The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review
Abstract
:1. Introduction
2. Material and Methods
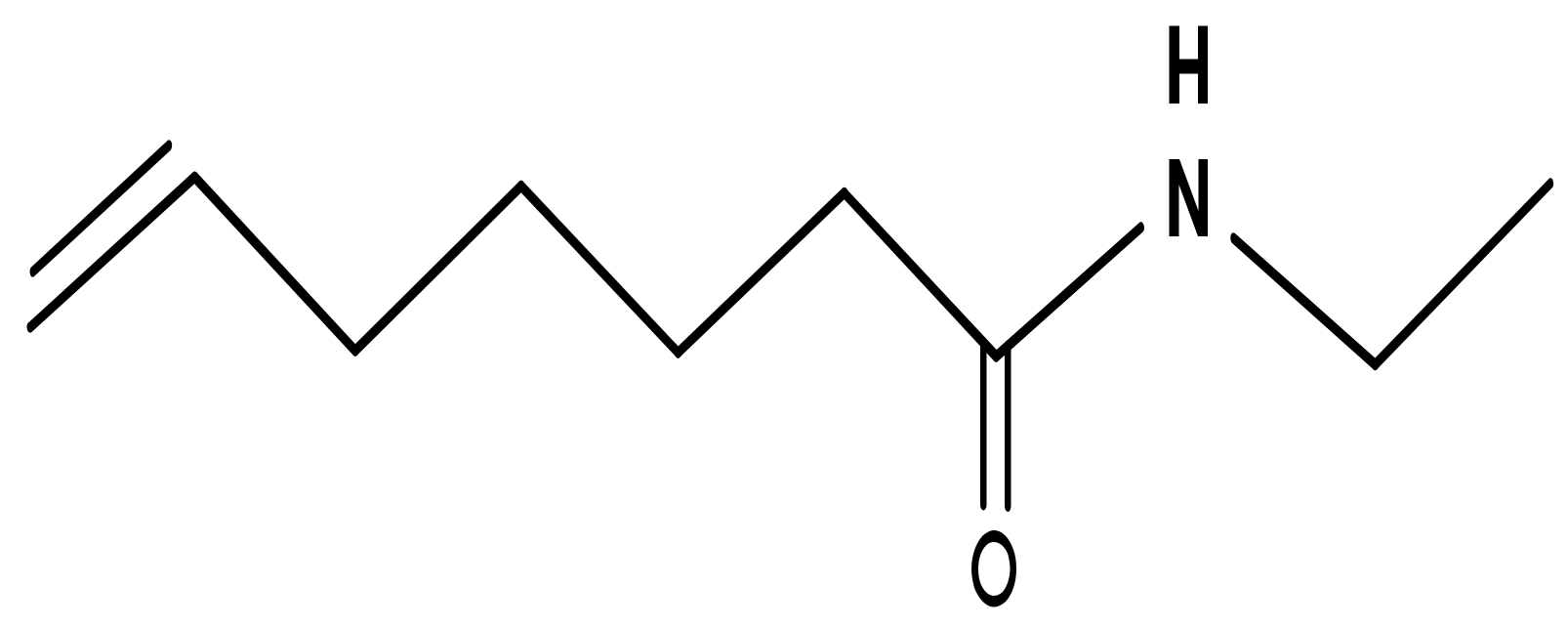
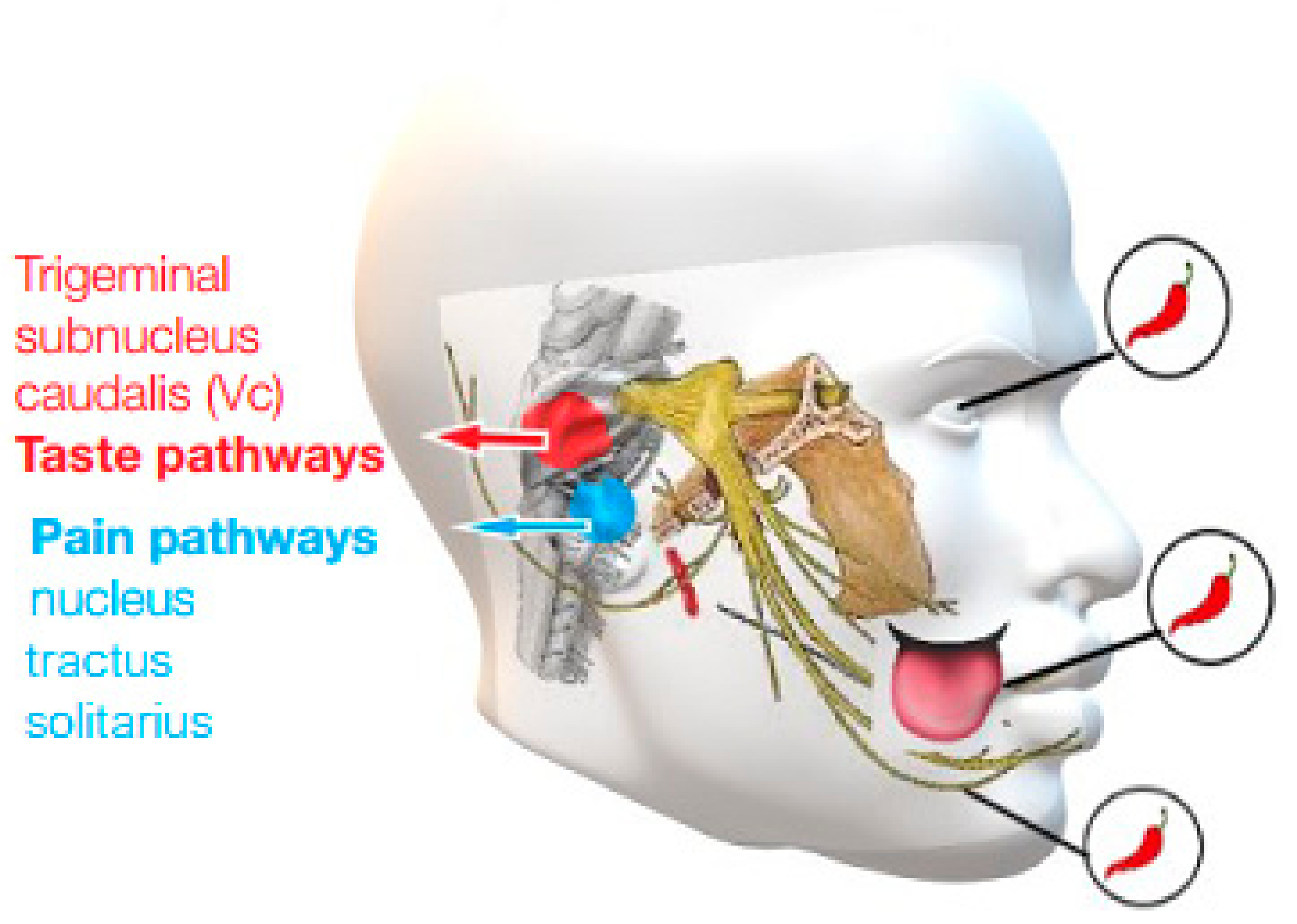
3. Mechanism of Action
- Repeated applications of Capsaicin deplete neurons’ SP [22].
- An excess of ion calcium influx causes the loss of mitochondrial functions, making the afferent fiber inoperative [23].
- High levels of intracellular calcium have cytotoxic effects, leading to the selective death of neuronal cells expressing TRPV1 receptors [24].
4. Pharmacological Aspects
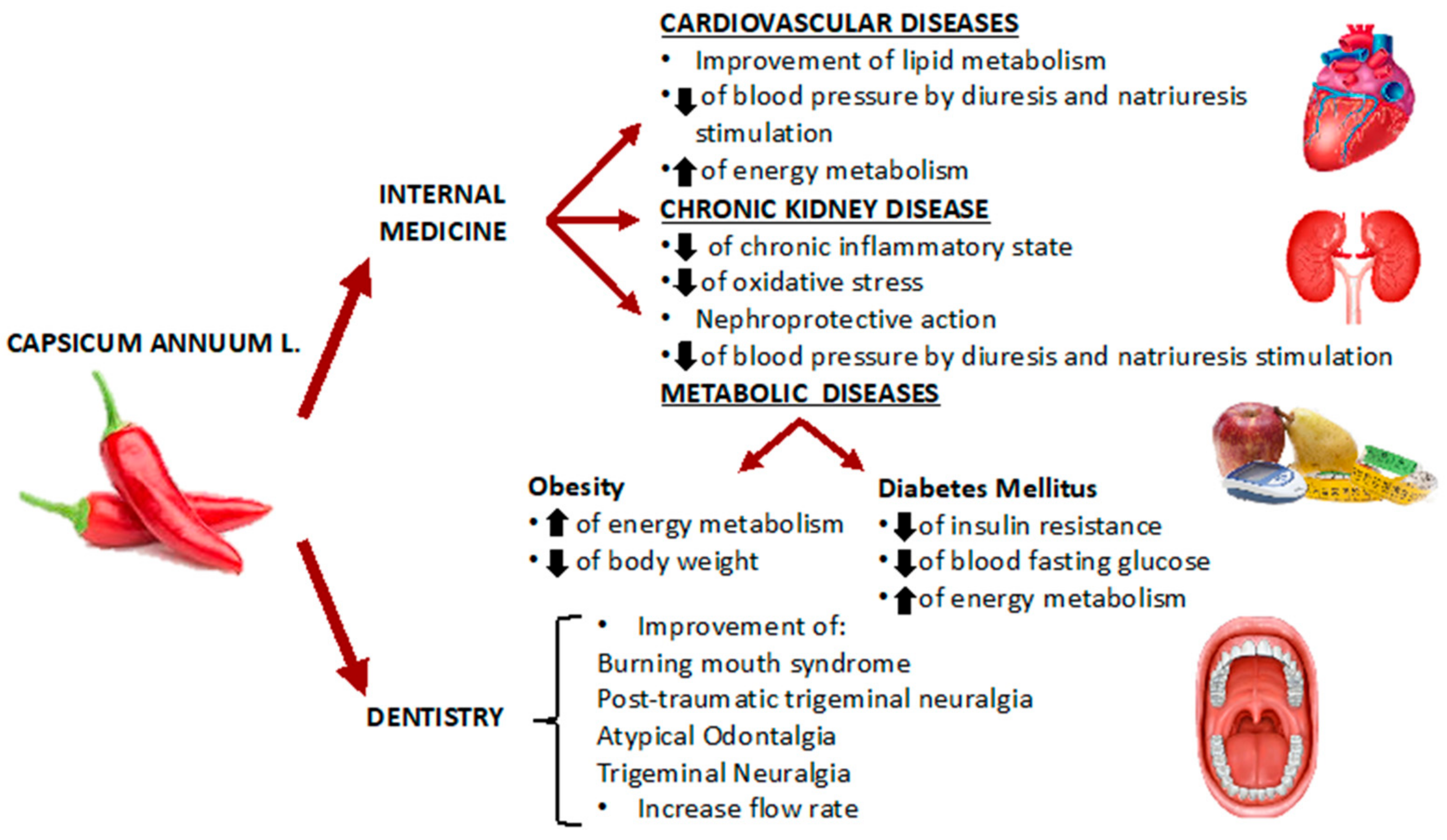
5. Effects of Capsicum annuum L. in Internal Medicine
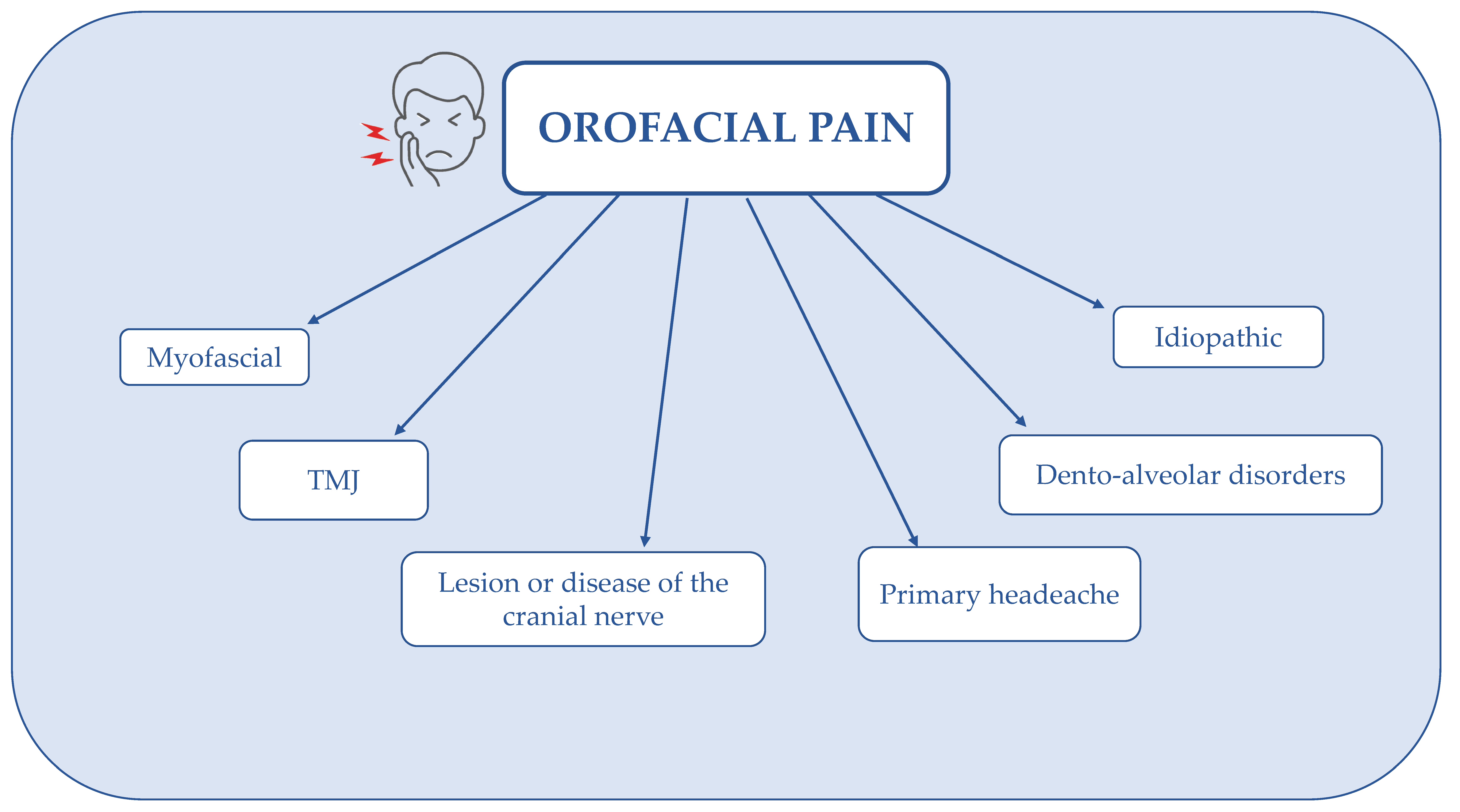
6. Effects of Capsicum annuum L. in Dentistry
Our Experience with Capsaicin in Dentistry
- Mucosal hyperemia.
- Acupressure with fovea sign or transient ischemia of the oral mucosa.
- Swollen or raised mucosa.
- Tactile allodynia.
- Thermic allodynia performed with a wet cotton wad at a known temperature (42 °C for hot and 2–4 °C for cold).
7. Conclusions
List of Abbreviations
| ACE | Angiotensin-converting-enzyme |
| AH | Arterial hypertension |
| AO | Atypical odontalgia |
| BMS | Burning mouth syndrome |
| CACC | Calcium-activated chloride channels |
| CGRP | Calcitonin gene-related peptide |
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| EGF | Epidermal growth factor |
| FDA | Food and drug administration |
| GFR | Glomerular filtration rate |
| GECs | Gingival epithelial cells |
| GLUT2 | Glucose transporter 2 |
| HDL | High-density lipoproteins |
| HIV-DSPN | HIV-associated distal sensory polyneuropathy |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| LDL | Low-density lipoproteins |
| LXR | Liver X receptor |
| mAChRs | Muscarinic acetylcholine receptors |
| NF-kB | Nuclear factor-kB |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| OH-1 | Heme oxygenase-1 |
| OED | Oral epithelial dysplasia |
| PDX-1 | Pancreatic duodenal homebox-1 |
| pERK | phosphorylated kinase |
| PHN | Post-herpetic neuropathy |
| PI3/AKT | Phosphoinositide 3-kinase/protein kinase B |
| PPARα | Peroxisome proliferators-activated receptor-α |
| PTNN | Painful post-traumatic trigeminal neuropathyoma |
| SMG | Submandibular gland |
| SNT | Solitary nucleus tract |
| SP | Substance P |
| TAK1/MAPK | Transforming growth factor-β-activated kinase 1/mitogen-activated protein-kinase |
| TG | Triglycerides |
| TJs | Tight junctions |
| TM1-6 | 6 transmembrane regions |
| TMD | Temporomandibular disorder |
| TN | Trigeminal neuralgia |
| TNF-α | Tumor necrosis factor-α |
| TRCs | Taste receptor cells |
| TRPV | Transient receptor potential vanilloid |
| Vc | Caudal trigeminal subnucleus |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Rosani, A.; Quick, J. Capsaicin; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yeam, C.T.; Yo, T.E.; Tan, Y.L.C.; Liew, A.; Seng, J.J.B. Complementary and alternative medicine therapies for uremic—A systematic review of randomized controlled trials. Complement. Ther. Med. 2021, 56, 102609. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Capsaicin 8% Dermal Patch: A Review in Peripheral Neuropathic Pain. Drugs 2018, 78, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Stevens, R.; Hanson, P.; Connolly, J.; Meske, D.S.; Chung, M.K.; Lascelles, B.D.X. Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules 2021, 26, 778. [Google Scholar] [CrossRef]
- Filipczak-Bryniarska, I.; Krzyzewski, R.M.; Kucharz, J.; Michalowska-Kaczmarczyk, A.; Kleja, J.; Woron, J.; Strzepek, K.; Kazior, L.; Wordliczek, J.; Grodzicki, T.; et al. High-dose 8% capsaicin patch in treatment of chemotherapy-induced peripheral neuropathy: Single-center experience. Med. Oncol. 2017, 34, 162. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.N.C.; Evans, R.J. The postmastectomy pain syndrome and topical capsaicin: A randomized trial. Pain 1992, 51, 375–379. [Google Scholar] [CrossRef]
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Saberski, L.; Bartoshuk, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain Symptom Manag. 1995, 10, 243–248. [Google Scholar] [CrossRef]
- Romero, V.; Lara, J.R.; Otero-Espinar, F.; Salgado, M.H.; Modolo, N.S.P.; Barros, G.A.M. [Capsaicin topical cream (8%) for the treatment of myofascial pain syndrome]. Braz J. Anesth. 2019, 69, 432–438. [Google Scholar] [CrossRef]
- Kim, D.Y.; Chancellor, M.B. Intravesical neuromodulatory drugs: Capsaicin and resiniferatoxin to treat the overactive bladder. J. Endourol. 2000, 14, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Galvez, R.; Navez, M.L.; Moyle, G.; Maihofner, C.; Stoker, M.; Ernault, E.; Nurmikko, T.J.; Attal, N. Capsaicin 8% Patch Repeat Treatment in Nondiabetic Peripheral Neuropathic Pain: A 52-Week, Open-Label, Single-Arm, Safety Study. Clin. J. Pain 2017, 33, 921–931. [Google Scholar] [CrossRef]
- Zebda, D.; Jiang, Z.Y.; Gibson, M.M.; Pham, C.; Ahmadi, S.; Floren, S.; Yao, W.C.; Citardi, M.J.; Luong, A.U. Double-blinded randomized prospective trial of intranasal capsaicin treatment for nonallergic rhinitis. Int. Forum. Allergy Rhinol. 2021, 11, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Jankovskis, V.; Selga, G. Vitamin B and Zinc Supplements and Capsaicin Oral Rinse Treatment Options for Burning Mouth Syndrome. Medicina 2021, 57, 391. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, M.; Basilicata, M.; Marrone, G.; Di Lauro, M.; Campolattano, V.; Bollero, P.; Docimo, R.; Di Daniele, N.; Noce, A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients 2022, 14, 2002. [Google Scholar] [CrossRef] [PubMed]
- Salerno, C.; Di Stasio, D.; Petruzzi, M.; Lauritano, D.; Gentile, E.; Guida, A.; Maio, C.; Tammaro, M.; Serpico, R.; Lucchese, A. An overview of burning mouth syndrome. Front. Biosci. 2016, 8, 213–218. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Q.; Chen, C.; Yang, X.; Xie, R.; Xu, J. The Role of Transient Receptor Potential Vanilloid 1 in Common Diseases of the Digestive Tract and the Cardiovascular and Respiratory System. Front. Physiol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Alawi, K.; Keeble, J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol. Ther. 2010, 125, 181–195. [Google Scholar] [CrossRef]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. Immunotargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef]
- Burks, T.F.; Buck, S.H.; Miller, M.S. Mechanisms of depletion of substance P by capsaicin. Fed. Proc. 1985, 44, 2531–2534. [Google Scholar]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Gregor, D.M.; Zuo, W.; Fu, R.; Bekker, A.; Ye, J.H. Elevation of Transient Receptor Potential Vanilloid 1 Function in the Lateral Habenula Mediates Aversive Behaviors in Alcohol-withdrawn Rats. Anesthesiology 2019, 130, 592–608. [Google Scholar] [CrossRef]
- Chiang, H.; Chang, K.C.; Kan, H.W.; Wu, S.W.; Tseng, M.T.; Hsueh, H.W.; Lin, Y.H.; Chao, C.C.; Hsieh, S.T. Physiological and pathological characterization of capsaicin-induced reversible nerve degeneration and hyperalgesia. Eur. J. Pain 2018, 22, 1043–1056. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef]
- Koplas, P.A.; Rosenberg, R.L.; Oxford, G.S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997, 17, 3525–3537. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8, ra15. [Google Scholar] [CrossRef] [PubMed]
- Peltier, A.C.; Wood, D. Management of Neuropathic Pain in Polyneuropathy. Continuum 2020, 26, 1299–1322. [Google Scholar] [CrossRef]
- Sanati, S.; Razavi, B.M.; Hosseinzadeh, H. A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iran. J. Basic Med. Sci. 2018, 21, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, M.; Liu, J. Chili Intake Is Inversely Associated with Chronic Kidney Disease among Adults: A Population-Based Study. Nutrients 2019, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses. 2012, 37, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Virus, R.M.; Knuepfer, M.M.; McManus, D.Q.; Brody, M.J.; Gebhart, G.F. Capsaicin treatment in adult Wistar-Kyoto and spontaneously hypertensive rats: Effects on nociceptive behavior and cardiovascular regulation. Eur. J. Pharmacol. 1981, 72, 209–217. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, H.J.; Oh, G.S.; Shen, A.; Lee, S.; Choe, S.K.; Park, R.; So, H.S. Capsaicin ameliorates cisplatin-induced renal injury through induction of heme oxygenase-1. Mol. Cells 2014, 37, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.H. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol. Res. 2008, 57, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Wang, D.H. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension 2005, 46, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Rios-Silva, M.; Santos-Alvarez, R.; Trujillo, X.; Cardenas-Maria, R.Y.; Lopez-Zamudio, M.; Bricio-Barrios, J.A.; Leal, C.; Saavedra-Molina, A.; Huerta-Trujillo, M.; Espinoza-Mejia, K.; et al. Effects of Chronic Administration of Capsaicin on Biomarkers of Kidney Injury in Male Wistar Rats with Experimental Diabetes. Molecules 2018, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Munjuluri, S.; Wilkerson, D.A.; Sooch, G.; Chen, X.; White, F.A.; Obukhov, A.G. Capsaicin and TRPV1 Channels in the Cardiovascular System: The Role of Inflammation. Cells 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Beck, V.; Jungbauer, A. PPARalpha activation by culinary herbs and spices. Planta Med. 2011, 77, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Negulesco, J.A.; Noel, S.A.; Newman, H.A.; Naber, E.C.; Bhat, H.B.; Witiak, D.T. Effects of pure capsaicinoids (capsaicin and dihydrocapsaicin) on plasma lipid and lipoprotein concentrations of turkey poults. Atherosclerosis 1987, 64, 85–90. [Google Scholar] [CrossRef]
- Lim, J.H.; Jung, E.S.; Choi, E.K.; Jeong, D.Y.; Jo, S.W.; Jin, J.H.; Lee, J.M.; Park, B.H.; Chae, S.W. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Kempaiah, R.K.; Srinivasan, K. Beneficial influence of dietary curcumin, capsaicin and garlic on erythrocyte integrity in high-fat fed rats. J. Nutr. Biochem. 2006, 17, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A.; Oloyede, O.B.; Oladiji, A.T.; Afolayan, A.J. Selected spices and their combination modulate hypercholesterolemia-induced oxidative stress in experimental rats. Biol. Res. 2014, 47, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, Y.J.; Yang, S.O.; Kim, S.H.; Hyun, S.H.; Cho, S.; Kim, Y.S.; Kwon, D.Y.; Cha, Y.S.; Chae, S.; et al. Hypoxanthine levels in human urine serve as a screening indicator for the plasma total cholesterol and low-density lipoprotein modulation activities of fermented red pepper paste. Nutr. Res. 2010, 30, 455–461. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Rioux, F.; Lemieux, M.; Roy, G. Capsaicin-sensitive primary afferents are involved in the hypotensive effect of neurotensin in ganglion-blocked guinea pigs. Peptides 1989, 10, 1033–1040. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. The effects of neonatal capsaicin on plasma levels and tissue contents of CGRP. Peptides 1993, 14, 247–252. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xiong, S.; Zhu, Z. Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction. Nutrients 2016, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Watcharachaisoponsiri, T.; Sornchan, P.; Charoenkiatkul, S.; Suttisansanee, U. The α-glucosidase and α-amylase inhibitory activity from different chili pepper extracts. Int. Food Res. J. 2016, 23, 1439–1445. [Google Scholar]
- Earnest, E.; Lawrence, E.; Ilevbare, F.; Okparume, D. The roles of capsicum in diabetes mellitus. Cont. J. Pharmacol. Toxicol. Res. 2013, 6, 22–27. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, J.T.; Park, H.S.; Kwon, D.Y.; Kim, M.S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013, 439, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Monsereenusorn, Y.; Glinsukon, T. Inhibitory effect of capsaicin on intestinal glucose absorption in vitro. Food Cosmet. Toxicol. 1978, 16, 469–473. [Google Scholar] [CrossRef]
- Han, J.; Isoda, H. Capsaicin induced the upregulation of transcriptional and translational expression of glycolytic enzymes related to energy metabolism in human intestinal epithelial cells. J. Agric. Food Chem. 2009, 57, 11148–11153. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Goto, T.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, X.; Zhang, L.; Sun, H.; Liu, X. Capsaicin Reduces Blood Glucose by Increasing Insulin Levels and Glycogen Content Better than Capsiate in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2017, 65, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.M.; Tu, T.H.; Noh, H.J.; Kim, C.S.; Choe, S.Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Hansen, A.J.; Wilken, M.; Elm, T.; Svendsen, O.; Carr, R.D.; Ahren, B.; Brand, C.L. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur. J. Endocrinol. 2005, 153, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yan, Z.; Zhong, J.; Chen, J.; Ni, Y.; Li, L.; Ma, L.; Zhao, Z.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Domotor, A.; Szolcsanyi, J.; Mozsik, G. Capsaicin and glucose absorption and utilization in healthy human subjects. Eur. J. Pharmacol. 2006, 534, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai. 2009, 92, 108–113. [Google Scholar]
- Lim, K.; Yoshioka, M.; Kikuzato, S.; Kiyonaga, A.; Tanaka, H.; Shindo, M.; Suzuki, M. Dietary red pepper ingestion increases carbohydrate oxidation at rest and during exercise in runners. Med. Sci. Sports Exerc. 1997, 29, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; Zhang, Q.Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, J.; Kim, K.; Kim, T.; Kim, D.; Kim, C.; Tsutomu, K.; Ochir, S.; Lee, K.; Park, C.H.; et al. Inhibitory effects of Capsicum annuum L. water extracts on lipoprotein lipase activity in 3T3-L1 cells. Nutr. Res. Pract. 2013, 7, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.M.; Kang, J.H.; Kawada, T.; Yoo, H.; Sung, M.K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Reinbach, H.C.; Smeets, A.; Martinussen, T.; Moller, P.; Westerterp-Plantenga, M.S. Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance. Clin. Nutr. 2009, 28, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Menichini, F.; Conforti, F. Hypolipidemic and Antioxidant Properties of Hot Pepper Flower (Capsicum annuum L.). Plant Foods Hum. Nutr. 2016, 71, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Do, M.-S.; Hong, S.-E.; Ha, J.-H.; Park, S.-M.; Ahn, I.S.; Yoon, J.-Y.; Park, K.-Y. Increased Lipolytic Activity by High-pungency Red Pepper Extract (var. Chungyang) in Rat Adipocytes in vitro. Prev. Nutr. Food Sci. 2004, 9, 34–38. [Google Scholar] [CrossRef]
- Saito, M. Capsaicin and Related Food Ingredients Reducing Body Fat Through the Activation of TRP and Brown Fat Thermogenesis. Adv. Food Nutr. Res. 2015, 76, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef]
- Baboota, R.K.; Singh, D.P.; Sarma, S.M.; Kaur, J.; Sandhir, R.; Boparai, R.K.; Kondepudi, K.K.; Bishnoi, M. Capsaicin induces “brite” phenotype in differentiating 3T3-L1 preadipocytes. PLoS ONE 2014, 9, e103093. [Google Scholar] [CrossRef]
- Kawada, T.; Hagihara, K.; Iwai, K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J. Nutr. 1986, 116, 1272–1278. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37, BSR20170286. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Farzin, N.; Taheri, S.; Mahlouji, M.; Akbari, H.; Karamali, F.; Asemi, Z. The Effect of Dietary Supplements Containing Green Tea, Capsaicin and Ginger Extracts on Weight Loss and Metabolic Profiles in Overweight Women: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Ann. Nutr. Metab. 2017, 70, 277–285. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite 2014, 77, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Falchi, M.; Bertelli, A.; Ferrara, F.; Galazzo, R.; Galazzo, S.; Gharib, C.; Dib, B. Intracerebroventricular capsaicin influences the body weight increasing of rats. Brain Res. Bull. 2008, 77, 253–256. [Google Scholar] [CrossRef]
- Lee, G.R.; Shin, M.K.; Yoon, D.J.; Kim, A.R.; Yu, R.; Park, N.H.; Han, I.S. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 21, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, A.; Himms-Hagen, J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes. Res. 1995, 3, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Whittle, T.; Murray, G.M.; Peck, C.C. The effects of capsaicin-induced intraoral mucosal pain on jaw movements in humans. J. Orofac. Pain 2012, 26, 277–287. [Google Scholar] [PubMed]
- Hammond, J.; Hammond, R.W. Molecular cloning, sequencing and expression in Escherichia coli of the bean yellow mosaic virus coat protein gene. J. Gen. Virol. 1989, 70 Pt 8, 1961–1974. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, K.; Li, H.Y.; Chung, G.; Park, C.K.; Kim, J.S.; Jung, S.J.; Lee, M.K.; Ahn, D.K.; Hwang, S.J.; et al. Selectively targeting pain in the trigeminal system. Pain 2010, 150, 29–40. [Google Scholar] [CrossRef]
- Sessle, B.J. Acute and chronic craniofacial pain: Brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit. Rev. Oral Biol. Med. 2000, 11, 57–91. [Google Scholar] [CrossRef]
- Trulsson, M.; Essick, G.K. Sensations evoked by microstimulation of single mechanoreceptive afferents innervating the human face and mouth. J. Neurophysiol. 2010, 103, 1741–1747. [Google Scholar] [CrossRef]
- Moayedi, Y.; Duenas-Bianchi, L.F.; Lumpkin, E.A. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci. Rep. 2018, 8, 9975. [Google Scholar] [CrossRef]
- Toda, K.; Ishii, N.; Nakamura, Y. Characteristics of mucosal nociceptors in the rat oral cavity: An in vitro study. Neurosci. Lett. 1997, 228, 95–98. [Google Scholar] [CrossRef]
- Urata, K.; Shinoda, M.; Honda, K.; Lee, J.; Maruno, M.; Ito, R.; Gionhaku, N.; Iwata, K. Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain. J. Dent. Res. 2015, 94, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kichko, T.I.; Neuhuber, W.; Kobal, G.; Reeh, P.W. The roles of TRPV1, TRPA1 and TRPM8 channels in chemical and thermal sensitivity of the mouse oral mucosa. Eur. J. Neurosci. 2018, 47, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia 2017, 37, 613–626. [Google Scholar] [CrossRef]
- Carstens, E.; Kuenzler, N.; Handwerker, H.O. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J. Neurophysiol. 1998, 80, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Noma, N.; Tsuboi, Y.; Kondo, M.; Matsumoto, M.; Sessle, B.J.; Kitagawa, J.; Saito, K.; Iwata, K. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J. Comp. Neurol. 2008, 507, 1428–1440. [Google Scholar] [CrossRef]
- Martin Carreras-Presas, C.; Amaro Sanchez, J.; Lopez-Sanchez, A.F.; Jane-Salas, E.; Somacarrera Perez, M.L. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021, 27 (Suppl. 3), 710–712. [Google Scholar] [CrossRef] [PubMed]
- Basilicata, M.; Zarone, F.; Leone, R.; Guerriero, C.; Di Lauro, M.; Franco, R.; Bernardini, S.; Noce, A.; Bollero, P.; Sorrentino, R. Impact of SARS-CoV-2 on dentistry: A review of literature. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3386–3398. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral Oncol. 2010, 46, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Nodai, T.; Hitomi, S.; Ono, K.; Masaki, C.; Harano, N.; Morii, A.; Sago-Ito, M.; Ujihara, I.; Hibino, T.; Terawaki, K.; et al. Endothelin-1 Elicits TRP-Mediated Pain in an Acid-Induced Oral Ulcer Model. J. Dent. Res. 2018, 97, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ono, K.; Hitomi, S.; Nodai, T.; Sago, T.; Yamaguchi, K.; Harano, N.; Gunnjigake, K.; Hosokawa, R.; Kawamoto, T.; et al. Prostanoid-dependent spontaneous pain and PAR2-dependent mechanical allodynia following oral mucosal trauma: Involvement of TRPV1, TRPA1 and TRPV4. Mol. Pain 2017, 13, 1744806917704138. [Google Scholar] [CrossRef] [PubMed]
- Haviv, Y.; Zadik, Y.; Sharav, Y.; Benoliel, R. Painful traumatic trigeminal neuropathy: An open study on the pharmacotherapeutic response to stepped treatment. J. Oral Facial. Pain Headache 2014, 28, 52–60. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H.J.; Tramer, M.; Nye, B.A.; Carroll, D.; Wiffen, P.J.; Moore, R.A. A systematic review of antidepressants in neuropathic pain. Pain 1996, 68, 217–227. [Google Scholar] [CrossRef]
- Niki, Y.; Kanai, A.; Hoshi, K.; Okamoto, H. Immediate analgesic effect of 8% lidocaine applied to the oral mucosa in patients with trigeminal neuralgia. Pain Med. 2014, 15, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Herrero Babiloni, A.; Kapos, F.P.; Nixdorf, D.R. Intraoral administration of botulinum toxin for trigeminal neuropathic pain. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, e148-153. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, X.; Yang, G.; Shen, J.; Xie, P.; Zuo, X.; Xia, L.; Han, Q.; Zhao, Y. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: A meta-analysis of randomized controlled trials. Brain Behav. 2019, 9, e01409. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Marcoe, J.H. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 135–140. [Google Scholar] [CrossRef]
- Gaul, C.; Resch, S. Application of the capsaicin 8% cutaneous patch in neuropathic pain of the head and face: A case series. Cephalalgia 2015, 35, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Korczeniewska, O.A.; Katzmann Rider, G.; Gajra, S.; Narra, V.; Ramavajla, V.; Chang, Y.J.; Tao, Y.; Soteropoulos, P.; Husain, S.; Khan, J.; et al. Differential gene expression changes in the dorsal root versus trigeminal ganglia following peripheral nerve injury in rats. Eur. J. Pain 2020, 24, 967–982. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; List, T.; Jensen, T.S.; Svensson, P. Increased pain sensitivity to intraoral capsaicin in patients with atypical odontalgia. J. Orofac. Pain 2006, 20, 107–114. [Google Scholar] [PubMed]
- Moisset, X.; Calbacho, V.; Torres, P.; Gremeau-Richard, C.; Dallel, R. Co-occurrence of Pain Symptoms and Somatosensory Sensitivity in Burning Mouth Syndrome: A Systematic Review. PLoS ONE 2016, 11, e0163449. [Google Scholar] [CrossRef]
- Beneng, K.; Yilmaz, Z.; Yiangou, Y.; McParland, H.; Anand, P.; Renton, T. Sensory purinergic receptor P2X3 is elevated in burning mouth syndrome. Int. J. Oral Maxillofac. Surg. 2010, 39, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, F.J.; Silvestre-Rangil, J.; Tamarit-Santafe, C.; Bautista, D. Application of a capsaicin rinse in the treatment of burning mouth syndrome. Med. Oral Patol. Oral Y Cir. Bucal 2012, 17, e1–e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauria, G.; Majorana, A.; Borgna, M.; Lombardi, R.; Penza, P.; Padovani, A.; Sapelli, P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 2005, 115, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Penza, P.; Majorana, A.; Lombardi, R.; Camozzi, F.; Bonadeo, S.; Sapelli, P.; Lauria, G. “Burning tongue” and “burning tip”: The diagnostic challenge of the burning mouth syndrome. Clin. J. Pain 2010, 26, 528–532. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Egbuniwe, O.; Renton, T. The Detection of Small-Fiber Neuropathies in Burning Mouth Syndrome and Iatrogenic Lingual Nerve Injuries: Use of Quantitative Sensory Testing. J. Oral Facial. Pain Headache 2016, 30, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Croveri, F.; Pasina, L.; Porrini, M.; Vinci, R.; Manfredini, M.; Tettamanti, L.; Tagliabue, A.; Silvestre-Rangil, J.; Spadari, F. A burning therapy for burning mouth syndrome: Preliminary results with the administration of topical capsaicin. J. Biol. Regul. Homeost. Agents 2017, 31, 89–95. [Google Scholar]
- Austah, O.N.; Ruparel, N.B.; Henry, M.A.; Fajardo, R.J.; Schmitz, J.E.; Diogenes, A. Capsaicin-sensitive Innervation Modulates the Development of Apical Periodontitis. J. Endod. 2016, 42, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Lan, S.H.; Huang, A.C.; Yang, J.S.; Chen, Y.Y.; Huang, H.Y.; Lin, Z.P.; Hsu, Y.M.; Yang, M.D.; Chiu, C.F.; et al. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria-dependent and -independent pathways. Environ. Toxicol. 2012, 27, 332–341. [Google Scholar] [CrossRef]
- Liu, N.C.; Hsieh, P.F.; Hsieh, M.K.; Zeng, Z.M.; Cheng, H.L.; Liao, J.W.; Chueh, P.J. Capsaicin-mediated tNOX (ENOX2) up-regulation enhances cell proliferation and migration in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Matsuda, Y.; Yamada, H.; Tabeta, K.; Nakajima, T.; Murakami, S.; Yamazaki, K. Epithelial TRPV1 signaling accelerates gingival epithelial cell proliferation. J. Dent. Res. 2014, 93, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.W.; Zhang, Y.; Wang, Y.; Wang, Y.N.; Zhang, L.; Ding, C.; Wu, L.L.; Yu, G.Y. Functional vanilloid receptor-1 in human submandibular glands. J. Dent. Res. 2010, 89, 711–716. [Google Scholar] [CrossRef]
- Duner-Engstrom, M.; Fredholm, B.B.; Larsson, O.; Lundberg, J.M.; Saria, A. Autonomic mechanisms underlying capsaicin induced oral sensations and salivation in man. J. Physiol. 1986, 373, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kanehira, T.; Hongou, H.; Asano, K.; Morita, M.; Maeshima, E.; Matsuda, A.; Sakamoto, W. A simple test for salivary gland function measuring resting and stimulated submandibular and sublingual secretions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kanehira, T.; Yamaguchi, T.; Asano, K.; Morita, M.; Maeshima, E.; Matsuda, A.; Fujii, Y.; Sakamoto, W. A screening test for capsaicin-stimulated salivary flow using filter paper: A study for diagnosis of hyposalivation with a complaint of dry mouth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Han, J.; Bellila, A.; Isoda, H.; Tanaka, T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem. Biophys. Res. Commun. 2008, 377, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zhang, Y.; Shi, L.; Yang, N.Y.; Ding, C.; Li, J.; Ding, Q.W.; Su, Y.C.; Xiang, R.L.; Wu, L.L.; et al. Activation of transient receptor potential vanilloid subtype 1 increases expression and permeability of tight junction in normal and hyposecretory submandibular gland. Lab. Investig. 2012, 92, 753–768. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, X.; Shi, L.; Xiang, B.; Li, Y.M.; Ding, Q.W.; Ding, C.; Wu, L.L.; Yu, G.Y. Activation of transient receptor potential vanilloid subtype 1 increases secretion of the hypofunctional, transplanted submandibular gland. Am. J. Physiol. -Gastrointest. Liver Physiol. 2010, 299, G54–G62. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.E.; Buccafusco, J.J.; Chapple, C.; de Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006, 148, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.E.; Yule, D.; Shuttleworth, T.; Begenisich, T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005, 67, 445–469. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Salivary secretion: Mechanism and neural regulation. Monogr. Oral Sci. 2014, 24, 14–29. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Shin, Y.H.; Namkoong, E.; Hwang, S.M.; Cong, X.; Yu, G.; Park, K. TRPV1 in Salivary Gland Epithelial Cells Is Not Involved in Salivary Secretion via Transcellular Pathway. Korean J. Physiol. Pharm. 2014, 18, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef]
- National Cancer Institution. Cancer Stat Facts: Tongue Cancer. Available online: https://seer.cancer.gov/statfacts/html/tongue.html (accessed on 29 August 2022).
- International Agency for Research on Cancer. Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 29 August 2022).
- Aguilar-Meléndez, A.; Vásquez-Dávila, M.A.; Manzanero-Medina, G.I.; Katz, E. Chile (Capsicum spp.) as Food-Medicine Continuum in Multiethnic Mexico. Foods 2021, 10, 2502. [Google Scholar] [CrossRef]
- Surh, Y.J.; Lee, S.S. Capsaicin in hot chili pepper: Carcinogen, co-carcinogen or anticarcinogen? Food Chem. Toxicol. 1996, 34, 313–316. [Google Scholar] [CrossRef]
- Modly, C.E.; Das, M.; Don, P.S.; Marcelo, C.L.; Mukhtar, H.; Bickers, D.R. Capsaicin as an in vitro inhibitor of benzo(a)pyrene metabolism and its DNA binding in human and murine keratinocytes. Drug Metab. Dispos. 1986, 14, 413–416. [Google Scholar]
- Mosqueda-Solis, A.; Lafuente-Ibanez de Mendoza, I.; Aguirre-Urizar, J.M.; Mosqueda-Taylor, A. Capsaicin intake and oral carcinogenesis: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e261–e268. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalfamo, L.M.; Marrone, G.; Basilicata, M.; Vivarini, I.; Paolino, V.; Della-Morte, D.; De Ponte, F.S.; Di Daniele, F.; Quattrone, D.; De Rinaldis, D.; et al. The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 11187. https://doi.org/10.3390/ijerph191811187
Catalfamo LM, Marrone G, Basilicata M, Vivarini I, Paolino V, Della-Morte D, De Ponte FS, Di Daniele F, Quattrone D, De Rinaldis D, et al. The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review. International Journal of Environmental Research and Public Health. 2022; 19(18):11187. https://doi.org/10.3390/ijerph191811187
Chicago/Turabian StyleCatalfamo, Luciano Maria, Giulia Marrone, Michele Basilicata, Ilaria Vivarini, Vincenza Paolino, David Della-Morte, Francesco Saverio De Ponte, Francesca Di Daniele, Domenico Quattrone, Danilo De Rinaldis, and et al. 2022. "The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review" International Journal of Environmental Research and Public Health 19, no. 18: 11187. https://doi.org/10.3390/ijerph191811187
APA StyleCatalfamo, L. M., Marrone, G., Basilicata, M., Vivarini, I., Paolino, V., Della-Morte, D., De Ponte, F. S., Di Daniele, F., Quattrone, D., De Rinaldis, D., Bollero, P., Di Daniele, N., & Noce, A. (2022). The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review. International Journal of Environmental Research and Public Health, 19(18), 11187. https://doi.org/10.3390/ijerph191811187








