Association between Short-Term Exposure to Ozone and Heart Rate Variability: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Question
2.2. Search Strategy
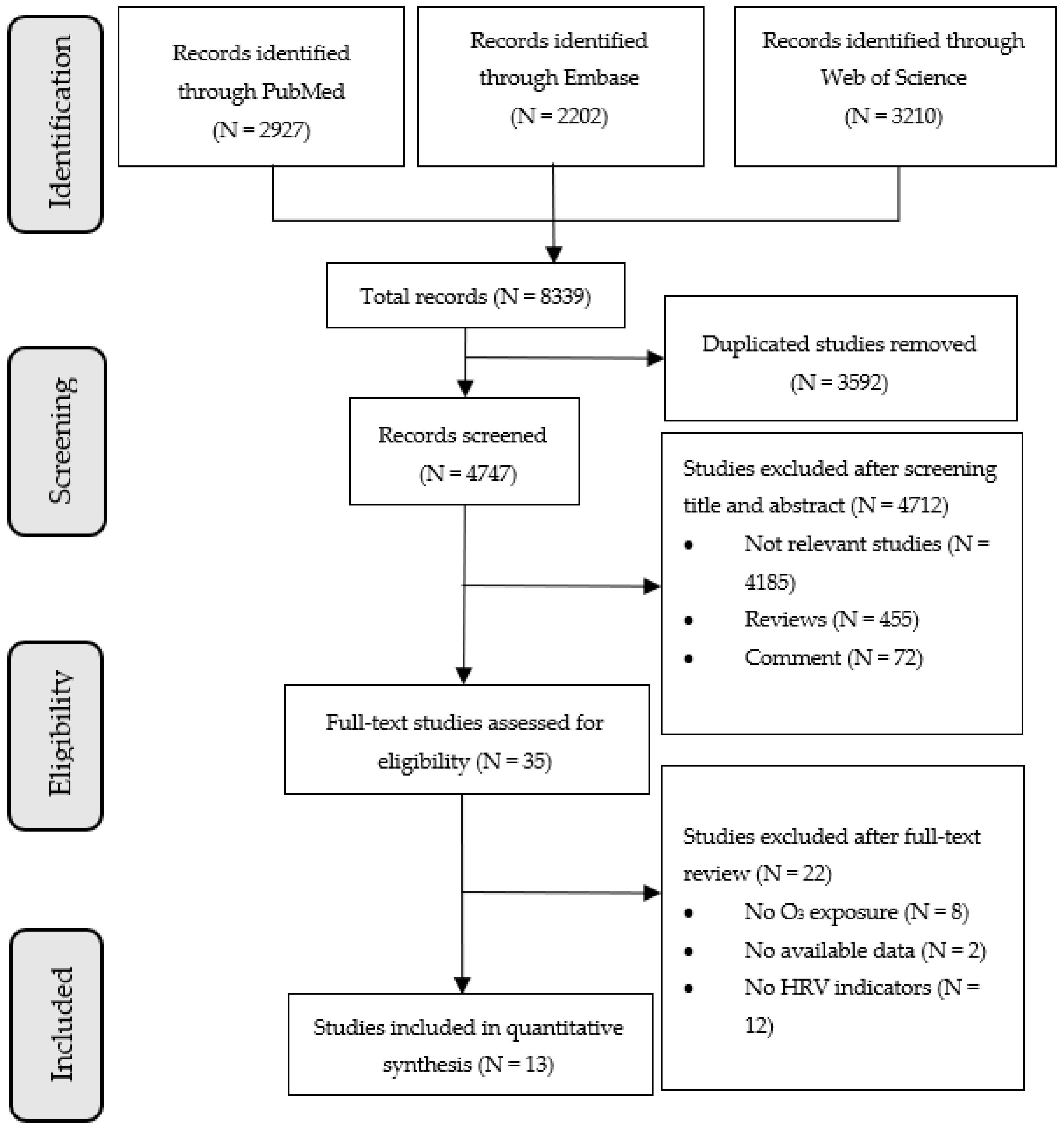
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
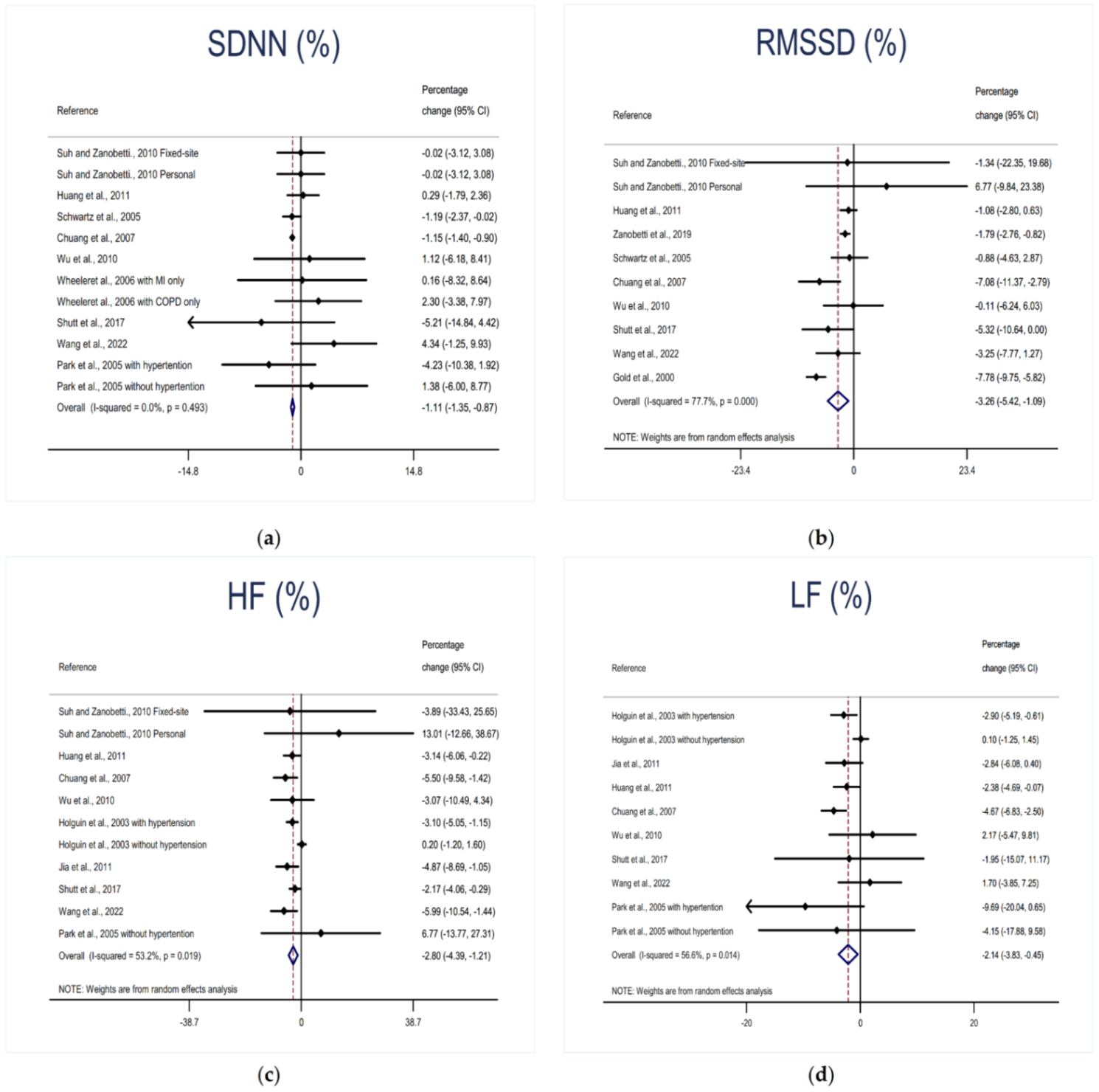
3.2. Association between O3 Exposure and HRV
3.3. Subgroup Analysis
3.4. Sensitivity Analysis
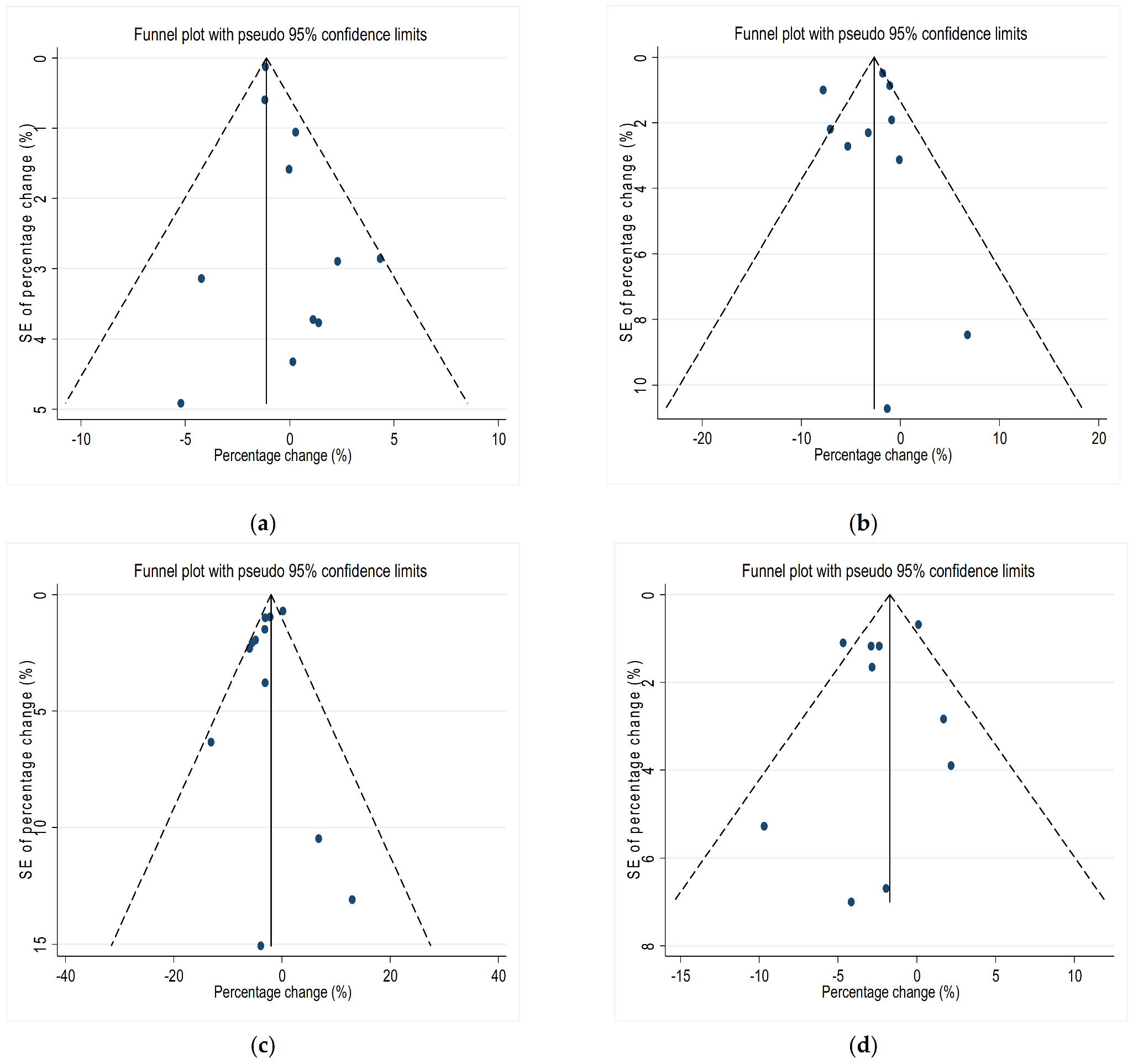
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Jin, Y.; Andersson, H.; Zhang, S. Air Pollution Control Policies in China: A Retrospective and Prospects. Int. J. Environ. Res. Public Health 2016, 13, 1219. [Google Scholar] [CrossRef]
- García-Serna, A.M.; Hernández-Caselles, T.; Jiménez-Guerrero, P.; Martín-Orozco, E.; Pérez-Fernández, V.; Cantero-Cano, E.; Muñoz-García, M.; Ballesteros-Meseguer, C.; Pérez de Los Cobos, I.; García-Marcos, L.; et al. Air pollution from traffic during pregnancy impairs newborn′s cord blood immune cells: The NELA cohort. Environ. Res. 2021, 198, 110468. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, K.; Ayoub, M.A.; Iratni, R. Vascular Inflammation in Cardiovascular Disease: Is Immune System Protective or Bystander? Curr. Pharm. Des. 2021, 27, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020, 17, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Thomas, L.; Meredith, L.; Rebecca, D.; Lance, A.; Michelle, B. Integrated Science Assessment (ISA) for Ozone and Related Photochemical Oxidants; U.S. Environmental Protection Agency: Washington, DC, USA, 2020. [Google Scholar]
- Barath, S.; Langrish, J.P.; Lundbäck, M.; Bosson, J.A.; Goudie, C.; Newby, D.E.; Sandström, T.; Mills, N.L.; Blomberg, A. Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men. Toxicol. Sci. 2013, 135, 292–299. [Google Scholar] [CrossRef]
- Balmes, J.R.; Arjomandi, M.; Bromberg, P.A.; Costantini, M.G.; Dagincourt, N.; Hazucha, M.J.; Hollenbeck-Pringle, D.; Rich, D.Q.; Stark, P.; Frampton, M.W. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The Multicenter Ozone Study in oldEr Subjects (MOSES). PLoS ONE 2019, 14, e0222601. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.B.; Duncan, K.E.; Jardim, M.; Schmitt, M.T.; Rappold, A.G.; Diaz-Sanchez, D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 2012, 126, 104–111. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Yuda, E. Assessment of autonomic function by long-term heart rate variability: Beyond the classical framework of LF and HF measurements. J. Physiol. Anthropol. 2021, 40, 21. [Google Scholar] [CrossRef] [PubMed]
- Martín-Montero, A.; Gutiérrez-Tobal, G.C.; Kheirandish-Gozal, L.; Jiménez-García, J.; Álvarez, D.; Del Campo, F.; Gozal, D.; Hornero, R. Heart rate variability spectrum characteristics in children with sleep apnea. Pediatr. Res. 2021, 89, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Shaffer, F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef]
- Rich, D.Q.; Balmes, J.R.; Frampton, M.W.; Zareba, W.; Stark, P.; Arjomandi, M.; Hazucha, M.J.; Costantini, M.G.; Ganz, P.; Hollenbeck-Pringle, D.; et al. Cardiovascular function and ozone exposure: The Multicenter Ozone Study in oldEr Subjects (MOSES). Environ. Int. 2018, 119, 193–202. [Google Scholar] [CrossRef]
- Correll, C.U.; Solmi, M.; Croatto, G.; Schneider, L.K.; Rohani-Montez, S.C.; Fairley, L.; Smith, N.; Bitter, I.; Gorwood, P.; Taipale, H.; et al. Mortality in people with schizophrenia: A systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry 2022, 21, 248–271. [Google Scholar] [CrossRef]
- Wang, F.; Liang, Q.; Sun, M.; Ma, Y.; Lin, L.; Li, T.; Duan, J.; Sun, Z. The relationship between exposure to PM2.5 and heart rate variability in older adults: A systematic review and meta-analysis. Chemosphere 2020, 261, 127635. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Association of thyroid disorders with gestational diabetes mellitus: A meta-analysis. Endocrine 2021, 73, 550–560. [Google Scholar] [CrossRef]
- Chuang, K.J.; Chan, C.C.; Su, T.C.; Lee, C.T.; Tang, C.S. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef]
- Gold, D.R.; Litonjua, A.; Schwartz, J.; Lovett, E.; Larson, A.; Nearing, B.; Allen, G.; Verrier, M.; Cherry, R.; Verrier, R. Ambient pollution and heart rate variability. Circulation 2000, 101, 1267–1273. [Google Scholar] [CrossRef]
- Holguín, F.; Téllez-Rojo, M.M.; Hernández, M.; Cortez, M.; Chow, J.C.; Watson, J.G.; Mannino, D.; Romieu, I. Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology 2003, 14, 521–527. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, T.; Pan, X.; Hu, M.; Lu, S.E.; Lin, Y.; Wang, T.; Zhang, Y.; Tang, X. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: Interactions of systemic inflammation, overweight, and gender. Am. J. Epidemiol. 2012, 176, 117–126. [Google Scholar] [CrossRef]
- Jia, X.; Song, X.; Shima, M.; Tamura, K.; Deng, F.; Guo, X. Acute effect of ambient ozone on heart rate variability in healthy elderly subjects. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 541–547. [Google Scholar] [CrossRef]
- Park, S.K.; O’Neill, M.S.; Vokonas, P.S.; Sparrow, D.; Schwartz, J. Effects of air pollution on heart rate variability: The VA normative aging study. Environ. Health Perspect. 2005, 113, 304–309. [Google Scholar] [CrossRef]
- Schwartz, J.; Litonjua, A.; Suh, H.; Verrier, M.; Zanobetti, A.; Syring, M.; Nearing, B.; Verrier, R.; Stone, P.; MacCallum, G.; et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax 2005, 60, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Shutt, R.H.; Kauri, L.M.; Weichenthal, S.; Kumarathasan, P.; Vincent, R.; Thomson, E.M.; Liu, L.; Mahmud, M.; Cakmak, S.; Dales, R. Exposure to air pollution near a steel plant is associated with reduced heart rate variability: A randomised crossover study. Environ. Health 2017, 16, 4. [Google Scholar] [CrossRef]
- Suh, H.H.; Zanobetti, A. Exposure error masks the relationship between traffic-related air pollution and heart rate variability. J. Occup. Environ. Med. 2010, 52, 685–692. [Google Scholar] [CrossRef]
- Wang, C.; Lin, J.; Niu, Y.; Wang, W.; Wen, J.; Lv, L.; Liu, C.; Du, X.; Zhang, Q.; Chen, B.; et al. Impact of ozone exposure on heart rate variability and stress hormones: A randomized-crossover study. J. Hazard. Mater. 2022, 421, 126750. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.; Zanobetti, A.; Gold, D.R.; Schwartz, J.; Stone, P.; Suh, H.H. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ. Health Perspect. 2006, 114, 560–566. [Google Scholar] [CrossRef]
- Wu, C.F.; Kuo, I.C.; Su, T.C.; Li, Y.R.; Lin, L.Y.; Chan, C.C.; Hsu, S.C. Effects of Personal Exposure to Particulate Matter and Ozone on Arterial Stiffness and Heart Rate Variability in Healthy Adults. Am. J. Epidemiol. 2010, 171, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Zanobetti, A.; Gold, D.R.; Stone, P.H.; Suh, H.H.; Schwartz, J.; Coull, B.A.; Speizer, F.E. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ. Health Perspect. 2010, 118, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Orellano, P.; Lin, H.L.; Jiang, M.; Guan, W.J. Short-term exposure to ozone, nitrogen dioxide, and sulphur dioxide and emergency department visits and hospital admissions due to asthma: A systematic review and meta-analysis. Environ. Int. 2021, 150, 106435. [Google Scholar] [CrossRef]
- Nhung, N.T.T.; Amini, H.; Schindler, C.; Kutlar Joss, M.; Dien, T.M.; Probst-Hensch, N.; Perez, L.; Künzli, N. Short-term association between ambient air pollution and pneumonia in children: A systematic review and meta-analysis of time-series and case-crossover studies. Environ. Pollut. 2017, 230, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Li, X.; Wang, X.; Nie, S. Air pollution increases the risk of pulmonary embolism: A meta-analysis. Rev. Environ. Health 2022, 37, 259–266. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, T.; Korantzopoulos, P.; Zhang, Z.; Zhao, J.; Li, G. Association between air pollution and development of atrial fibrillation: A meta-analysis of observational studies. Heart Lung 2016, 45, 557–562. [Google Scholar] [CrossRef]
- Halaris, A. Comorbidity between depression and cardiovascular disease. Int. Angiol. 2009, 28, 92–99. [Google Scholar]
- Sangaleti, C.T.; Katayama, K.Y.; De Angelis, K.; Lemos de Moraes, T.; Araújo, A.A.; Lopes, H.F.; Camacho, C.; Bortolotto, L.A.; Michelini, L.C.; Irigoyen, M.C.; et al. The Cholinergic Drug Galantamine Alleviates Oxidative Stress Alongside Anti-inflammatory and Cardio-Metabolic Effects in Subjects With the Metabolic Syndrome in a Randomized Trial. Front. Immunol. 2021, 12, 613979. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Huang, Y.C.; Huang, W.L. Heart rate variability in patients with dementia or neurocognitive disorders: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2022, 56, 16–27. [Google Scholar] [CrossRef]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Larson, M.G.; Venditti FJJr Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of reduced heart rate variability on risk for cardiac events. Fram. Heart Study. Circ. 1996, 94, 2850–2855. [Google Scholar] [CrossRef] [Green Version]
- Kop, W.J.; Verdino, R.J.; Gottdiener, J.S.; O’Leary, S.T.; Bairey Merz, C.N.; Krantz, D.S. Changes in heart rate and heart rate variability before ambulatory ischemic events. J. Am. Coll. Cardiol. 2001, 38, 742–749. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Kino, T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann. N. Y. Acad. Sci. 2009, 1179, 153–166. [Google Scholar] [CrossRef]
- Snow, S.J.; Henriquez, A.R.; Costa, D.L.; Kodavanti, U.P. Neuroendocrine Regulation of Air Pollution Health Effects: Emerging Insights. Toxicol. Sci. 2018, 164, 9–20. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- Fowler, D.; Brimblecombe, P.; Burrows, J.; Heal, M.R.; Grennfelt, P.; Stevenson, D.S.; Jowett, A.; Nemitz, E.; Coyle, M.; Lui, X.; et al. A chronology of global air quality. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190314. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Wang, L.; Gao, M.; Li, K.; Zhang, Y.; Yue, X. Rapid Increases in Warm-Season Surface Ozone and Resulting Health Impact in China Since 2013. Environ. Sci. Technol. Lett. 2020, 7, 240–247. [Google Scholar] [CrossRef]
- Evangelopoulos, D.; Chatzidiakou, L.; Walton, H.; Katsouyanni, K.; Kelly, F.J.; Quint, J.K.; Jones, R.L.; Barratt, B. Personal exposure to air pollution and respiratory health of COPD patients in London. Eur. Respir. J. 2021, 58, 2003432. [Google Scholar] [CrossRef] [PubMed]
- Vural, C.; Dinleyici, E.C.; Kosger, P.; Bolluk, O.; Kilic, Z.; Ucar, B. Evaluation of cardiac autonomic function using heart rate variability in children with acute carbon monoxide poisoning. Cardiol. Young 2017, 27, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Liu, F.; Yang, X.; Liu, Q.; Wang, X.; Lin, Z.; Huang, K.; Cao, J.; Li, J.; Fan, M.; et al. Declines in heart rate variability associated with short-term PM2.5 exposure were modified by blood pressure control and treatment: A multi-city panel study in China. Environ. Pollut. 2021, 287, 117572. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, D.F.; Tang, S.Y. Effect of diltiazem on exercise tolerance in patients with stable coronary artery disease and hypertension. J. Physiol. Pharmacol. 2021, 72. [Google Scholar] [CrossRef]
- Zhong, J.; Colicino, E.; Lin, X.; Mehta, A.; Kloog, I.; Zanobetti, A.; Byun, H.M.; Bind, M.A.; Cantone, L.; Prada, D.; et al. Cardiac autonomic dysfunction: Particulate air pollution effects are modulated by epigenetic immunoregulation of Toll-like receptor 2 and dietary flavonoid intake. J. Am. Heart Assoc. 2015, 4, e001423. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| PECOS Element | Evidence |
|---|---|
| Population | General population, of all ages, developed and developing areas, both urban and rural. No geographical restrictions. |
| Exposure | Exposure to ambient O3 pollution. Exposure was expressed in continuous. |
| Comparator | A comparation population exposed to lower levels of O3 pollution. |
| Outcomes | Heart rate variability including four common indicators (RMSSD, SDNN, LF, and HF). |
| Study design | Cohort, nested or not nested case–control, case–cohort, or cross-sectional study designs, were considered. |
| Author and Year of Publication | Study Location, Period and Design | Study Population | Outcome Assessment | Ozone Exposure Time | Monitoring Type | Adjusted Covariates | Heart Rate Variability Indicators and Percentage Change (%) | NOS Score |
|---|---|---|---|---|---|---|---|---|
| Suh and Zanobetti, 2010 [28] | Atlanta (USA), Fall 1999 and Spring 2000 Panel study | 30 subjects: 12 with a recent myocardial infarction and 18 with chronic obstructive pulmonary disease Mean age: 65 year, 57% male | min ECG daily on seven consecutive days in one or both seasons. The ECG protocol involved 5 min of rest, 5 min of standing, 5 min of exercise out- doors, 5 min of recovery, and 20 cycles of slow breathing | 24 h | Fixed-site; Personal exposure | Body mass index (BMI), temperature, relative humidity, sex, age, season, hour of day, day of week, medications use (beta-blockers, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, and bronchodilators) | Per 16.02 ppb increase: SDNN: −0.03 (−8.40, 9.10) RMSSD: 10.83 (−12.63, 40.58) HF: 20.84 (−13.47, 68.76) | 8 |
| Huang et al., 2011 [23] | Beijing (China), during summer 2007 and summer 2008 Panel study | 40 nonsmoking CVD patients (mean age = 65.6 years (standard deviation, 5.8) recruited through the on-campus clinic of Peking University Health Science Center (PKUHSC. A subset of 23 patients participated in 24-h ambulatory blood pressure monitoring | Consecutive 5-min measurements of heart rate and various measures of HRV were calculated for each monitoring session of each subject using personal computer-based software | 12 h | Fixed-site | Age, BMI, gender, time of day, day of the week, visit, temperature, and relative humidity | Per 27.7 ppb increase SDNN: 0.8 (−1.8, 3.5) RMSSD: −3.0 (−7.6, 1.9) HF: −8.7 (−16.4, −0.2) LF: −6.6 (−12.8, −0.01) | 8 |
| Zanobetti et al., 2010 [32] | Boston (USA), 1999–2003 Panel study | 46 patients with coronary artery disease, mean age: 57 year, 80% male, non-smoking | 24 h ambulatory ECG, up to four with approximately 3-month intervals between visits | 120 h | Fixed-site | Day of the week, traffic, average heart rate, hour of the day, date, mean temperature | Per 19 ppb increase RMSSD: −3.4 (−5.2, −1.5) | 8 |
| Wheeler et al., 2006 [30] | Atlanta (USA), Fall 1999 and Spring 2000 Panel study | 30 subjects: 12 with a recent myocardial infarction and 18 with chronic obstructive pulmonary disease Mean age: 65 year, 57% male | min ECG daily on seven consecutive days in one or both seasons The ECG protocol involved 5 min of rest, 5 min of standing, 5 min of exercise out- doors, 5 min of recovery, and 20 cycles of slow breathing | 4 h | Fixed-site | BMI, temperature, relative humidity, sex, age, season, hour of day, day of week, medications use (beta-blockers, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, and bronchodilators) | Total (per 9.61 ppb increase) SDNN: 0.75 (−3.6, 5.3) With MI (per 8.08 ppb increase) SDNN: 0.13 (−6.5, 7.2) With COPD (per 10.66 increase) SDNN: 2.45 (−3.4, 8.7) | 7 |
| Schwartz et al., 2005 [26] | Boston (USA), Summer 1999 Panel study | 28 subjects living near the exposure and health monitoring site, 61–89 year, 25% male myocardial infarction (n = 3), congestive heart failure (n = 2), chronic pulmonary disease (n = 2) | 30-min ECG weekly over 12 weeks The ECG protocol involved 5 min of rest, 5 min of standing, 5 min of exercise outdoors, 5 min of recovery, and 3 min and 20 s of slow breathing | 24 h | Fixed-site | Temperature, day of the week, hour of the day, medication use, time trend | Per 26 ppb increase SDNN: −1.5 (−5.7, 2.9) RMSSD: −2.3 (−11.6, 7.9) | 6 |
| Holguin et al., 2003 [22] | Mexico City (Mexico), 8 February–30 April 2000 Panel study | 34 elderly residents of a nursing home, hypertension (n = 13), diabetes mellitus (n = 6), Parkinson’s disease (n = 4), chronic bronchitis (n = 4), 60–96 year, 44% male | 5-min resting ECG in supine position, every other day be- tween 8:00 a.m. and 1:00 p.m. for three months | 1 h | Fixed-site | Age, heart rate | Per 10 ppb increase Total HF: −0.1 (−0.016, 0.013) LF: −0.5 (−0.019, 0.009) With hypertension HF: −1.4 (−4.0, 1.2) LF: −2.1 (−0.045, 0.003) Without hypertension HF: 0.007 (−0.010, 0.024) LF: 0.005 (−0.011, 0.022) | 6 |
| Jia et al., 2011 [24] | Beijing (China), Summer 2008 and Winter 2009 Panel study | 20 healthy elderlies, mean age 58.7 year, living near busy road, 25% male, non-smoking | Two 24 h ambulatory ECGs: one in summer 2008; one in winter 2009 | 2 h | Fixed-site | PM2.5, NOx, temperature, relative humidity, gender, age, BMI, survey number, activity | Per 10 ppb increase HF: −4.87 (−8.62, −0.97) LF: −2.84 (−6.03, 0.46) | 7 |
| Chuang et al., 2007 [20] | Taipei (China), April–June of 2004 or 2005 Panel study | 76 healthy college students, no history of cardiovascular disease and of smoking, mean age: 21 year, 60% male | One monthly 16 min resting ECG in the sitting position, during daytime (8 a.m. to 2 p.m.), for three months (~30 days between measurements) | 72 h | Fixed-site | Sex, age, BMI, weekday, temperature of day before, relative humidity | Per 12.0 ppb increase SDNN: −8.3 (−10.1, −6.5) RMSSD: −8.5 (−13.6, −3.3) HF: −6.6 (−11.8, −1.4) LF: −5.6 (−8.2, −3.0) | 6 |
| Wu et al., 2010 [31] | Taipei (China), February–March 2007 Panel study | 17 healthy mail carriers, 32.4 year, 100% male, non-smoking | Ambulatory electrocardiographic data were collected continuously during their working periods, starting and ending 30 min before and after the mail delivery periods | 24 h | Personal exposure | Age, BMI, second-hand smoke exposure, temperature during the working period | Per 17.6 ppb increase SDNN: 1.97 (−10.06, 15.62) RMSSD: −0.19 (−10.40, 11.19) HF: 5.41 (−7.60, 20.25) LF: 3.82 (−8.76, 18.13) | 6 |
| Shutt et al., 2017 [27] | Ottawa (Canada), Summer 2010 Case–crossover study | 60 healthy adults, 24.2 ± 5.8 year, 46 male, 14 female | HRV analysis was undertaken on a segment of the ambulatory ECG recording during a 15 min rest period, near the end of the 8-h on-site day | 120 h | Fixed-site | Age, heart rate, sex, BMI, temperature and relative humidity | Per 8.7 ppb increase SDNN: −5.59 (−10.01, 1.18) RMSSD: −6.11 (−10.87, 1.36) HF: −2.50 (−4.67, −0.33) LF: −2.24 (−17.32, 12.84) | 7 |
| Wang et al., 2022 [29] | Shanghai (China) October to November 2018 Case–crossover study | 22 young participants (10 males and 12 females, 18–30 year) with complete data for final analyses | 24-h ECG monitoring was performed using a 3-lead electrographic Holter monitor (Seer Light, GE Medical Systems) with a sampling rate of 128 Hz | 2 h | Fixed-site | Age, sex, BMI, the collinearity between ozone and relative humidity in chamber | Per 10 ppb increase SDNN: 4.34 (−1.15, 10.14) RMSSD: −3.25 (−7.66, 1.38) HF: −5.99 (−10.44, −1.33) LF: 1.7 (−3.71, 7.40) | 8 |
| Gold et al., 2000 [21] | Boston (USA) May to July 1997 Panel study | 21 volunteers, 73.3 year, 10 males and 11 females | 25 min per week of continuous ECG monitoring, including 5 min of rest, 5 min of standing, 5 min of exercise outdoors, and 5 min of recovery | 1 h | Fixed-site | Age, BMI, sex, smoking status, race, medication use, hypertension, coronary artery disease (history of angina or heart attack), history of congestive heart failure | Per 23.0 ppb increase RMSSD: −17.9 (−7.66, 1.38) | 6 |
| Park et al., 2005 [25] | Boston (USA) 14 November 2000–30 October 2003 Cohort study | 497 elderly men, 72.7 ± 6.6 | After the participants had rested for 5 min, the ECG was recorded for approximately 7 min with the subject seated. The best 4-consecutive-minute interval was used for the HRV calculations | 4 h | Fixed-site | Age, BMI, mean arterial blood pressure (MAP), fasting blood glucose (FBG), cigarette smoking, use of beta-blocker, calcium-channel blocker, and/or ACE inhibitor, room temperature, season, and cubic smoothing splines (3 df) for moving averages of apparent temperature corresponding for the predictor | Per 13.0 ppb increase With hypertension SDNN: −5.5 (−15.7, 0.3) HF: −17.0 (−31.6, 0.7) LF: −12.6 (−25.0, 1.9) Without hypertension SDNN: 1.8 (−7.4, 11.8) HF: 8.8 (−14.7, 38.7) LF: −5.4 (−21.6, 14.1) | 7 |
| HRV Indices | Subgroup | Subgroup Criteria | Pooled Percentage Changes (%) with 95%CI | No. of Effect Estimates | No. of Studies | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | p Value for Heterogeneity | ||||||
| SDNN | Age of participants | ≤35 year | −0.15 (−3.09, 2.79) | 4 | 4 | 36.8 | 0.191 |
| ≥55 year | −0.65 (−1.54, 0.24) | 8 | 5 | 0.0 | 0.710 | ||
| Study location | North America | −0.91 (−1.89, 0.08) | 8 | 5 | 0.0 | 0.733 | |
| East Asia | −1.12 (−1.37, −0.87) | 4 | 4 | 48.8 | 0.119 | ||
| ECG recording length | Length of ECG ≤ 30 min | −0.89 (−1.88, 0.09) | 5 | 3 | 0.0 | 0.541 | |
| Others | −1.12 (−1.37, −0.87) | 6 | 4 | 19.0 | 0.290 | ||
| O3 exposure time | O3 exposure < 24 h | −0.90 (−0.90, 2.70) | 4 | 3 | 0.0 | 0.563 | |
| Others | −1.14 (−1.39, −0.90) | 8 | 6 | 0.0 | 0.863 | ||
| Exposure assessment | Fixed-site exposure | −1.12 (−1.36, −0.87) | 10 | 8 | 6.2 | 0.385 | |
| Personal exposure | −0.16 (−2.70, 3.01) | 2 | 2 | 0.0 | 0.778 | ||
| Quality of study | High | −0.23 (−1.09, 1.55) | 9 | 6 | 0.0 | 0.650 | |
| Medium | −1.15 (−1.40, −0.91) | 3 | 3 | 0.0 | 0.828 | ||
| RMSSD | Age of participants | ≤35 year | −4.36 (−7.13, −1.59) | 4 | 4 | 19.9 | 0.290 |
| ≥55 year | −2.67 (−5.55, 0.21) | 6 | 5 | 85.4 | <0.001 | ||
| Study location | North America | −3.43 (−7.02, 0.16) | 6 | 5 | 84.3 | <0.001 | |
| East Asia | −2.81 (−5.78, 0.17) | 4 | 4 | 58.0 | 0.067 | ||
| ECG recording length | Length of ECG ≤ 30 min | −3.78 (−8.20, 0.67) | 4 | 4 | 88.9 | <0.001 | |
| Others | −2.52 (−4.50, −0.54) | 6 | 5 | 31.3 | 0.201 | ||
| O3 exposure time | O3 exposure < 24 h | −4.08 (−9.01, 0.85) | 3 | 3 | 92.1 | <0.001 | |
| Others | −2.55 (−4.56, −0.54) | 7 | 6 | 32.1 | 0.183 | ||
| Exposure assessment | Fixed-site exposure | −3.69 (−5.98, −1.39) | 8 | 8 | 81.8 | <0.001 | |
| Personal exposure | −0.72 (−5.04, 6.47) | 2 | 2 | 0.0 | 0.446 | ||
| Quality of study | High | −1.74 (−2.56, −0.92) | 6 | 5 | 0.0 | 0.586 | |
| Medium | −4.38 (−8.42, −0.33) | 4 | 4 | 78.7 | 0.003 | ||
| HF | Age of participants | ≤35 year | −3.56 (−5.61, −1.51) | 4 | 4 | 20.9 | 0.285 |
| ≥55 year | −2.54 (−4.90, −0.17) | 8 | 5 | 62.1 | 0.014 | ||
| Study location | North America | −1.75 (−3.89, 0.39) | 7 | 4 | 56.4 | 0.032 | |
| East Asia | −4.11 (−6.20, −2.62) | 5 | 5 | 0.0 | 0.802 | ||
| ECG recording length | Length of ECG ≤ 30 min | −2.10 (−3.88, −0.32) | 7 | 5 | 57.4 | 0.029 | |
| Others | −5.22 (−7.58, −2.86) | 5 | 4 | 0.0 | 0.716 | ||
| O3 exposure time | O3 exposure < 24 h | −2.92 (−5.23, −0.62) | 5 | 4 | 75.1 | 0.003 | |
| Others | −3.28 (−5.75, −0.81) | 7 | 5 | 14.6 | 0.318 | ||
| Exposure assessment | Fixed-site exposure | −3.10 (−4.83, −1.37) | 10 | 8 | 60.9 | 0.006 | |
| Personal exposure | 0.08 (−12.44, 12.60) | 2 | 2 | 28.2 | 0.238 | ||
| Quality of study | High | −3.42 (−5.15, −1.68) | 8 | 6 | 14.3 | 0.318 | |
| Medium | −2.43 (−5.20, 0.34) | 4 | 3 | 74.9 | 0.007 | ||
| LF | Age of participants | ≤35 year | −1.33 (−5.70, 3.03) | 4 | 4 | 54.8 | 0.084 |
| ≥55 year | −2.02 (−3.80, −0.25) | 6 | 4 | 51.9 | 0.065 | ||
| Study location | North America | −1.86 (−4.51, 0.78) | 5 | 5 | 50.1 | 0.091 | |
| East Asia | −2.50 (−4.52, −0.49) | 5 | 5 | 43.4 | 0.133 | ||
| ECG recording length | Length of ECG ≤ 30 min | −1.62 (−3.43, 0.19) | 7 | 5 | 40.8 | 0.119 | |
| Others | −2.79 (−5.77, 0.19) | 3 | 3 | 56.8 | 0.099 | ||
| O3 exposure time | O3 exposure < 24 h | −1.49 (−3.14, 0.16) | 5 | 4 | 53.8 | 0.070 | |
| Others | −4.29 (−6.37, −2.20) | 5 | 4 | 0.8 | 0.402 | ||
| Exposure assessment | Fixed-site exposure | −2.33 (−4.07, −0.58) | 9 | 7 | 59.4 | 0.011 | |
| Personal exposure | -- | -- | -- | -- | -- | ||
| Quality of study | High | −2.34 (−4.07, −0.62) | 6 | 5 | 81.2 | 0.001 | |
| Medium | −1.94 (−4.76, 0.87) | 4 | 4 | 0.0 | 0.530 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, Z.; Zhang, M.; Xu, K.; Zhang, Y.; Hu, C. Association between Short-Term Exposure to Ozone and Heart Rate Variability: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11186. https://doi.org/10.3390/ijerph191811186
Zong Z, Zhang M, Xu K, Zhang Y, Hu C. Association between Short-Term Exposure to Ozone and Heart Rate Variability: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(18):11186. https://doi.org/10.3390/ijerph191811186
Chicago/Turabian StyleZong, Zhiqiang, Mengyue Zhang, Kexin Xu, Yunquan Zhang, and Chengyang Hu. 2022. "Association between Short-Term Exposure to Ozone and Heart Rate Variability: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 18: 11186. https://doi.org/10.3390/ijerph191811186
APA StyleZong, Z., Zhang, M., Xu, K., Zhang, Y., & Hu, C. (2022). Association between Short-Term Exposure to Ozone and Heart Rate Variability: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(18), 11186. https://doi.org/10.3390/ijerph191811186







