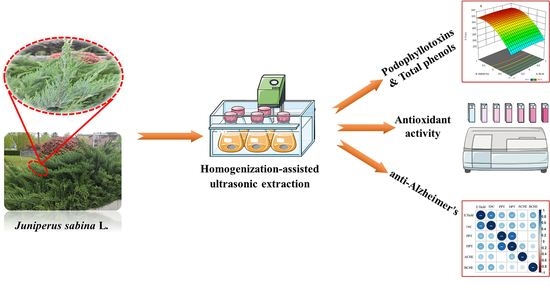
Juniperus sabina L. as a Source of Podophyllotoxins: Extraction Optimization and Anticholinesterase Activities
Abstract
:1. Introduction
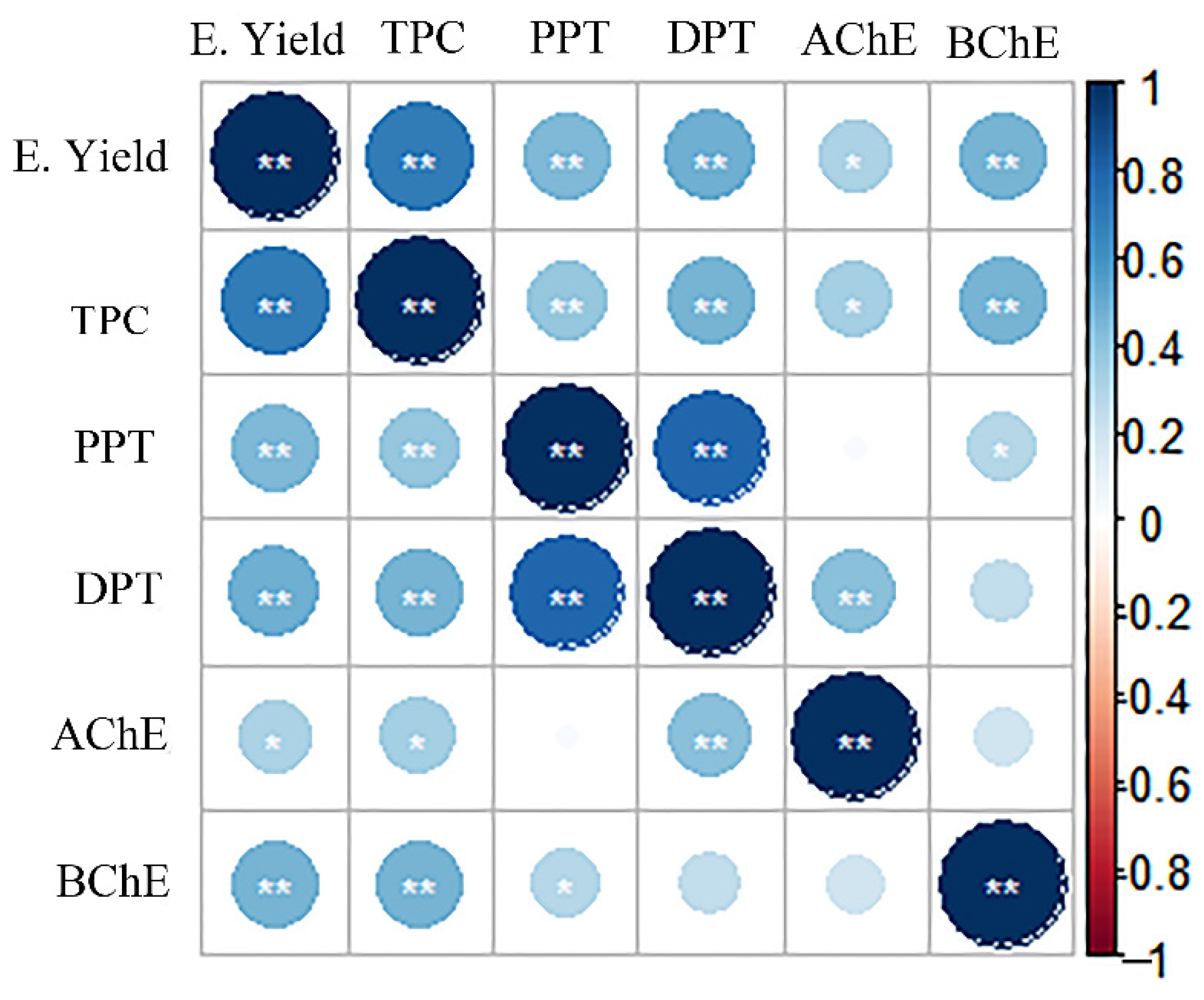
2. Results and Discussion
2.1. Optimization of the Extraction Conditions
2.1.1. Total Phenol Content (TPC)
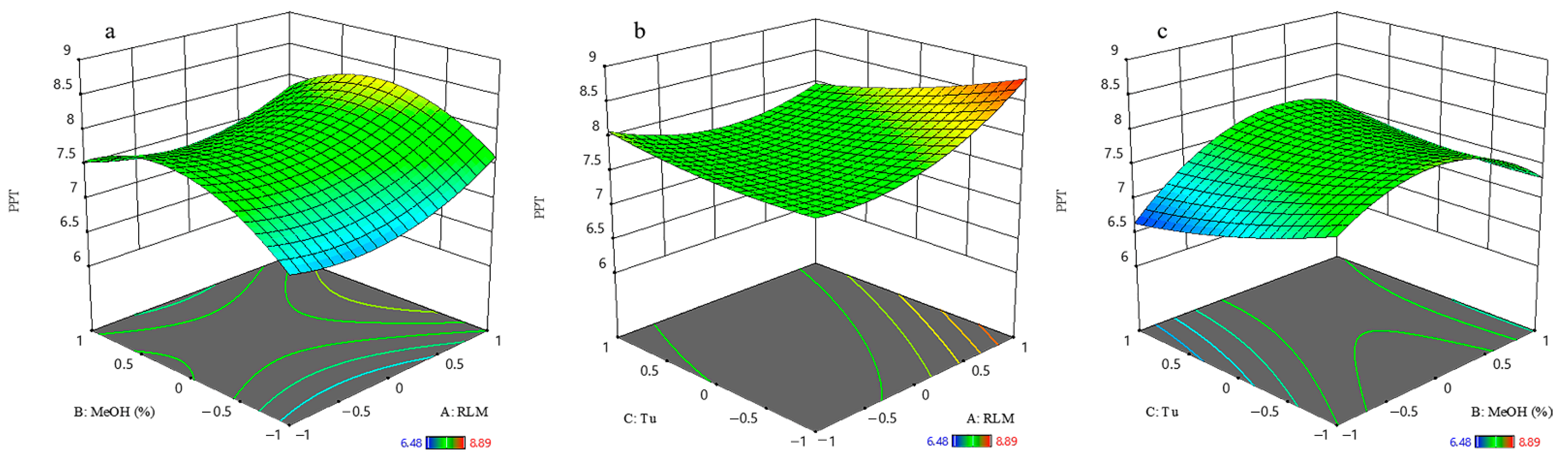
2.1.2. Podophyllotoxin and Deoxypodophyllotoxin
2.1.3. Extraction Yield (E. Yield)
2.1.4. Antioxidant Activity In Vitro
2.1.5. Process Optimization and Model Validation
2.2. Anticholinesterase Activity
3. Material and Methods
3.1. Chemicals
3.2. Plant Material
3.3. HPLC Analysis
3.4. Extraction of J. sabina
3.5. Experimental Design
3.6. Determine Optimal Conditions and Validate the Model
3.7. Determination of Total Phenol Content (TPC)
3.8. Evaluation of In Vitro Biological Activity
3.8.1. Antioxidant Activity In Vitro
3.8.2. Cholinesterase Inhibitory Activity
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canel, C.; Moraes, R.M.; Dayan, F.; Ferreira, D. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef]
- Guerram, M.; Jiang, Z.Z.; Zhang, L.Y. Podophyllotoxin, a medicinal agent of plant origin: Past, present and future. Chin. J. Nat. Med. 2012, 10, 161–169. [Google Scholar] [CrossRef]
- Zi, C.T.; Yang, L.; Kong, Q.H.; Li, H.M.; Yang, X.Z.; Ding, Z.T.; Jiang, Z.H.; Hu, J.M.; Zhou, J. Glucoside derivatives of podophyllotoxin: Synthesis, physicochemical properties, and cytotoxicity. Drug Des. Dev. Ther. 2019, 13, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Maulin, P.; Eduardo, D.; Daniel, S.; Parag, D.; Ashwin, C.; Michael, B.; Roberto, C.; Gerard, C.J. Etoposide as salvage therapy for cytokine storm due to coronavirus disease, 2019. Chest 2021, 159, E7–E11. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupré, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagège, D.; Lamblin, F.; Lainé, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12 other juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef]
- Hartwell, J.L.; Johnson, J.M.; Fitzgerald, D.B.; Belkin, M. Podophyllotoxin from Juniperus species; savinin. J. Am. Chem. Soc. 1953, 75, 235–236. [Google Scholar] [CrossRef]
- Och, M.; Och, A.; Cieśla, L.; Kubrak, T.; Pecio, L.; Stochmal, A.; Kocki, J.; Bogucka-Kocka, A. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm. Biol. 2014, 53, 831–837. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Zheljazkov, V.D.; Osbrink, W.L.; Castro, A.; Maddox, V.; Craker, L.E.; Astatkie, T. Podophyllotoxin and essential oil profile of Juniperus and related species. Ind. Crops Prod. 2013, 43, 668–676. [Google Scholar] [CrossRef]
- Biswas, D.; Biswas, P.; Nandy, S.; Mukherjee, A.; Dey, A. Endophytes producing podophyllotoxin from Podophyllum sp. and other plants: A review on isolation, extraction and bottlenecks. S. Afr. J. Bot. 2020, 134, 303–313. [Google Scholar] [CrossRef]
- Doussot, J.; Mathieu, V.; Colas, C.; Molinié, R.; Corbin, C.; Montguillon, J.; Banuls, L.M.Y.; Renouard, S.; Lamblin, F.; Dupré, P.; et al. Investigation of the lignan content in extractss from Linum, Callitris and Juniperus species in relation to their in vitro antiproliferative activities. Planta Med. 2016, 83, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Cushman, K.E.; Maqbool, M.; Gerard, P.D.; Bedir, E.; Lata, H.; Moraes, R.M. Variation of podophyllotoxin in leaves of Eastern red cedar (Juniperus virginiana). Planta Med. 2003, 69, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Gawde, A.; Cantrell, C.L.; Zheljazkov, V.D. Dual extraction of essential oil and podophyllotoxin from Juniperus virginiana. Ind. Crops Prod. 2009, 30, 276–280. [Google Scholar] [CrossRef]
- Gawde, A.; Zheljazkov, V.D.; Maddox, V.; Cantrell, C.L. Bioprospection of Eastern red cedar from nine physiographic regions in Mississippi. Ind. Crops Prod. 2009, 30, 59–64. [Google Scholar] [CrossRef]
- Kusari, S.; Zühlke, S.; Spiteller, M. Chemometric evaluation of the anti-cancer pro-drug podophyllotoxin and potential therapeutic analogues in Juniperus and Podophyllum species. Phytochem. Anal. 2010, 22, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Cantrell, C.L.; Donega, M.A.; Astatkie, T.; Heidel, B. Podophyllotoxin concentration in Junipers in the Big Horn Mountains in Wyoming. HortScience 2012, 47, 1696–1697. [Google Scholar] [CrossRef]
- Benzina, S.; Harquail, J.; Jean, S.; Beauregard, A.-P.; Colquhoun, C.D.; Carroll, M.; Bos, A.; Gray, C.A.; Robichaud, G.A. Deoxypodophyllotoxin isolated from Juniperus communis induces apoptosis in breast cancer cells. Anti-Cancer Agents Med. Chem. 2014, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.I.; Tashev, A.N.; Nedialkov, P.T.; Ilieva, Y.E.; Atanassova, T.N.; Olech, M.; Nowak, R.; Angelov, G.; Tsvetanova, F.V.; Iliev, I.A.; et al. Antioxidant and antiproliferative activity of Juniperus L. species of Bulgarian and foreign origin and their anticancer metabolite identification. Bulg. Chem. Commun. 2018, 50, 144–150. [Google Scholar]
- Zheljazkov, V.; Cantrell, C.; Semerdjieva, I.; Radoukova, T.; Stoyanova, A.; Maneva, V.; Kačániová, M.; Astatkie, T.; Borisova, D.; Dincheva, I.; et al. Essential oil composition and bioactivity of two Juniper Species from Bulgaria and Slovakia. Molecules 2021, 26, 3659. [Google Scholar] [CrossRef]
- Tavares, W.R.; Seca, A.M. The current status of the pharmaceutical potential of Juniperus L. metabolites. Medicines. 2018, 5, 81. [Google Scholar] [CrossRef]
- Li, G.Z.; He, J.; Yan, H.Y.; Feng, R.H.; Feng, J.T. Advances in the studies on chemical constituents and bioactivities of Sabina vulgaris Ant. J. Northwest Sci.-Tech. Univ. Agric. For. 2006, 34, 133–139. [Google Scholar] [CrossRef]
- Guerram, M.; Jiang, Z.; Sun, L.; Zhu, X.; Zhang, Y.L. Antineoplastic effects of deoxypodophyllotoxin, a potent cytotoxic agent of plant origin, on glioblastoma U-87 MG and SF126 cells. Pharmacol. Rep. 2015, 67, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Wang, G.; Cai, Q.; Zheng, X.; Zhang, J.; Chen, Q.; Wu, B.; Zhu, X.; Hao, H.; Zhou, F. A promising microtubule inhibitor deoxypodophyllotoxin exhibits better efficacy to multidrug-resistant breast cancer than paclitaxel via avoiding efflux transport. Drug Metab. Dispos. 2018, 46, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.P.; Oliveira, R.R.; Figueiredo, M.R. Isolation of limonoids from seeds of Carapa guianensis Aublet (Meliaceae) by high-speed counter current chromatography. Phytochem. Anal. 2009, 20, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Park, D.K.; Park, H.J. Hair growth-promoting activity of hot water extracts of Thuja orientalis. BMC Complem. Altern. Med. 2013, 13, 9. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, G.L.; Zhang, B.; Sun, Z.G.; Li, Y. Study progress of oriental Arborvitae pharmacological effects. Lishizhen Med. Mater. Med. Res. 2013, 24, 2231–2233. [Google Scholar] [CrossRef]
- Ivanova, D.I.; Nedialkov, P.T.; Tashev, A.N.; Olech, M.; Nowak, R.; Ilieva, Y.E.; Najdenski, H.M. Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin. Molecules 2021, 26, 5179. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Ding, J.W.; Sun, L.L.; Mai Maiti, A. Research summary of common medicinal materials of ethnic medicine in Xinjiang. Chin. J. Ethnomedicine Ethnopharmacy 2021, 30, 49–59. [Google Scholar]
- Orhan, N.; Orhan, I.E.; Ergun, F. Insights into cholinesterase inhibitory and antioxidant activities of five Juniperus species. Food Chem. Toxicol. 2011, 49, 2305–2312. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Li, D.; Shuai, Z. Quality evaluation of Juniperus rigida sieb. et zucc. based on phenolic profiles, bioactivity, and HPLC fingerprint combined with chemometrics. Front. Pharmacol. 2017, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Trovato, A.; Marino, A.; Bellinghieri, V.; Melchini, A.; Dugo, P.; Cacciola, F.; Donato, P.; Mondello, L.; Güvenç, A.; et al. Phenolic composition and biological activities of Juniperus drupacea labill. berries from turkey. Food Chem. Toxicol. 2011, 49, 2600–2608. [Google Scholar] [CrossRef]
- Oliveira, B.D.; Rodrigues, A.C.; Cardoso, B.M.I.; Ramos, A.L.C.C.; Bertoldi, M.C.; Taylor, J.G.; da Cunha, L.R.; Pinto, U.M. Antioxidant, antimicrobial and anti-quorum sensing activities of Rubus rosaefolius phenolic extract. Ind. Crops Prod. 2016, 84, 59–66. [Google Scholar] [CrossRef]
- Piwowarska, N.; González-Alvarez, J. Extraction of antioxidants from forestry biomass: Kinetics and optimization of extraction conditions. Biomass Bioenerg. 2012, 43, 42–51. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Abas, F.; Lai, O.M.; Ho, C.W.; Tan, C.P. Optimal binary solvent extraction system for phenolic antioxidants from mengkudu (Morinda citrifolia) fruit. Molecules 2013, 18, 7004–7022. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Darra, N.E.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Darra, N.E.; Tannous, J.; Mouncef, P.B.; Palge, J.; Yaghi, J.; Vorobiev, E.; Louka, N.; Maroun, R.G. A Comparative study on antiradical and antimicrobial properties of red grapes extracts obtained from different Vitis vinifera varieties. Food Nutr. Sci. 2012, 3, 1420–1432. [Google Scholar] [CrossRef]
- Pope, C.N.; Brimijoin, S. Cholinesterases and the fine line between poison and remedy. Biochem. Pharmacol. 2018, 153, 205–216. [Google Scholar] [CrossRef]
- Cieśla, L.; Moaddel, R. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat. Prod. Rep. 2016, 33, 1131–1145. [Google Scholar] [CrossRef]
- Ballard, C.G.; Greig, N.H.; Guillozet-Bongaarts, A.L.; Enz, A.; Darvesh, S. Cholinesterases: Roles in the brain during health and disease. Alzh. Res. 2005, 2, 307–318. [Google Scholar] [CrossRef]
- Burcu, B.; Aysel, U.; Nurdan, S. Antimicrobial, antioxidant, antimutagenic activities, and phenolic compounds of iris germanica. Ind. Crops Prod. 2014, 61, 526–530. [Google Scholar] [CrossRef]
- Mocan, A.; Crișan, G.; Vlase, L.; Crișan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Feng, C.; Wang, X.; Gu, H.; Yang, L. Chemical composition and antioxidant activity of essential oils from barks of pinus pumila using microwave-assisted hydrodistillation after screw extrusion treatment. Ind. Crops Prod. 2021, 166, 113489. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]


| NO. | Compound Name | Structure | |
|---|---|---|---|
| 1 | Podophyllotoxin |  | 1 R1=R3=H, R2=OH 2 R1=R2=H, R3=OH 3 R1=R2=H, R3=OAc 4 R1=R2=R3=H 5 R1=OMe, R2=OH, R3=H 6 R1=OMe, R2=R3=H |
| 2 | Epipodophyllotoxin | ||
| 3 | Epipodophyllotoxin acetate | ||
| 4 | Deoxypodophyllotoxin | ||
| 5 | 2′-Methoxy-podophyllotoxin | ||
| 6 | β-Methylpeltatin A | ||
| 7 | Deoxy-picropodophyllotoxin | 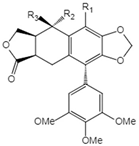 | 7 R1=R2=R3=H 8 R1=R3=H, R2=OH 9 R1=R3=H, R2=OAc 10 R1=R2=H, R3=OH 11 R1=R2=H, R3=OAc 12 R1=OMe, R2=OH, R3=H 13 R1=OMe, R2=H, R3=OH |
| 8 | Picropodophyllotoxin | ||
| 9 | Picropodophyllotoxin acetate | ||
| 10 | Epipicropodophyllotoxin | ||
| 11 | Epipicropodophyllotoxin acetate | ||
| 12 | 2′-Methoxy-picropodophyllotoxin | ||
| 13 | 2′-Methoxy-epipicropodophyllotoxin | ||
| 14 | Dehydropodophyllotoxin | 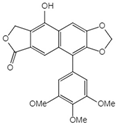 | |
| 15 | Acetyl epipodophyllotoxin | 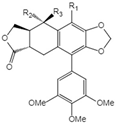 | 15 R1=R2=H, R3=OAc 16 R1=O, R2=R3=H 17 R1=H, R3=O |
| 16 | β-peltatin-A methylether | ||
| 17 | Podophyllotoxone | ||
| 18 | Acetyl epipicropodophyllotoxin | 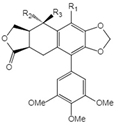 | 18 R1=R2=H, R3=OAc 19 R1=OMe, R2= H, R3=OH 20 R1=H, R2=R3=O |
| 19 | β-peltatin-B methylether | ||
| 20 | Picropodophyllotoxone | ||
| 21 | Junaphthoic acid | 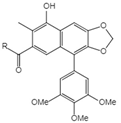 | 21 R=OH 22 R=OAc |
| 22 | 4-Acetyl junaphtholic acid | ||
| EC | RLM | Methanol (%) | TU (min) | TPC | PPT | DPT | E. Yield | ABTS | FRAP | DPPH |
|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | Dependent Variables | |||||||||
| x1 | x2 | x3 | y1 | y2 | y3 | y4 | y5 | y6 | y7 | |
| 1 | 1:40 (1) | 60 (−1) | 10 (0) | 483.27 ± 40.96 | 7.61 ± 0.32 | 2.20 ± 0.07 | 253.85 ± 9.25 | 12,462.3 ± 602.4 | 7880.3 ± 158.6 | 194.0 ± 69.0 |
| 2 | 1:40 (1) | 100 (1) | 10 (0) | 611.27 ± 91.04 | 7.61 ± 0.33 | 2.46 ± 0.11 | 322.30 ± 3.60 | 14,606.7 ± 107.4 | 10,584.9 ± 284.5 | 2129.5 ± 88.4 |
| 3 | 1:30 (0) | 80 (0) | 10 (0) | 546.53 ± 44.48 | 7.82 ± 0.09 | 2.33 ± 0.09 | 316.60 ± 3.90 | 15,454.3 ± 612.0 | 10,139.1 ± 85.5 | 645.7 ± 145.3 |
| 4 | 1:30 (0) | 80 (0) | 10 (0) | 537.60 ± 44.30 | 7.91 ± 0.14 | 2.20 ± 0.06 | 314.60 ± 7.00 | 15,922.9 ± 285.2 | 10,855.9 ± 383.9 | 444.8 ± 6.0 |
| 5 | 1:20 (−1) | 100 (1) | 10 (0) | 546.76 ± 88.62 | 7.57 ± 0.28 | 2.03 ± 0.07 | 318.50 ± 2.60 | 12,281.6 ± 721.9 | 9880.6 ± 262.1 | 2086.3 ± 271.3 |
| 6 | 1:40 (1) | 80 (0) | 5 (−1) | 582.62 ± 36.37 | 8.89 ± 0.46 | 2.49 ± 0.13 | 307.50 ± 3.90 | 14,940.4 ± 926.5 | 11,009.4 ± 713.0 | 324.4 ± 158.9 |
| 7 | 1:30 (0) | 100 (1) | 5 (−1) | 587.09 ± 68.29 | 7.49 ± 0.57 | 2.01 ± 0.16 | 329.15 ± 2.75 | 13,477.8 ± 1040.0 | 11,155.0 ± 316.3 | 2360.2 ± 62.4 |
| 8 | 1:30 (0) | 80 (0) | 10 (0) | 515.27 ± 46.66 | 7.41 ± 0.65 | 2.02 ± 0.21 | 314.55 ± 0.85 | 13,291.0 ± 910.0 | 9623.9 ± 664.1 | 311.0 ± 105.8 |
| 9 | 1:20 (−1) | 80 (0) | 15 (1) | 515.27 ± 35.77 | 8.03 ± 0.22 | 2.23 ± 0.05 | 305.05 ± 1.05 | 10,652.7 ± 305.0 | 9873.9 ± 384.6 | 280.8 ± 111.4 |
| 10 | 1:30 (0) | 60 (−1) | 5 (−1) | 412.95 ± 29.03 | 7.51 ± 0.10 | 2.12 ± 0.09 | 266.60 ± 1.10 | 9903.6 ± 376.8 | 7823.2 ± 112.6 | 138.0 ± 56.1 |
| 11 | 1:20 (−1) | 80 (0) | 5 (−1) | 538.72 ± 45.32 | 7.70 ± 0.31 | 2.37 ± 0.09 | 307.65 ± 6.45 | 14,467.8 ± 488.1 | 11,343.7 ± 754.0 | 192.1 ± 121.2 |
| 12 | 1:20 (−1) | 60 (−1) | 10 (0) | 422.25 ± 25.22 | 7.28 ± 0.19 | 2.01 ± 0.06 | 251.30 ± 0.80 | 11,013.7 ± 365.2 | 7368.7 ± 86.5 | 122.7 ± 18.1 |
| 13 | 1:30 (0) | 100 (1) | 15 (1) | 596.02 ± 99.99 | 7.64 ± 0.57 | 2.28 ± 0.26 | 324.55 ± 3.25 | 9949.0 ± 134.1 | 11,657.2 ± 221.5 | 1689.3 ± 41.1 |
| 14 | 1:30 (0) | 80 (0) | 10 (0) | 543.18 ± 50.87 | 8.08 ± 0.26 | 2.40 ± 0.15 | 308.80 ± 2.20 | 13,122.1 ± 234.7 | 11,144.7 ± 86.5 | 301.9 ± 143.8 |
| 15 | 1:30 (0) | 60 (−1) | 15 (1) | 388.39 ± 24.49 | 6.48 ± 0.12 | 1.75 ± 0.11 | 222.10 ± 1.30 | 9523.8 ± 183.6 | 6930.0 ± 135.6 | 8.7 ± 1.2 |
| 16 | 1:30 (0) | 80 (0) | 10 (0) | 536.11 ± 55.07 | 7.57 ± 0.51 | 2.24 ± 0.22 | 319.50 ± 2.90 | 12,741.9 ± 353.5 | 11,096.4 ± 513.9 | 293.5 ± 212.9 |
| 17 | 1:40 (1) | 80 (0) | 15 (1) | 543.18 ± 43.06 | 8.16 ± 0.03 | 2.38 ± 0.00 | 314.30 ± 3.90 | 14,436.9 ± 557.3 | 11,702.4 ± 347.5 | 339.1 ± 8.7 |
| TPC | PPT | DPT | E. Yield | ABTS | FRAP | DPPH | |
|---|---|---|---|---|---|---|---|
| y2 | y3 | y4 | y1 | y5 | y6 | y7 | |
| β0 | 535.74 | 7.76 | 2.24 | 313.32 | 14,106.40 | 10,572.00 | 399.38 |
| β1 | 22.42 b | 0.21 c | 0.11 b | 1.93 | 1003.81 b | 338.76 | 38.14 |
| β2 | 81.53 a | 0.18 | 0.09 c | 37.58 a | 926.46 c | 1659.44 a | 975.24 a |
| β3 | −9.81 | −0.16 | −0.04 | −5.61 c | −1028.40 b | −145.98 | −87.10 |
| β12 | −3.63 | −0.07 | 0.06 | 0.31 | 219.13 | 48.18 | −7.03 |
| β13 | −4.00 | −0.27 c | 0.01 | 2.35 | 827.90 | 540.70 | −18.50 |
| β23 | 8.37 | 0.30 c | 0.16 b | 9.98 b | −787.25 | 348.85 | −135.40 |
| β11 | 16.74 | 0.34 b | 0.13 c | −2.83 | 697.77 | −26.19 | −15.60 |
| β22 | −32.09 b | −0.58 a | −0.20 b | −25.86 a | −2213.13 a | −1617.19 a | 749.35 a |
| β33 | −7.53 | 0.10 | − | − | −1179.76 c | 436.54 | −99.68 |
| R2 | 0.9612 | 0.8642 | 0.8319 | 0.9627 | 0.8687 | 0.9283 | 0.9812 |
| PPT | DPT | TPC | E. Yield | ABTS | FRAP | DPPH | |
|---|---|---|---|---|---|---|---|
| Predicted value | 8.24 | 2.45 | 464.90 | 324.52 | 15,638.40 | 11,092.20 | 1506.86 |
| Experimental value | 7.51 | 2.35 | 540.1 | 309.31 | 17,242.14 | 10,963.49 | 2211.19 |
| Std Dev | 0.52 | 0.07 | 53.11 | 10.76 | 1134.01 | 91.01 | 2211.19 |
| RSD (%) | 6.88 | 3.15 | 9.84 | 3.48 | 6.58 | 0.83 | 22.52 |
| EC | AChE (mg GALE/g Extract) | BChE (mg GALE/g Extract) |
|---|---|---|
| JS-1 | 9.64 ± 2.69 | 63.95 ± 10.37 |
| JS-2 | 28.79 ± 4.14 | 282.99 ± 47.85 |
| JS-3 | 2.61 ± 1.28 | 91.18 ± 14.77 |
| JS-4 | 2.29 ± 1.17 | 86.36 ± 13.99 |
| JS-5 | 2.06 ± 1.09 | 351.8 ± 60.4 |
| JS-6 | 8.43 ± 2.53 | 284.77 ± 48.17 |
| JS-7 | 9.26 ± 2.64 | 361.18 ± 160.67 |
| JS-8 | 3.86 ± 1.64 | 434.77 ± 80.93 |
| JS-9 | 1.99 ± 1.06 | 254.69 ± 100.49 |
| JS-10 | 0.96 ± 0.63 | 173.4 ± 28.55 |
| JS-11 | 8.28 ± 2.5 | 384.58 ± 66.48 |
| JS-12 | 2.74 ± 1.32 | 125.96 ± 20.51 |
| JS-13 | 17.01 ± 3.4 | 242.55 ± 40.62 |
| JS-14 | 18.48 ± 3.5 | 520.15 ± 92.2 |
| JS-15 | 5.02 ± 1.91 | 37.67 ± 6.21 |
| JS-16 | 13.5 ± 3.11 | 109.39 ± 17.76 |
| JS-17 | 7.07 ± 2.31 | 185.64 ± 30.65 |
| JS-Y | 16.78 ± 4.61 | 208.79 ± 41.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Li, X.; Liu, S.; Tian, P.; Li, D. Juniperus sabina L. as a Source of Podophyllotoxins: Extraction Optimization and Anticholinesterase Activities. Int. J. Mol. Sci. 2022, 23, 10205. https://doi.org/10.3390/ijms231810205
Xu S, Li X, Liu S, Tian P, Li D. Juniperus sabina L. as a Source of Podophyllotoxins: Extraction Optimization and Anticholinesterase Activities. International Journal of Molecular Sciences. 2022; 23(18):10205. https://doi.org/10.3390/ijms231810205
Chicago/Turabian StyleXu, Shengnan, Xinru Li, Shi Liu, Peilin Tian, and Dengwu Li. 2022. "Juniperus sabina L. as a Source of Podophyllotoxins: Extraction Optimization and Anticholinesterase Activities" International Journal of Molecular Sciences 23, no. 18: 10205. https://doi.org/10.3390/ijms231810205
APA StyleXu, S., Li, X., Liu, S., Tian, P., & Li, D. (2022). Juniperus sabina L. as a Source of Podophyllotoxins: Extraction Optimization and Anticholinesterase Activities. International Journal of Molecular Sciences, 23(18), 10205. https://doi.org/10.3390/ijms231810205