 13 | Portulene | 66.3 | 337.02 | [22] |
| 14 | Lupeol | 66.5 | 427.5 | [22] |
| 15 | (3S)-3-O-(β-D-Glucopyranosyl)-3,7-dimethylocta-1,6-dien-3-ol | 67.8 | 318.12 | [23] |
| 16 | Friedelane | 54.9 | 413.34 | [24] |
| 17 | Quercetin | 39.4 | 303.18 | [25] |
| 18 | Myricetin | 55.1 | 318.24 | [25] |
| 19 | Genistin | 65.4 | 433.20 | [26] |
| 20 | Indole-3-carboxylic acid | 77.8 | 162.90 | [16] |
| 21 | Palmitic acid | 62.2 | 256.14 | [27] |
| 22 | Stearic acid | 37.8 | 285.18 | [27] |
| 23 | Caffeic acid | 65.8 | 181.08 | [28] |
| 24 | Riboflavin | 35.0 | 376.62 | [29] |
| 25 | Vitamin C | 28.5 | 177.00 | [29] |
| 26 | α-Tocopherol | 67.1 | 431.22 | [27] |
| 27 | Hesperidin | 76.8 | 611.58 | [30] |
| 28 | Portulacerebroside A | 64.6 | 843.18 | [24] |
| 29 | β-Sitosterol | 48.7 | 415.32 | [22] |
| 30 | β-Carotene | 37.5 | 538.74 | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhshandeh, H.; Rajabi Khasevan, H.; Saviano, A.; Mahdinezhad, M.R.; Baradaran Rahimi, V.; Ehtiati, S.; Etemad, L.; Ebrahimzadeh-bideskan, A.; Maione, F.; Askari, V.R. Protective Effect of Portulaca oleracea on Streptozotocin-Induced Type I Diabetes-Associated Reproductive System Dysfunction and Inflammation. Molecules 2022, 27, 6075. https://doi.org/10.3390/molecules27186075
Rakhshandeh H, Rajabi Khasevan H, Saviano A, Mahdinezhad MR, Baradaran Rahimi V, Ehtiati S, Etemad L, Ebrahimzadeh-bideskan A, Maione F, Askari VR. Protective Effect of Portulaca oleracea on Streptozotocin-Induced Type I Diabetes-Associated Reproductive System Dysfunction and Inflammation. Molecules. 2022; 27(18):6075. https://doi.org/10.3390/molecules27186075
Chicago/Turabian StyleRakhshandeh, Hassan, Hamed Rajabi Khasevan, Anella Saviano, Mohammad Reza Mahdinezhad, Vafa Baradaran Rahimi, Sajjad Ehtiati, Leila Etemad, Alireza Ebrahimzadeh-bideskan, Francesco Maione, and Vahid Reza Askari. 2022. "Protective Effect of Portulaca oleracea on Streptozotocin-Induced Type I Diabetes-Associated Reproductive System Dysfunction and Inflammation" Molecules 27, no. 18: 6075. https://doi.org/10.3390/molecules27186075
APA StyleRakhshandeh, H., Rajabi Khasevan, H., Saviano, A., Mahdinezhad, M. R., Baradaran Rahimi, V., Ehtiati, S., Etemad, L., Ebrahimzadeh-bideskan, A., Maione, F., & Askari, V. R. (2022). Protective Effect of Portulaca oleracea on Streptozotocin-Induced Type I Diabetes-Associated Reproductive System Dysfunction and Inflammation. Molecules, 27(18), 6075. https://doi.org/10.3390/molecules27186075











