Membranes Based on Polyvinylidene Fluoride and Radiation-Grafted Sulfonated Polystyrene and Their Performance in Proton-Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Materials
3. Membrane Synthesis
4. Methods
4.1. Performance of Fuel Cell
4.1.1. Technique for the Formation of Membrane Electrode Assemblies
4.1.2. Testing of MEAs
5. Results and Discussions
5.1. Membranes Characterization
5.2. Testing of MEAs
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearre, N.; Adye, K.; Swan, L. Proportioning Wind, Solar, and in-Stream Tidal Electricity Generating Capacity to Co-Optimize Multiple Grid Integration Metrics. Appl. Energy 2019, 242, 69–77. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Digital Management of Solar Energy En Route to Energy Self-Sufficiency. Glob. Chall. 2019, 3. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in Carbon Capture Technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen Energy: Development Prospects and Materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Birol, F. The Future of Hydrogen: Seizing Today’s Opportunities. IEA Rep. Prep. G 2019. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Golubenko, D.V. Membrane Materials for Energy Production and Storage. Pure Appl. Chem. 2020, 92, 1147–1157. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of Energy Storage Systems and Environmental Challenges of Batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Lin, L.; Zhu, Q.; Wang, G.; Guo, H. Study of the Carbon Cycle of a Hydrogen Supply System over a Supported Pt Catalyst: Methylcyclohexane–Toluene–Hydrogen Cycle. Catal. Sci. Technol. 2020, 10, 1171–1181. [Google Scholar] [CrossRef]
- Seong, W.M.; Park, K.-Y.; Lee, M.H.; Moon, S.; Oh, K.; Park, H.; Lee, S.; Kang, K. Abnormal Self-Discharge in Lithium-Ion Batteries. Energy Environ. Sci. 2018, 11, 970–978. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-Exchange Membranes in the Chemical Process Industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Zabolotskii, V.; Sheldeshov, N.; Melnikov, S. Heterogeneous Bipolar Membranes and Their Application in Electrodialysis. Desalination 2014, 342, 183–203. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Pokhidnia, E.V.; Pourcelly, G.; Nikonenko, V.V. Can the Electrochemical Performance of Heterogeneous Ion-Exchange Membranes Be Better than That of Homogeneous Membranes? J. Membr. Sci. 2018, 566, 54–68. [Google Scholar] [CrossRef]
- Svoboda, M.; Beneš, J.; Vobecká, L.; Slouka, Z. Swelling Induced Structural Changes of a Heterogeneous Cation-Exchange Membrane Analyzed by Micro-Computed Tomography. J. Membr. Sci. 2017, 525, 195–201. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L.; Baklouti, L. Modelling of Anion-Exchange Membrane Transport Properties with Taking into Account the Change in Exchange Capacity and Swelling When Varying Bathing Solution Concentration and PH. J. Membr. Sci. 2019, 590, 117291. [Google Scholar] [CrossRef]
- Hodgdon, R.B. Polyelectrolytes Prepared from Perfluoroalkylaryl Macromolecules. J. Polym. Sci. Part A-1 Polym. Chem. 1968, 6, 171–191. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-Grafted Materials for Energy Conversion and Energy Storage Applications. Prog. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Sproll, V.; Schmidt, T.J.; Gubler, L. Grafting Design: A Strategy to Increase the Performance of Radiation-Grafted Membranes. Polym. Int. 2016, 65, 174–180. [Google Scholar] [CrossRef]
- Albert, A.; Lochner, T.; Schmidt, T.J.; Gubler, L. Stability and Degradation Mechanisms of Radiation-Grafted Polymer Electrolyte Membranes for Water Electrolysis. ACS Appl. Mater. Interfaces 2016, 8, 15297–15306. [Google Scholar] [CrossRef]
- Ben youcef, H.; Henkensmeier, D.; Balog, S.; Scherer, G.G.; Gubler, L. Copolymer Synergistic Coupling for Chemical Stability and Improved Gas Barrier Properties of a Polymer Electrolyte Membrane for Fuel Cell Applications. Int. J. Hydrogen Energy 2020, 45, 7059–7068. [Google Scholar] [CrossRef]
- Golubenko, D.; Yaroslavtsev, A. Development of Surface-Sulfonated Graft Anion-Exchange Membranes with Monovalent Ion Selectivity and Antifouling Properties for Electromembrane Processes. J. Membr. Sci. 2020, 612, 118408. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yaroslavtsev, A.B. New Approach to the Preparation of Grafted Ion Exchange Membranes Based on UV-Oxidized Polymer Films and Sulfonated Polystyrene. Mendeleev Commun. 2017, 27, 572–573. [Google Scholar] [CrossRef]
- Golubenko, D.V.; van der Bruggen, B.; Yaroslavtsev, A.B. Novel Anion Exchange Membrane with Low Ionic Resistance Based on Chloromethylated/Quaternized-grafted Polystyrene for Energy Efficient Electromembrane Processes. J. Appl. Polym. Sci. 2020, 137, 48656. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Pourcelly, G.; Yaroslavtsev, A.B. Permselectivity and Ion-Conductivity of Grafted Cation-Exchange Membranes Based on UV-Oxidized Polymethylpenten and Sulfonated Polystyrene. Sep. Purif. Technol. 2018, 207, 329–335. [Google Scholar] [CrossRef]
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface Modification of Polyvinylidene Fluoride (PVDF) Membrane via Radiation Grafting: Novel Mechanisms Underlying the Interesting Enhanced Membrane Performance. Sci. Rep. 2017, 7, 2721. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Zhang, F.; Gao, S.; Wang, A.; Fang, W.; Jin, J. Zwitterionic Nanohydrogel Grafted PVDF Membranes with Comprehensive Antifouling Property and Superior Cycle Stability for Oil-in-Water Emulsion Separation. Adv. Funct. Mater. 2018, 28, 1804121. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, Y.; Wang, R.; Chen, D. Poly(Vinylidene Fluoride) Grafted Polystyrene (PVDF-g-PS) Membrane Based on in Situ Polymerization for Solvent Resistant Nanofiltration. RSC Adv. 2017, 7, 33201–33207. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Chen, S.T.; Ang, M.B.M.Y.; Patra, T.; Castilla-Casadiego, D.A.; Fan, R.; Almodovar, J.; Hung, W.S.; Wickramasinghe, S.R. High-Performance Polyacrylic Acid-Grafted Pvdf Nanofiltration Membrane with Good Antifouling Property for the Textile Industry. Polymers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Sezgin, S.; Sinirlioglu, D.; Muftuoglu, A.E.; Bozkurt, A. An Investigation of Proton Conductivity of Vinyltriazole-Grafted PVDF Proton Exchange Membranes Prepared via Photoinduced Grafting. J. Chem. 2014, 2014, 963131. [Google Scholar] [CrossRef] [Green Version]
- Lepit, A.; Aini, N.A.; Jaafar, N.K.; Hashim, N.; Ali, A.M.M.; Dahlan, K.Z.M.; Yahya, M.Z.A. Influences of Co-Polymerization 1-Vinylimidazole onto γ-Irradiated Poly(Vinylidene Flouride) Membranes. Int. J. Electrochem. Sci. 2012, 7, 8560–8577. [Google Scholar]
- Li, S.; Li, X.; Fu, P.; Zhang, Y. Alkali-Grafting Proton Exchange Membranes Based on Co-Grafting of α-Methylstyrene and Acrylonitrile into PVDF. Polymers 2022, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Khan, H.; Raj, S.K.; Kothandaraman, R.; Kulshrestha, V. Styrene-Co-DVB Grafted PVDF Proton Exchange Membranes for Vanadium Redox Flow Battery Applications. Mater. Adv. 2020, 1, 1930–1938. [Google Scholar] [CrossRef]
- Sharma, P.P.; Yadav, V.; Gahlot, S.; Lebedeva, O.V.; Chesnokova, A.N.; Srivastava, D.N.; Raskulova, T.V.; Kulshrestha, V. Acid Resistant PVDF-Co-HFP Based Copolymer Proton Exchange Membrane for Electro-Chemical Application. J. Membr. Sci. 2019, 573, 485–492. [Google Scholar] [CrossRef]
- Chavan, V.; Agarwal, C.; Adya, V.C.; Pandey, A.K. Hybrid Organic-Inorganic Anion-Exchange Pore-Filled Membranes for the Recovery of Nitric Acid from Highly Acidic Aqueous Waste Streams. Water Res. 2018, 133, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Choi, Y.W.; Choi, J.; Jeong, N.; Kim, H.; Jeong, H.; Byeon, S.Y.; Yoon, H.; Kim, Y.H. Green Fabrication of Pore-Filling Anion Exchange Membranes Using R2R Processing. J. Membr. Sci. 2019, 584, 181–190. [Google Scholar] [CrossRef]
- Xiao, X.; Shehzad, M.A.; Yasmin, A.; Zhang, Z.; Liang, X.; Ge, L.; Zhang, J.; Wu, L.; Xu, T. Covalent Bonding-Triggered Pore-Filled Membranes for Alkaline Fuel Cells. J. Membr. Sci. 2020, 597, 117776. [Google Scholar] [CrossRef]
- Song, H.-B.; Kim, D.-H.; Kang, M.-S. Thin-Reinforced Anion-Exchange Membranes with High Ionic Contents for Electrochemical Energy Conversion Processes. Membranes 2022, 12, 196. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yurova, P.A.; Desyatov, A.V.; Stenina, I.A.; Kosarev, S.A.; Yaroslavtsev, A.B. Pore-Filled Ion Conducting Membranes Based on Track-Etched Membranes and Sulfonated Polystyrene. Membr. Membr. Technol. 2022, in press.
- Mohamed, N.H.; Tamada, M.; Ueki, Y.; Seko, N. Emulsion Graft Polymerization of 4-Chloromethylstyrene on Kenaf Fiber by Pre-Irradiation Method. Radiat. Phys. Chem. 2013, 82, 63–68. [Google Scholar] [CrossRef]
- Ueki, Y.; Mohamed, N.H.; Seko, N.; Tamada, M. Rapid Biodiesel Fuel Production Using Novel Fibrous Catalyst Synthesized by Radiation-Induced Graft Polymerization. Int. J. Org. Chem. 2011, 1, 20–25. [Google Scholar] [CrossRef]
- Wang, L.; Magliocca, E.; Cunningham, E.L.; Mustain, W.E.; Poynton, S.D.; Escudero-Cid, R.; Nasef, M.M.; Ponce-González, J.; Bance-Souahli, R.; Slade, R.C.T.; et al. An Optimized Synthesis of High Performance Radiation-Grafted Anion-Exchange Membranes. Green Chem. 2017, 19, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Golubenko, D.V.; Malakhova, V.R.; Yurova, P.A.; Evsiunina, M.V.; Stenina, I.A. Effect of Sulfonation Conditions on Properties of Ion-Conducting Membranes Based on Polystyrene Grafted on Gamma-Irradiated Polyvinylidene Fluoride Films. Membr. Membr. Technol. 2022, 4, 267–275. [Google Scholar] [CrossRef]
- Volkov, A.O.; Golubenko, D.V.; Yaroslavtsev, A.B. Development of Solid Polymer Composite Membranes Based on Sulfonated Fluorocopolymer for Olefin/Paraffin Separation with High Permeability and Selectivity. Sep. Purif. Technol. 2021, 254, 117562. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Osipov, A.K.; Yaroslavtsev, A.B. Short Side Chain Aquivion Perfluorinated Sulfonated Proton-Conductive Membranes: Transport and Mechanical Properties. Pet. Chem. 2018, 58, 130–136. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O. Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms. Catalysts 2021, 11, 335. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.; Wang, H.; Mérida, W.; Zhu, H.; Shen, J.; Wu, S.; Zhang, J. A Review of Accelerated Stress Tests of MEA Durability in PEM Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 388–404. [Google Scholar] [CrossRef]
- Tarasevich, M.R.; Korchagin, O.V. Rapid Diagnostics of Characteristics and Stability of Fuel Cells with Proton-Conducting Electrolyte. Russ. J. Electrochem. 2014, 50, 737–750. [Google Scholar] [CrossRef]
- Shishlov, N.M.; Khursan, S.L. Effect of Ion Interactions on the IR Spectrum of Benzenesulfonate Ion. Restoration of Sulfonate Ion Symmetry in Sodium Benzenesulfonate Dimer. J. Mol. Struct. 2016, 1123, 360–366. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Safronova, E.Y.; Ilyin, A.B.; Shevlyakova, N.V.; Tverskoi, V.A.; Dammak, L.; Grande, D.; Yaroslavtsev, A.B. Influence of the Water State on the Ionic Conductivity of Ion-Exchange Membranes Based on Polyethylene and Sulfonated Grafted Polystyrene. Mater. Chem. Phys. 2017, 197, 192–199. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic Mobility in Ion-Exchange Membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.; Wu, C. Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(Vinylidene Fluoride) Membranes. Membranes 2013, 3, 389–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürsel, S.A.; Schneider, J.; ben Youcef, H.; Wokaun, A.; Scherer, G.G. Thermal Properties of Proton-Conducting Radiation-Grafted Membranes. J. Appl. Polym. Sci. 2008, 108, 3577–3585. [Google Scholar] [CrossRef]
- Holmberg, S.; Näsman, J.H.; Sundholm, F. Synthesis and Properties of Sulfonated and Crosslinked Poly [(Vinylidene Fluoride)-Graft-Styrene] Membranes. Polym. Adv. Technol. 1998, 9, 121–127. [Google Scholar] [CrossRef]
- McGrath, J.E.; Park, H.B.; Geise, G.M.; Sagle, A.C.; Freeman, B.D. Water Permeability and Water/Salt Selectivity Tradeoff in Polymers for Desalination. J. Membr. Sci. 2010, 369, 130–138. [Google Scholar] [CrossRef]
- Russell, S.T.; Pereira, R.; Vardner, J.T.; Jones, G.N.; Dimarco, C.; West, A.C.; Kumar, S.K. Hydration Effects on the Permselectivity-Conductivity Trade-Off in Polymer Electrolytes. Macromolecules 2020, 53, 1014–1023. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Sharma, R.; Andersen, S.M. Quantification on Degradation Mechanisms of Polymer Electrolyte Membrane Fuel Cell Catalyst Layers during an Accelerated Stress Test. Acs Catal. 2018, 8, 3424–3434. [Google Scholar] [CrossRef]
- Chu, T.; Zhang, R.; Wang, Y.; Ou, M.; Xie, M.; Shao, H.; Yang, D.; Li, B.; Ming, P.; Zhang, C. Performance Degradation and Process Engineering of the 10 KW Proton Exchange Membrane Fuel Cell Stack. Energy 2021, 219, 119623. [Google Scholar] [CrossRef]
- Gaumont, T.; Maranzana, G.; Lottin, O.; Dillet, J.; Didierjean, S.; Pauchet, J.; Guétaz, L. Measurement of Protonic Resistance of Catalyst Layers as a Tool for Degradation Monitoring. Int. J. Hydrog. Energy 2017, 42, 1800–1812. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Gubler, L.; Bonorand, L. Radiation Grafted Membranes for Fuel Cells: Strategies to Compete with PFSA Membranes. ECS Trans. 2013, 58, 149–162. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current Understanding of Chemical Degradation Mechanisms of Perfluorosulfonic Acid Membranes and Their Mitigation Strategies: A Review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Zhao, M.; Shi, W.; Wu, B.; Liu, W.; Liu, J.; Xing, D.; Yao, Y.; Hou, Z.; Ming, P.; Zou, Z. Influence of Membrane Thickness on Membrane Degradation and Platinum Agglomeration under Long-Term Open Circuit Voltage Conditions. Electrochim. Acta 2015, 153, 254–262. [Google Scholar] [CrossRef]
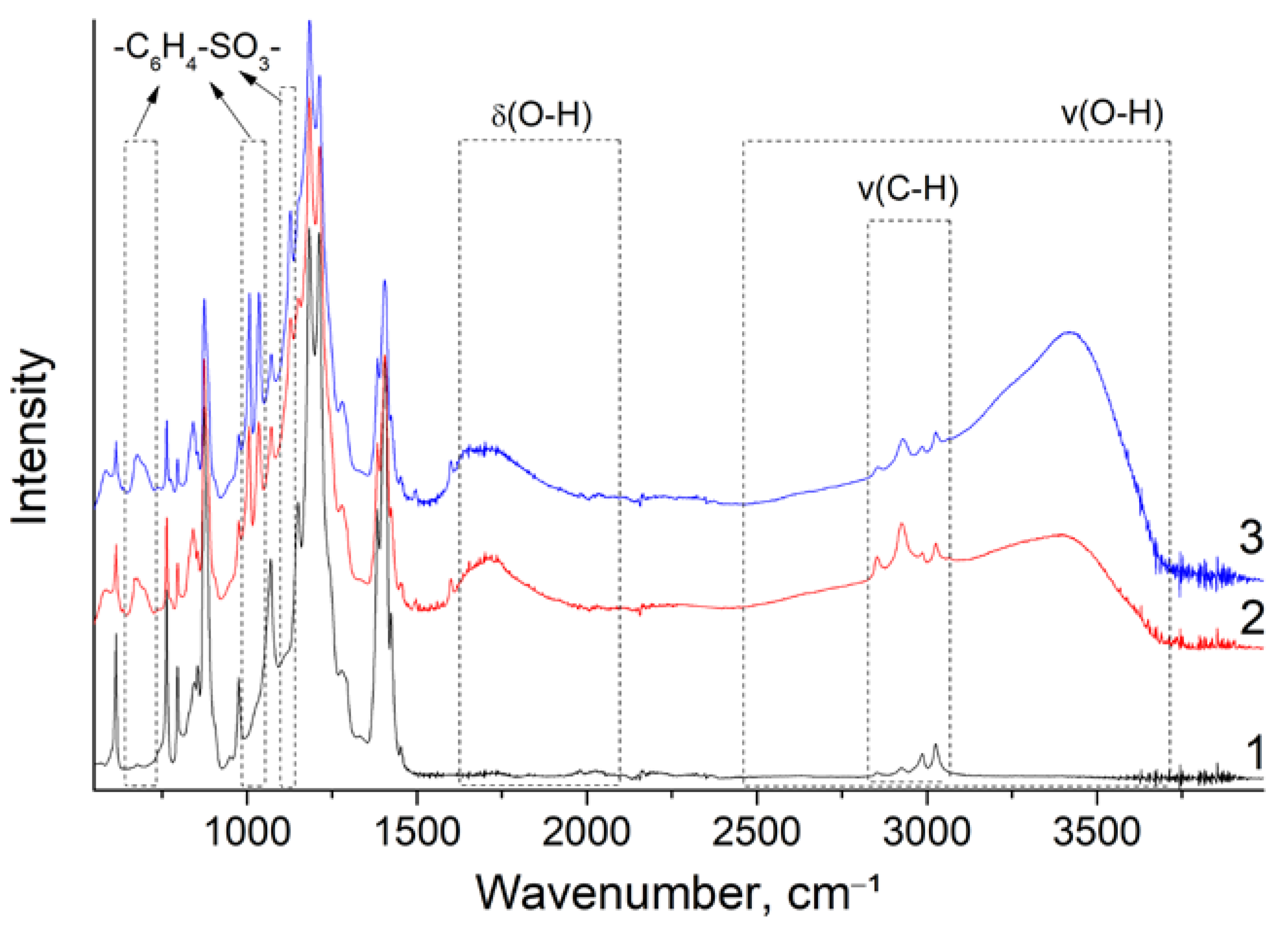
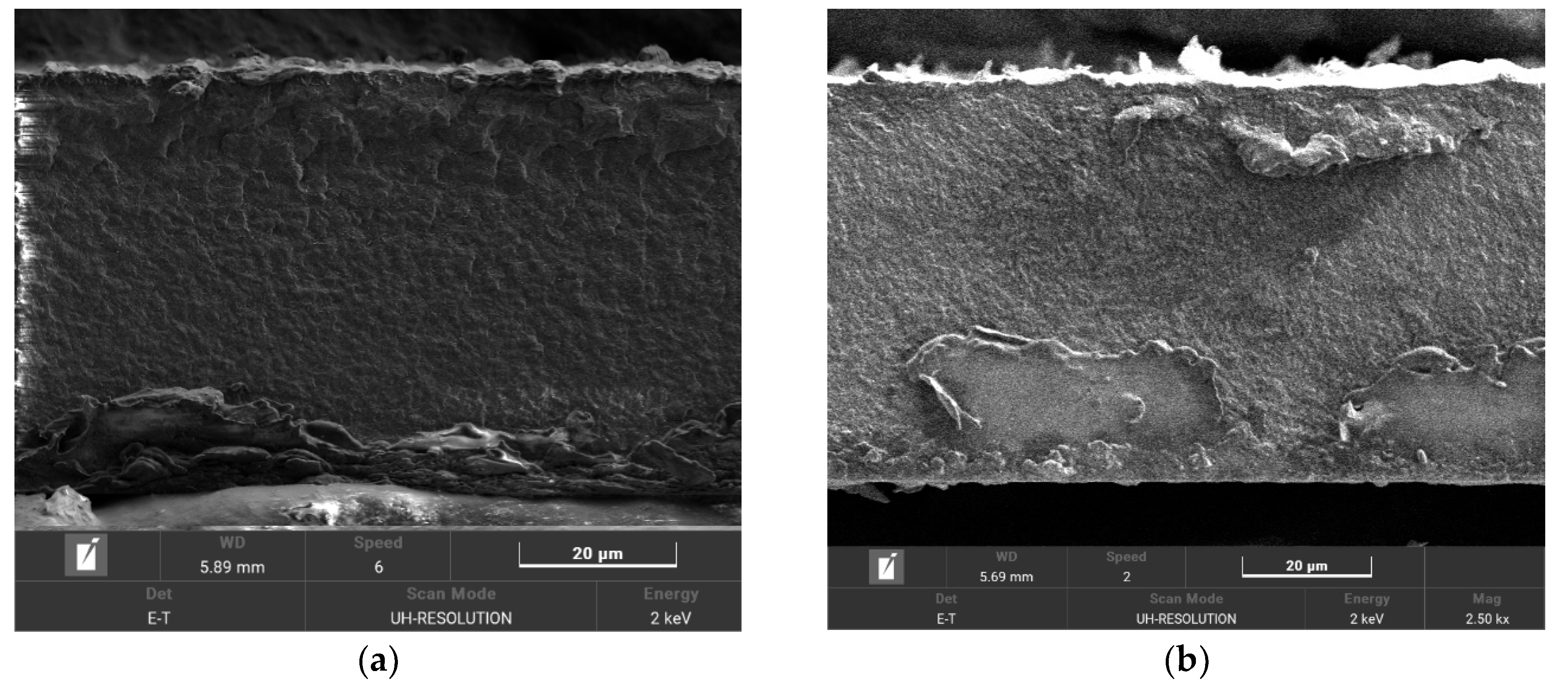
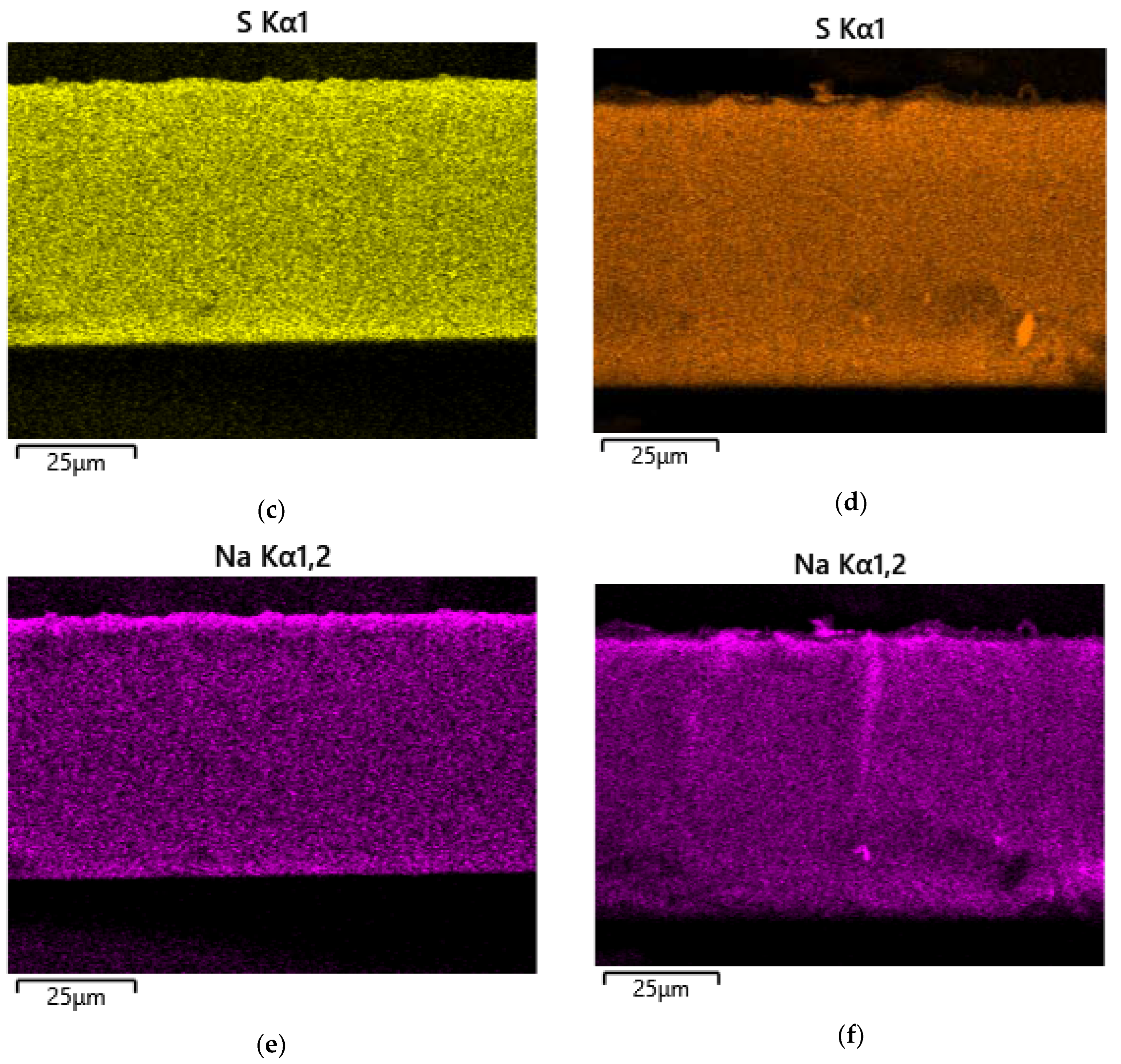
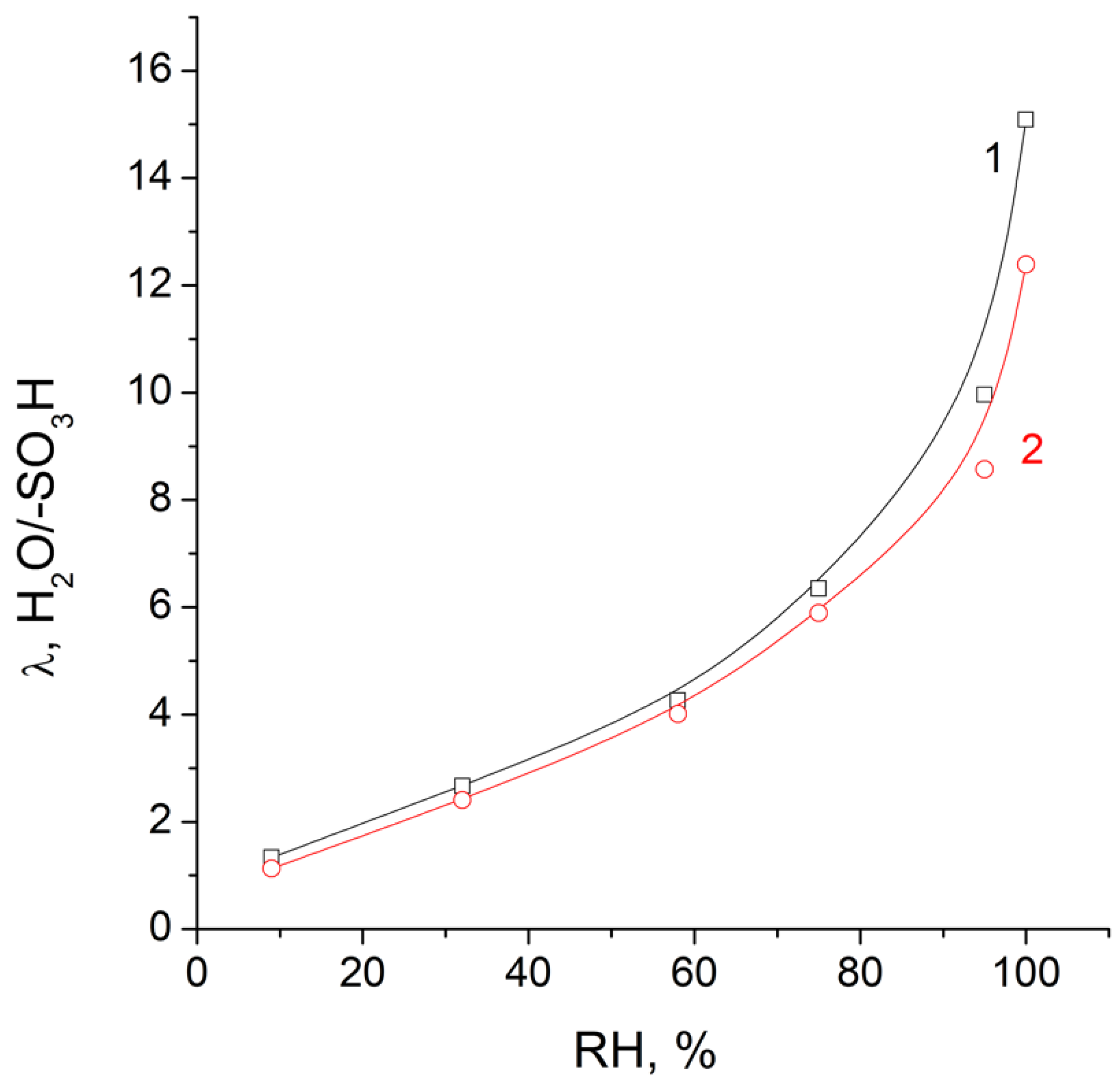
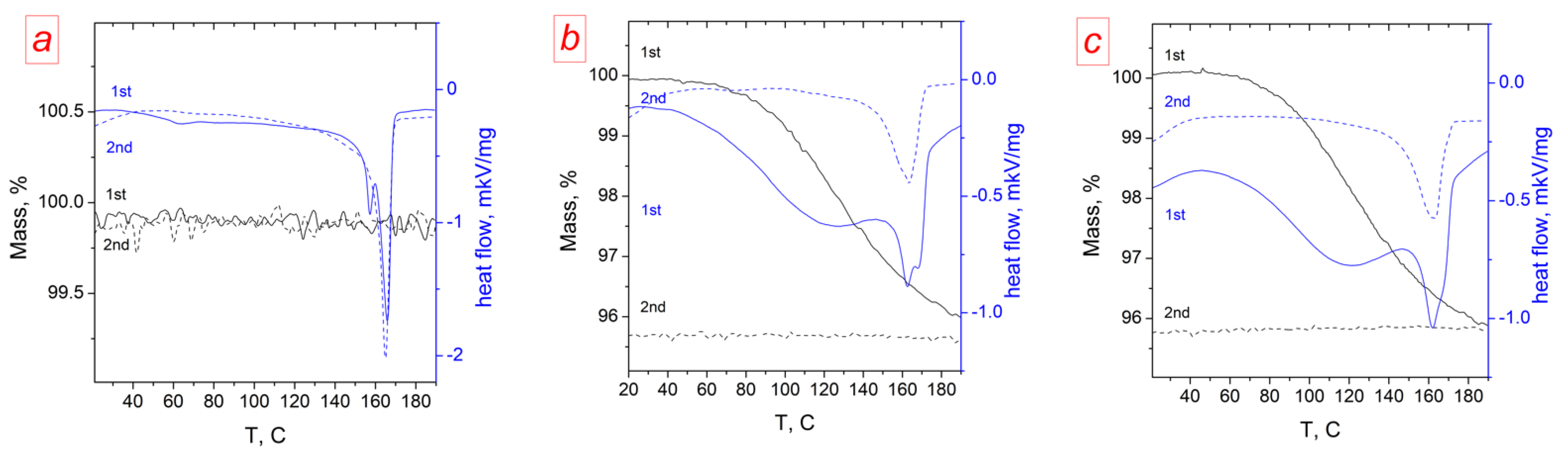
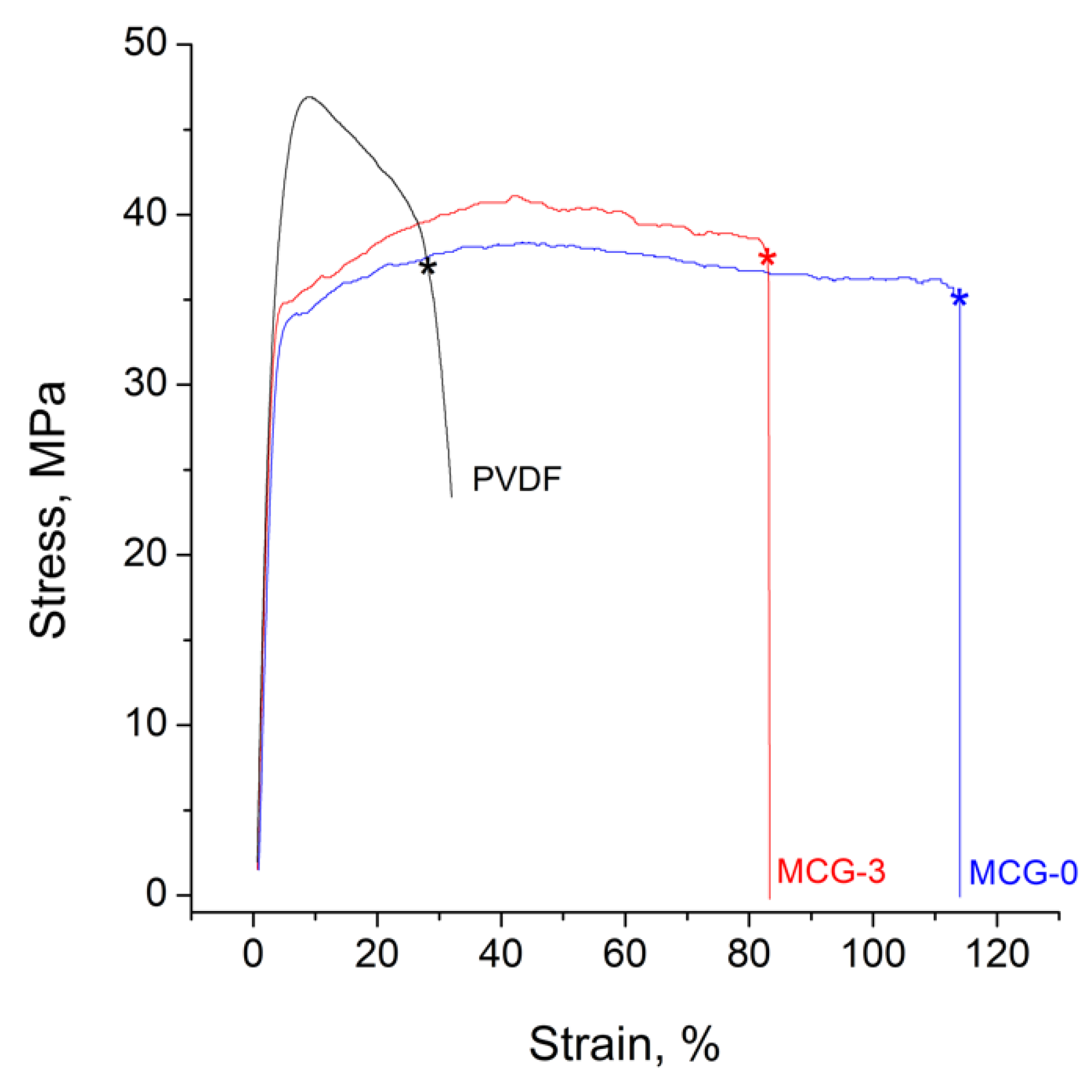
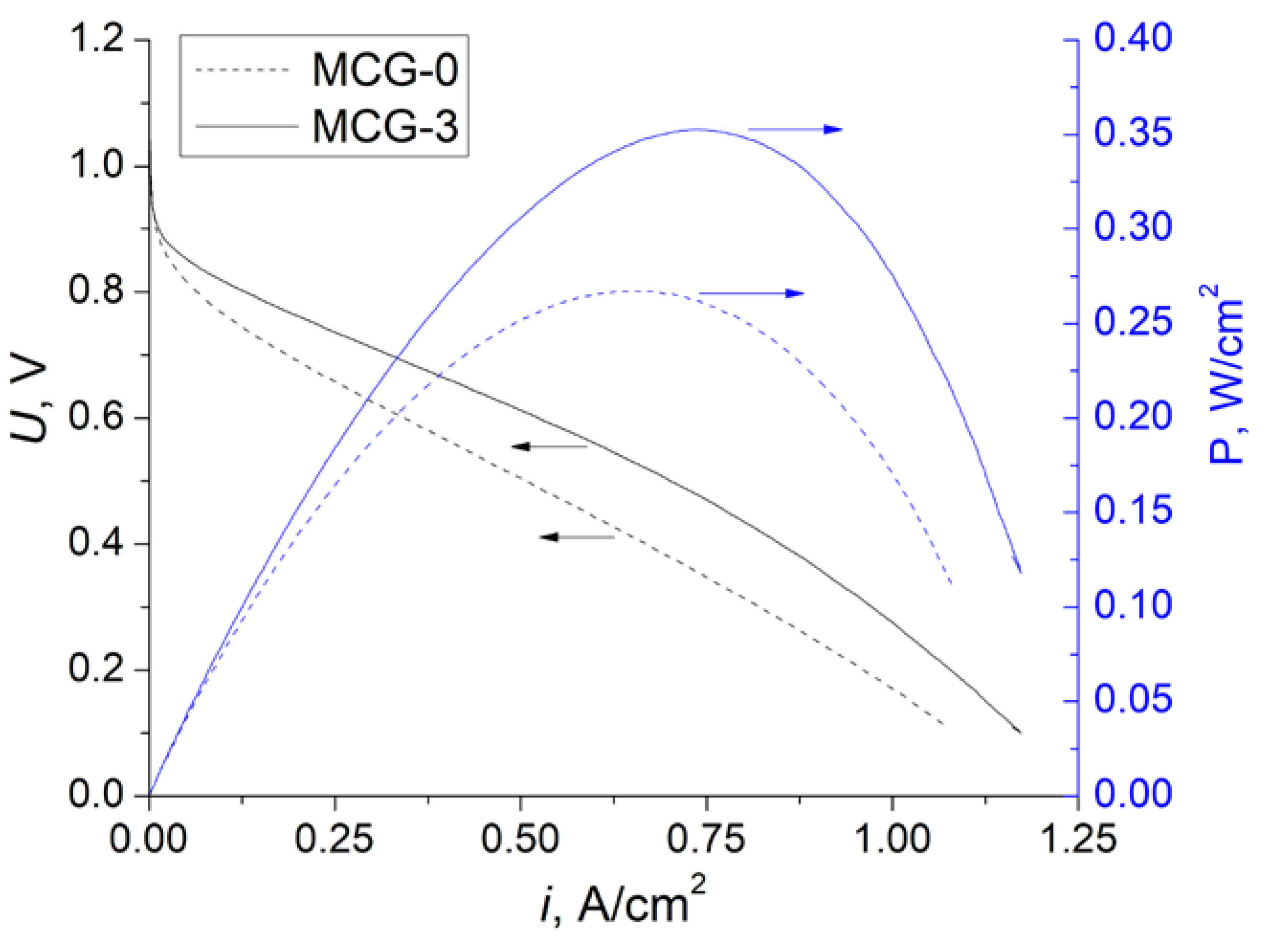

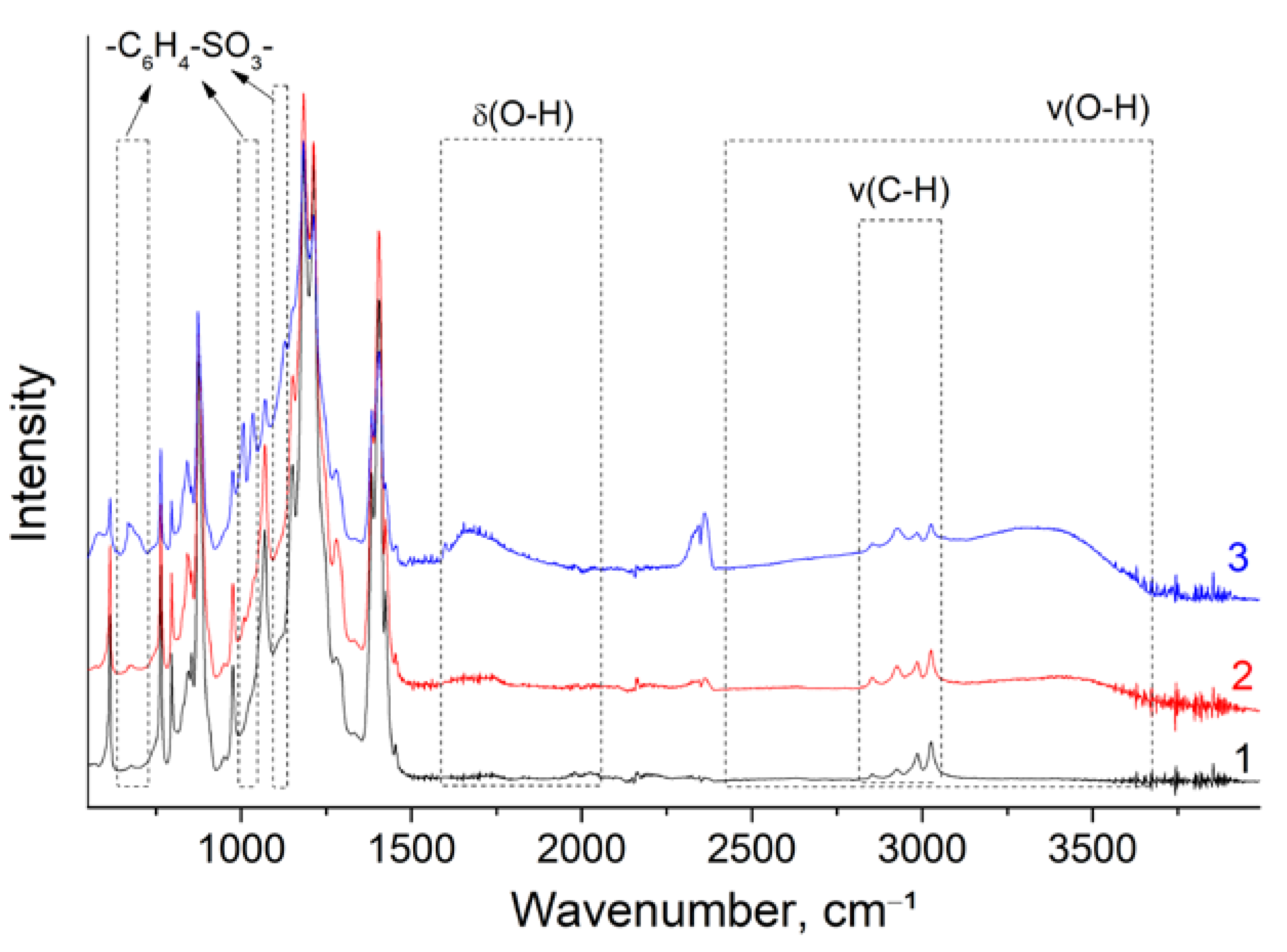
| Membrane | T, μm | DG,% | IEC, mg-eq/g | λ,H2O/ SO3H | P(H2)∙108, cm2/s | , mS/cm | , mS/cm |
|---|---|---|---|---|---|---|---|
| MCG-0 | 80 | 27 | 1.83 | 15 | 7.1 | 71 | 132 |
| MCG-3 | 74 | 24 | 1.82 | 12 | 5.2 | 47 | 85 |
| PVDF | 50 | 0 | - | - | 0.2 | - | - |
| Nafion®212 [45] | 50.8 | - | 0.87 | 13.8 | 21.4 | 17 | 25 |
| Sample | Tm1, °C | Tm2, °C | ∆H1, J/g | ∆H2, J/g | αmem *, % | αPVDF, % |
|---|---|---|---|---|---|---|
| PVDF (initial film) | 154–168 | 161–169 | 36.1 | 40.4 | 34.4 | 34.4 |
| MCG-0 | 157–173 | 145–170 | 16.9 | 17.5 | 16.1 | 24.0 |
| MCG-3 | 156–172 | 150–163 | 16.8 | 21.3 | 16.0 | 23.2 |
| Membrane/RH | Contact with Water | 95% | 30% |
|---|---|---|---|
| MCG-0 | 71 | 18 | 0.42 |
| MCG-3 | 47 | 25 | 0.44 |
| Sample | Young’s Modulus, % | Yield Strength, MPa | Tensile Strength at Break, MPa | Elongation on Break, % |
|---|---|---|---|---|
| MCG-0 | 1290 ± 60 | 34 ± 1 | 35 ± 1 | 107 ± 4 |
| MCG-3 | 1317 ± 160 | 34 ± 1 | 39 ± 1 | 71 ± 14 |
| PVDF | 1460 ± 140 | 45 ± 1 | 37 ± 1 | 30 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubenko, D.V.; Korchagin, O.V.; Voropaeva, D.Y.; Bogdanovskaya, V.A.; Yaroslavtsev, A.B. Membranes Based on Polyvinylidene Fluoride and Radiation-Grafted Sulfonated Polystyrene and Their Performance in Proton-Exchange Membrane Fuel Cells. Polymers 2022, 14, 3833. https://doi.org/10.3390/polym14183833
Golubenko DV, Korchagin OV, Voropaeva DY, Bogdanovskaya VA, Yaroslavtsev AB. Membranes Based on Polyvinylidene Fluoride and Radiation-Grafted Sulfonated Polystyrene and Their Performance in Proton-Exchange Membrane Fuel Cells. Polymers. 2022; 14(18):3833. https://doi.org/10.3390/polym14183833
Chicago/Turabian StyleGolubenko, Daniil V., Oleg V. Korchagin, Daria Yu. Voropaeva, Vera A. Bogdanovskaya, and Andrey B. Yaroslavtsev. 2022. "Membranes Based on Polyvinylidene Fluoride and Radiation-Grafted Sulfonated Polystyrene and Their Performance in Proton-Exchange Membrane Fuel Cells" Polymers 14, no. 18: 3833. https://doi.org/10.3390/polym14183833






