Recovery Strategies for Heavy Metal-Inhibited Biological Nitrogen Removal from Wastewater Treatment Plants: A Review
Abstract
:1. Introduction
2. Methods for Biological Nitrogen Removal
2.1. Nitrification and Denitrification
2.2. Anammox
2.3. Nutrient Removal by Algae/Microalgae-Based Processes
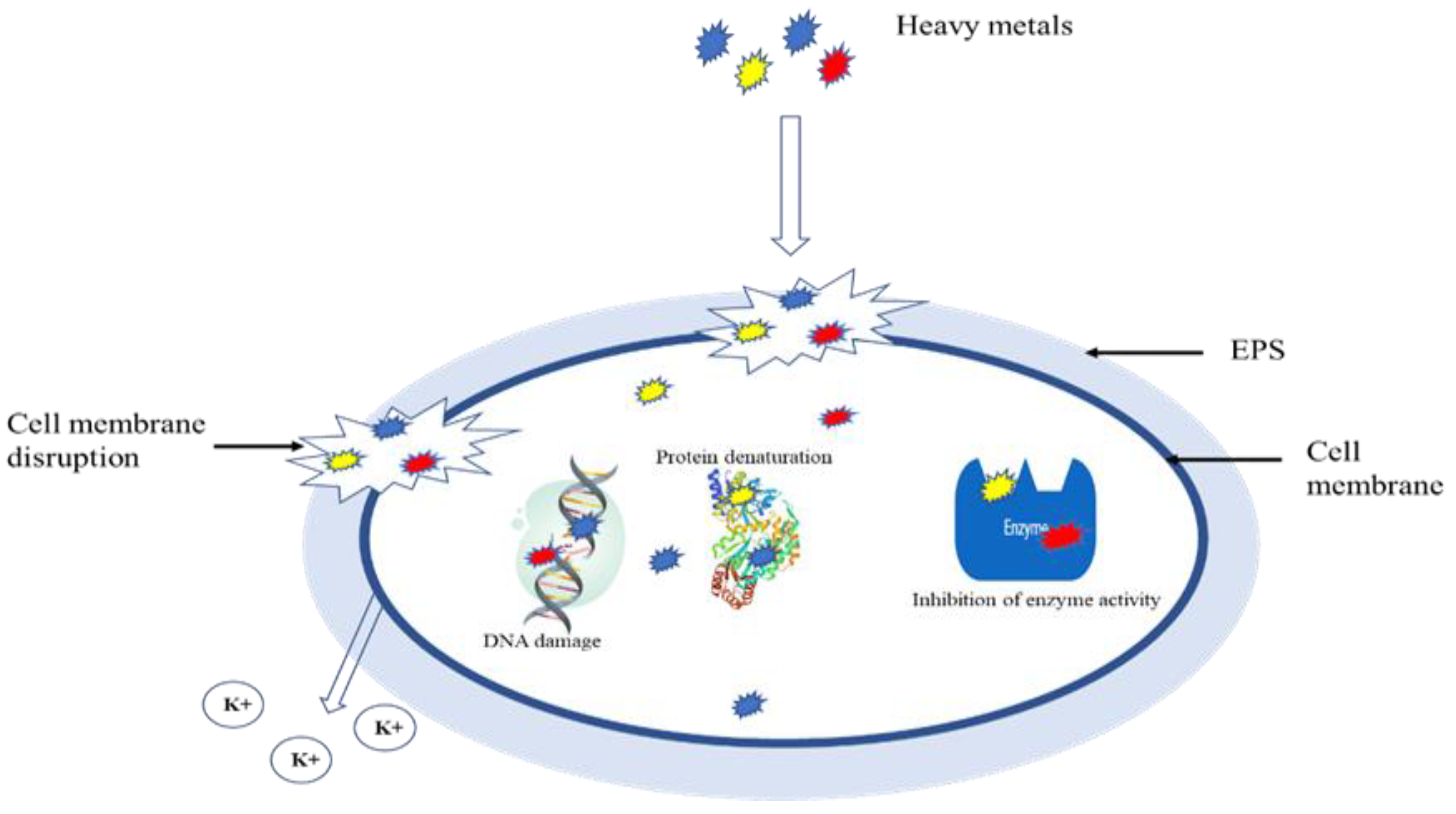
3. Inhibition of Biological Nitrogen Removal Process by Heavy Metals
4. Mechanism of Heavy Metal Inhibition of Biological Methods
4.1. Copper
4.2. Zinc
4.3. Cadmium
4.4. Lead
4.5. Nickel
4.6. Arsenic
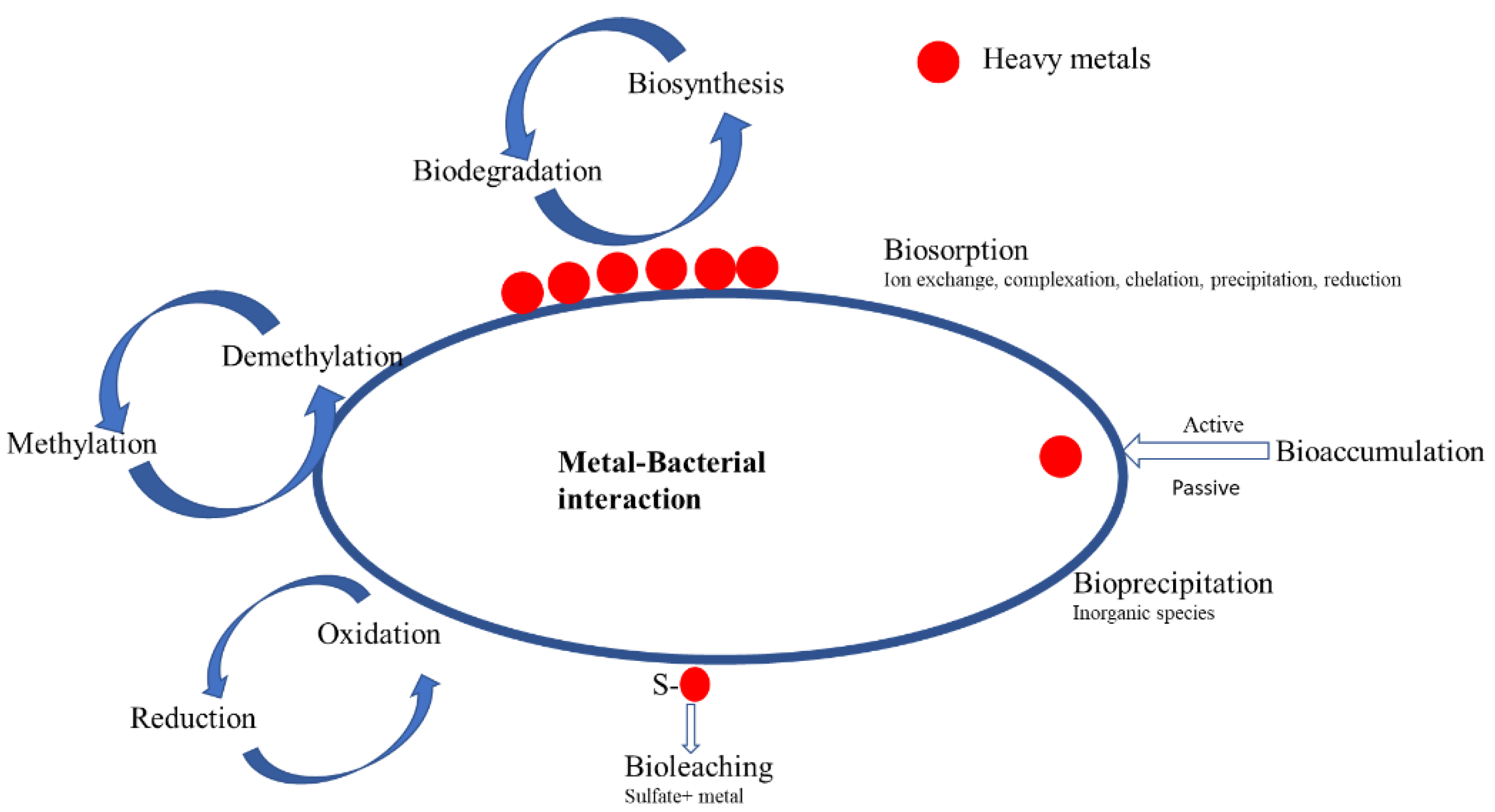
5. Current Strategies in Place to Prevent Inhibition of Biological Processes by Heavy Metals
6. Recovery Strategy for the Biological Wastewater Treatment Process
6.1. Physical Methods to Recover Biological Wastewater Treatment
6.2. Recovering of Anammox Using a Chelating Agent
Post-Treatment of Chelating-Metal Complex By-Products
6.3. Recovering of Anammox Using External Field Energy
6.4. Recovering of Anammox Using Biological Accelerants
6.5. Post-Treatment of Biological Accelerants By-Products
7. Drawbacks of These Recovery Strategies
8. Recent Work on Recovery Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sankhla, M.S.; Kumari, M.; Nandan, M.; Kumar, R.; Agrawal, P. Heavy metals contamination in water and their hazardous effect on human health-a review. Int. J. Curr. Microbiol. A Sci. 2016, 5, 759–766. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, A.; Yadav, S.; Singhal, M.K. Assessment of heavy metal contamination in water of Kali River using principle component and cluster analysis, India. Sustain. Water Resour. Manag. 2018, 4, 573–581. [Google Scholar] [CrossRef]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in Eastern Cape Province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Ferati, F.; Kerolli-Mustafa, M.; Kraja-Ylli, A. Assessment of heavy metal contamination in water and sediments of Trepça and Sitnica rivers, Kosovo, using pollution indicators and multivariate cluster analysis. Environ. Monit. Assess. 2015, 187, 338. [Google Scholar] [CrossRef]
- Kiran, M.G.; Pakshirajan, K.; Das, G. Heavy metal removal from aqueous solution using sodium alginate immobilized sulfate reducing bacteria: Mechanism and process optimization. J. Environ. Manag. 2018, 218, 486–496. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, H.; Chandran, K. Nitrification inhibition by hexavalent chromium Cr (VI)–Microbial ecology, gene expression and off-gas emissions. Water Res. 2016, 92, 254–261. [Google Scholar] [CrossRef]
- Zhou, X.; Zhai, S.; Zhao, Y.; Liu, D.; Wang, Q.; Ji, M. Rapid recovery of inhibited denitrification with cascade Cr (VI) exposure by bio-accelerant: Characterization of chromium distributions, EPS compositions and denitrifying communities. J. Hazard. Mater. 2021, 411, 125087. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, M.; Zhao, Y.; Zhai, H. Recovery of nitrification in cadmium-inhibited activated sludge system by bio-accelerators. Bioresour. Technol. 2016, 200, 812–819. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ji, M.; Zhai, H. Nitrification recovery behavior by bio-accelerators in copper-inhibited activated sludge system. Bioresour. Technol. 2015, 192, 748–755. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron robustly stimulates simultaneous nitrification and denitrification under aerobic conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Medhi, K.; Singhal, A.; Chauhan, D.K.; Thakur, I.S. Investigating the nitrification and denitrification kinetics under aerobic and anaerobic conditions by Paracoccus denitrificans ISTOD1. Bioresour. Technol. 2017, 242, 334–343. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, B.; Peng, Y.; Li, X.; Zhang, Q.; Hu, T. Enhanced nutrient removal of simultaneous partial nitrification, denitrification and phosphorus removal (SPNDPR) in a single-stage anaerobic/micro-aerobic sequencing batch reactor for treating real sewage with low carbon/nitrogen. Chemosphere 2020, 257, 127097. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Ye, M.; Ma, X.; Li, Y.Y. Towards advanced nitrogen removal and optimal energy recovery from leachate: A critical review of anammox-based processes. Crit. Rev. Environ. Sci. Technol. 2020, 50, 612–653. [Google Scholar] [CrossRef]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef]
- Ma, B.; Qian, W.; Yuan, C.; Yuan, Z.; Peng, Y. Achieving mainstream nitrogen removal through coupling anammox with denitratation. Environ. Sci. Technol. 2017, 51, 8405–8413. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Phycoremediation of nitrogen and phosphate from wastewater using Picochlorum sp.: A tenable approach. J. Basic Microbiol. 2022, 62, 279–295. [Google Scholar] [CrossRef]
- Škufca, D.; Kovačič, A.; Prosenc, F.; Bulc, T.G.; Heath, D.; Heath, E. Phycoremediation of municipal wastewater: Removal of nutrients and contaminants of emerging concern. Sci. Total Environ. 2021, 782, 146949. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Kumar, A.; Bharti, R.K. An integrated approach for phycoremediation of municipal wastewater and production of sustainable transportation fuel using oleaginous Chlorella sp. J. Water Process. Eng. 2021, 42, 102183. [Google Scholar] [CrossRef]
- Hu, R.; Cao, Y.; Chen, X.; Zhan, J.; Luo, G.; Ngo, H.H.; Zhang, S. Progress on microalgae biomass production from wastewater phycoremediation: Metabolic mechanism, response behavior, improvement strategy and principle. Chem. Eng. J. 2022, 137187. [Google Scholar] [CrossRef]
- Salama, E.S.; Roh, H.S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.; Chang, S.W.; Jeon, B.H. Algae is a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [CrossRef]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Santo Domingo, J.W. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K. Evaluation of inhibitory effects of heavy metals on anaerobic ammonium oxidation (anammox) by continuous feeding tests. Appl. Microbiol. Biotechnol. 2014, 98, 6965–6972. [Google Scholar] [CrossRef]
- Gutwiński, P.; Cema, G.; Ziembińska-Buczyńska, A.; Wyszyńska, K.; Surmacz-Gorska, J. Long-term effect of heavy metals Cr (III), Zn (II), Cd (II), Cu (II), Ni (II), Pb (II) on the anammox process performance. J. Water Process. Eng. 2021, 39, 101668. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Nguyen, H.N.; Li, S.; Rodrigues, D.F. Effect of cadmium on the performance of partial nitrification using sequencing batch reactor. Chemosphere 2019, 222, 913–922. [Google Scholar] [CrossRef]
- Rehman, K.; Ijaz, A.; Arslan, M.; Afzal, M. Floating treatment wetlands as biological buoyant filters for wastewater reclamation. Int. J. Phytoremediat. 2019, 21, 1273–1289. [Google Scholar] [CrossRef]
- Cho, S.; Kambey, C.; Nguyen, V.K. Performance of anammox processes for wastewater treatment: A critical review on effects of operational conditions and environmental stresses. Water 2019, 12, 20–27. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; León, G.; Banihani, Q.; Field, J.A.; Sierra-Alvarez, R. Toxicity of copper (II) ions to microorganisms in biological wastewater treatment systems. Sci. Total Environ. 2011, 412, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Cheng, Y.F.; Zhou, Y.H.; Buayi, X.; Jin, R.C. A novel strategy for accelerating the recovery of an anammox reactor inhibited by copper (II): EDTA washing combined with biostimulation via low-intensity ultrasound. Chem. Eng. J. 2015, 279, 912–920. [Google Scholar] [CrossRef]
- Buaisha, M.; Balku, S.; Özalp-Yaman, S. Heavy metal removal investigation in conventional activated sludge systems. Civ. Eng. J. 2020, 6, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Qiao, S.; Zhou, J.; Tang, X.; Cheng, Y. Inhibition and recovery of Anammox biomass subjected to short-term exposure of Cd, Ag, Hg and Pb. Chem. Eng. J. 2014, 244, 89–96. [Google Scholar] [CrossRef]
- Hou, J.; Miao, L.; Wang, C.; Wang, P.; Ao, Y.; Qian, J.; Dai, S. Inhibitory effects of ZnO nanoparticles on aerobic wastewater biofilms from oxygen concentration profiles determined by microelectrodes. J. Hazard. Mater. 2014, 276, 164–170. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, K.; Sun, H.; Ren, H.; Zhang, X.X.; Ye, L. Comparison of the impacts of zinc ions and zinc nanoparticles on nitrifying microbial community. J. Hazard. Mater. 2018, 343, 166–175. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Ma, B.; Guo, L.; She, Z.; Zhao, Y.; Gao, M.; Jin, C.; Dong, J.; Wan, Y. Performance evaluation and microbial community of a sequencing batch reactor under divalent cadmium (Cd (II)) stress. Chem. Eng. J. 2018, 336, 325–333. [Google Scholar] [CrossRef]
- Yamina, B.; Tahar, B.; Lila, M.; Hocine, H.; Laure, F.M. Study on cadmium resistant-bacteria isolated from hospital wastewaters. J. Adv. Biol. Biotechnol. 2014, 5, 47951. [Google Scholar] [CrossRef]
- Ren, Q.; Gao, J.; Wang, C. Effects of Heavy Metals on the Performance and Mechanism of Anaerobic Ammonium Oxidation for Treating Wastewater. Front. Chem. Sci. Eng. 2022, 53, 851822. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.F.; Huang, B.C.; Jin, R.C. A review of heavy metals inhibitory effects in the process of anaerobic ammonium oxidation. J. Hazard. Mater. 2022, 429, 128362. [Google Scholar] [CrossRef]
- Ma, B.; Li, Z.; Wang, S.; Liu, Z.; Li, S.; She, Z.; Yu, N.; Zhao, C.; Jin, C.; Zhao, Y.; et al. Insights into the effect of nickel (Ni (II)) on the performance, microbial enzymatic activity and extracellular polymeric substances of activated sludge. Environ. Pollut. 2019, 251, 81–89. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Wang, D.; Wang, S.; Chen, F.; Zhong, Y.; Yi, K.; Yao, F.; Jiang, C.; Li, S.; et al. Nickel toxicity to the performance and microbial community of enhanced biological phosphorus removal system. Chem. Eng. J. 2017, 313, 415–423. [Google Scholar] [CrossRef]
- Qu, C.; Duan, C.; Li, W.; Wu, X.; Liu, Z.; Feng, F.; Tang, X.; Chai, X.; Tang, C.J. Understanding the slight inhibition of high As (III) stress on nitritation process: Insights from arsenic speciation and microbial community analyses. J. Hazard. Mater. 2022, 435, 128957. [Google Scholar] [CrossRef]
- Papirio, S.; Zou, G.; Ylinen, A.; Di Capua, F.; Pirozzi, F.; Puhakka, J.A. Effect of arsenic on nitrification of simulated mining water. Bioresour. Technol. 2014, 164, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Ren, Z.Q.; Yu, L.Q.; Wu, X.X.; Yao, Y.X.; Zhang, J.T.; Guo, J.Y.; Fan, N.S.; Jin, R.C. Deciphering the response of anammox process to heavy metal and antibiotic stress: Arsenic enhances the permeability of extracellular polymeric substance and aggravates the inhibition of sulfamethoxazole. Chem. Eng. J. 2021, 426, 130815. [Google Scholar] [CrossRef]
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. J. Bioremed. Biodeg. 2015, 6, 315. [Google Scholar] [CrossRef]
- Azizi, S.; Kamika, I.; Tekere, M. Evaluation of heavy metal removal from wastewater in a modified packed bed biofilm reactor. PLoS ONE 2016, 11, e0155462. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process. Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Mpongwana, N.; Rathilal, S. A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment. Water 2022, 14, 1550. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M. An overview of heavy metal removal from wastewater using magnetotactic bacteria. J. Chem. Technol. Biotechnol. 2018, 93, 2817–2832. [Google Scholar] [CrossRef]
- Que, W.; Jiang, L.; Wang, C.; Liu, Y.; Zeng, Z.; Wang, X.; Ning, Q.; Liu, S.; Zhang, P.; Liu, S. Influence of sodium dodecyl sulfate coating on adsorption of methylene blue by biochar from aqueous solution. J. Environ. Sci. 2018, 70, 166–174. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhao, B.H.; Sun, Q.; Zhang, J.; Zhang, Y.Q.; Li, J. Responses of Anammox Process to Ni (II): Reactor Performance, Mechanisms and Inhibition Recovery. 2022, p. 51. Available online: https://www.researchsquare.com/article/rs-1611852/v1 (accessed on 8 August 2022).
- Feng, F.; Tang, X.; Qu, C.; Lu, X.; Liu, Z.; Tang, J.; Tang, C.J.; Chai, L. Hydroxylamine addition enhances fast recovery of anammox activity suffering Cr (VI) inhibition. Bioresour. Technol. 2021, 329, 124920. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Gao, H. A novel strategy for accelerating the recovery of a Fe (II)-inhibited anammox reactor by intermittent addition of betaine: Performance, kinetics and statistical analysis. Chemosphere 2020, 251, 126362. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, J.; Zhang, Y.; Lv, Y.; Dou, R.; Wen, S.; Li, L.; Chen, Y.; Hu, Y. Treatment of Ni-EDTA containing wastewater by electrocoagulation using iron scraps packed-bed anode. Chemosphere 2016, 164, 304–313. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Y.; Shan, C.; Li, X.; Zhang, W.; Pan, B. Coupled Cu (II)-EDTA degradation and Cu (II) removal from acidic wastewater by ozonation: Performance, products and pathways. Chem. Eng. J. 2016, 299, 23–29. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Pham, T.T.; Pham, H.G.; Hoang, B.L.; Nguyen, T.H.; Nguyen, T.H.; Tran, T.H.; Ngo, H.H. Fenton/ozone-based oxidation and coagulation processes for removing metals (Cu, Ni)-EDTA from plating wastewater. J. Water Process. Eng. 2021, 39, 101836. [Google Scholar] [CrossRef]
- Yin, X.; Qiao, S.; Zhou, J. Effects of cycle duration of an external electrostatic field on anammox biomass activity. Sci. Rep. 2016, 6, 19568. [Google Scholar] [CrossRef]
- Qiao, S.; Yin, X.; Zhou, J.; Furukawa, K. Inhibition and recovery of continuous electric field application on the activity of anammox biomass. Biodegradation 2014, 25, 505–513. [Google Scholar] [CrossRef]
- Duan, X.; Zhou, J.; Qiao, S.; Wei, H. Application of low intensity ultrasound to enhance the activity of anammox microbial consortium for nitrogen removal. Bioresour. Technol. 2011, 102, 4290–4293. [Google Scholar] [CrossRef]
- Ni, S.Q.; Wang, Y.; Wang, Z.; Cui, Z.; Zhang, J.; Ahmad, S.; Sung, S. Utilizing Infrared Electromagnetic Fields to Increase Anammox Activity and Growth Rates. ACS Es&T Water 2021, 1, 1220–1228. [Google Scholar]
- Zhang, C.; Li, L.; Wang, Y.; Hu, X. Enhancement of the ANAMMOX bacteria activity and granule stability through pulsed electric field at a lower temperature (16 ± 1 °C). Bioresour. Technol. 2019, 292, 121960. [Google Scholar] [CrossRef]
- Cheng, B.; Bao, J.; Du, J.; Tufail, H.; Xu, T.; Zhang, Y.; Mao, Q. Application of electric fields to mitigate inhibition on anammox consortia under long-term tetracycline stress. Bioresour. Technol. 2021, 341, 125730. [Google Scholar] [CrossRef]
- Madoni, P. Protozoa in wastewater treatment processes: A minireview. Ital. J. Zool. 2011, 78, 3–11. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Zhai, S.; Liu, D.; Zhou, X.; Wang, Y.; Cabrera, J.; Ji, M. Application of different redox mediators induced bio-promoters to accelerate the recovery of denitrification and denitrifying functional microorganisms inhibited by transient Cr (VI) shock. J. Hazard. Mater. 2021, 420, 126664. [Google Scholar] [CrossRef]
- Kim, B.; Nerenberg, R. Effects of eukaryotic predation on nitrifying MABR biofilms. Water Res. 2022, 209, 117911. [Google Scholar] [CrossRef]
- Hall, R.P. Growth-factors for protozoa. In Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 1943; Volume 1, pp. 249–268. [Google Scholar]
- Freudenthal, J.; Ju, F.; Bürgmann, H.; Dumack, K. Microeukaryotic gut parasites in wastewater treatment plants: Diversity, activity, and removal. Microbiome 2022, 10, 27. [Google Scholar] [CrossRef]
- Etemadzadeh, S.S.; Emtiazi, G. Generation of non-toxic, chemical functional bio-polymer for desalination, metal removal and antibacterial activities from animal meat by-product. Food Sci. Technol. 2021, 58, 159–165. [Google Scholar] [CrossRef]
- Vishali, S.; Kavitha, E. Application of membrane-based hybrid process on paint industry wastewater treatment. In Membrane-Based Hybrid Processes for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 97–117. ISBN 9780128241882. [Google Scholar]
- Mendes, S.S.; Miranda, V.; Saraiva, L.M. Hydrogen Sulfide and Carbon Monoxide Tolerance in Bacteria. Antioxidants 2021, 10, 729. [Google Scholar] [CrossRef]
- Santos, A.L.; Sodre, C.L.; Valle, R.S.; Silva, B.A.; Abi-Chacra, E.A.; Silva, L.V.; Souza-Goncalves, A.L.; Sangenito, L.S.; Goncalves, D.S.; Souza, L.O.; et al. Antimicrobial action of chelating agents: Repercussions on the microorganism development, virulence and pathogenesis. Curr. Med. Chem. 2012, 19, 2715–2737. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef]
- Arora, A.S.; Nawaz, A.; Qyyum, M.A.; Ismail, S.; Aslam, M.; Tawfik, A.; Yun, C.M.; Lee, M. Energy saving anammox technology-based nitrogen removal and bioenergy recovery from wastewater: Inhibition mechanisms, state-of-the-art control strategies, and prospects. Renew. Sust. Energ. Rev. 2021, 135, 110126. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, J.; Hu, B.; Zhao, J.; Yuan, C. Effective restoration of partial nitritation and anammox biofilm process by short-term hydroxylamine dosing: Mechanism and microbial interaction. Bioresour. Technol. 2021, 341, 125910. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Wang, Y.; Wang, H.; Yue, W.; Chen, Y.; Yu, D.; Chen, M.; Wei, Y. Roles of hydroxylamine and hydrazine in the in-situ recovery of one-stage partial nitritation-anammox process: Characteristics and mechanisms. Sci. Total Environ. 2020, 707, 135648. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Cheng, Y.F.; Jin, R.C. Comprehensive evaluation of the long-term effect of Cu2+ on denitrifying granular sludge and feasibility of in situ recovery by phosphate. J. Hazard. Mater. 2022, 422, 126901. [Google Scholar] [CrossRef] [PubMed]
| Reference | Heavy Metal | Inhibited Process | Metal-Inhibition Conc | Percent Inhibited | IC50 |
|---|---|---|---|---|---|
| Ochoa-Herrera et al. [28] | Cu (II) | denitrification | 50 mg/L | 100% | 0.95 mg/L |
| Zhang et al. [8] | Cu (II) | anammox | 16.3 mg/L | 20.1% | 30 mg/L |
| Zhang et al. [8] | Zn (II) | anammox | 20.0 mg/L | 20.1% | 25 mg/L |
| Kimura and Isaka, [23] | Ni | anammox | 10 mg/L | 87% | - |
| Kimura and Isaka [23] | Co | anammox | 12 mg/L | 64% | - |
| Kimura and Isaka [23] | Zn | anammox | 15 mg/L | 79% | - |
| Buaisha et al. [30] | Cu (II) | heterotrophic bacteria concentration | 0.7 mg/L | 25.00% | - |
| Buaisha et al. [30] | cadmium | heterotrophic bacteria concentration | 0.7 mg/L | 8.76% | - |
| Bi et al., [31] | Cd | anammox biomass | - | - | 11.16 ± 0.42 mg/L |
| Bi et al. [31] | Ag | anammox biomass | - | - | 11.52 ± 0.49 mg/L |
| Bi et al. [31] | Hg | anammox biomass | - | - | 60.35 ± 2.47 mg/L |
| Wang et al. [9] | Cu2+ | nitrification bioactivity | 50.00 mg/L | 80.00% | - |
| Ref | Inhibited Process | Inhibiting Metal | Method of Acceleration | Accelerant | Percent Recovered | Recovery Time | Recovery Cycle |
|---|---|---|---|---|---|---|---|
| Wang et al. [9] | AOB and NOB | copper | Bio-accelerators | biotin, l-aspartic acid and cytokinin, l-aspartic acid | 100% recovered | 8 days | - |
| Zhang etal. [29] | nitrogen removal rate | copper | chelating + bio-accelerator+ External field energy | EDTA + biostimulation+ low-intensity ultrasound | 100% recovered | 64 days | - |
| Zhang et al. [29] | dehydrogenase activity | copper | chelating + bio-accelerator+ External field energy | EDTA + biostimulation+ low-intensity ultrasound | 100% recovered | 110 days | - |
| Wang et al. [8] | nitrifying bacteria | cadmium | bio-accelerators | Biotin, l-aspartic acid, and cytokinin | 100% recovered | 7 days | - |
| Wang et al. [64] | denitrification | Cr (VI) | natural conditions | natural conditions | 89.28 ± 0.20% | - | 52 T |
| Wang et al. [64] | denitrification | Cr (VI) | redox mediators + bio-promoters | L-cysteine, flavin adenine dinucleotide (FAD), biotin, cytokinin, and redox mediators | <90.00% | - | >40 T |
| Wang et al. [64] | denitrification | Cr (VI) | redox mediators + bio-promoters | L-cysteine, FAD, biotin, and cytokinin | 90.3% | - | 42 T |
| Wang et al. [64] | denitrification | Cr (VI) | natural conditions | natural conditions | - | 63 T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpongwana, N.; Rathilal, S.; Tetteh, E.K. Recovery Strategies for Heavy Metal-Inhibited Biological Nitrogen Removal from Wastewater Treatment Plants: A Review. Microorganisms 2022, 10, 1834. https://doi.org/10.3390/microorganisms10091834
Mpongwana N, Rathilal S, Tetteh EK. Recovery Strategies for Heavy Metal-Inhibited Biological Nitrogen Removal from Wastewater Treatment Plants: A Review. Microorganisms. 2022; 10(9):1834. https://doi.org/10.3390/microorganisms10091834
Chicago/Turabian StyleMpongwana, Ncumisa, Sudesh Rathilal, and Emmanuel K. Tetteh. 2022. "Recovery Strategies for Heavy Metal-Inhibited Biological Nitrogen Removal from Wastewater Treatment Plants: A Review" Microorganisms 10, no. 9: 1834. https://doi.org/10.3390/microorganisms10091834
APA StyleMpongwana, N., Rathilal, S., & Tetteh, E. K. (2022). Recovery Strategies for Heavy Metal-Inhibited Biological Nitrogen Removal from Wastewater Treatment Plants: A Review. Microorganisms, 10(9), 1834. https://doi.org/10.3390/microorganisms10091834