Ameliorative Effect of Pomegranate Peel Extract (PPE) on Hepatotoxicity Prompted by Iron Oxide Nanoparticles (Fe2O3-NPs) in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Preparation of Fe2O3 Nanoparticles (Fe2O3-NPs)
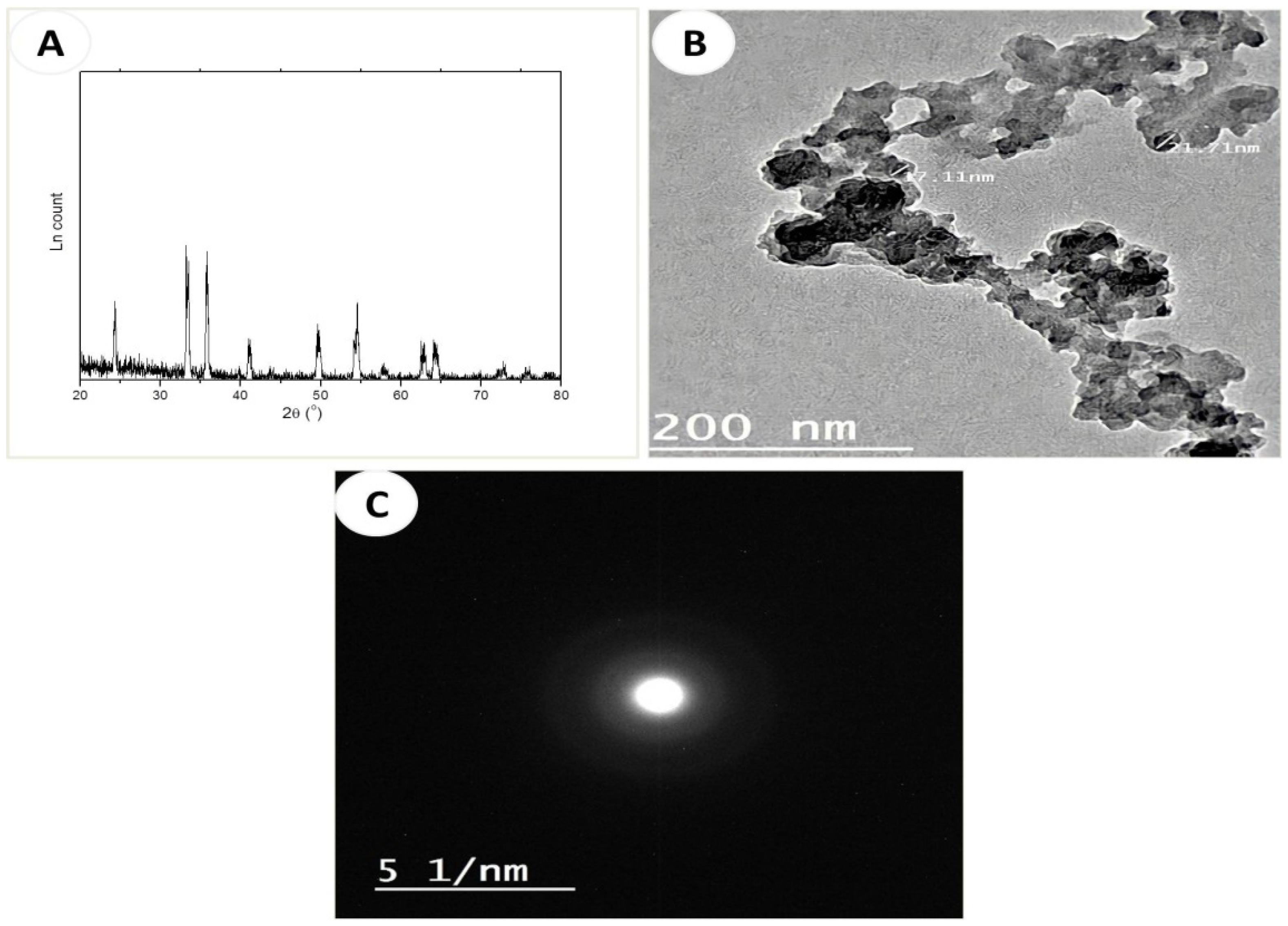
XRD and TEM Characterization of Fe2O3-NPs
2.3. Procedures of Pomegranate Peel Extract Preparation (PPE)
2.4. Experimental Animals
2.5. Histopathological Examinations
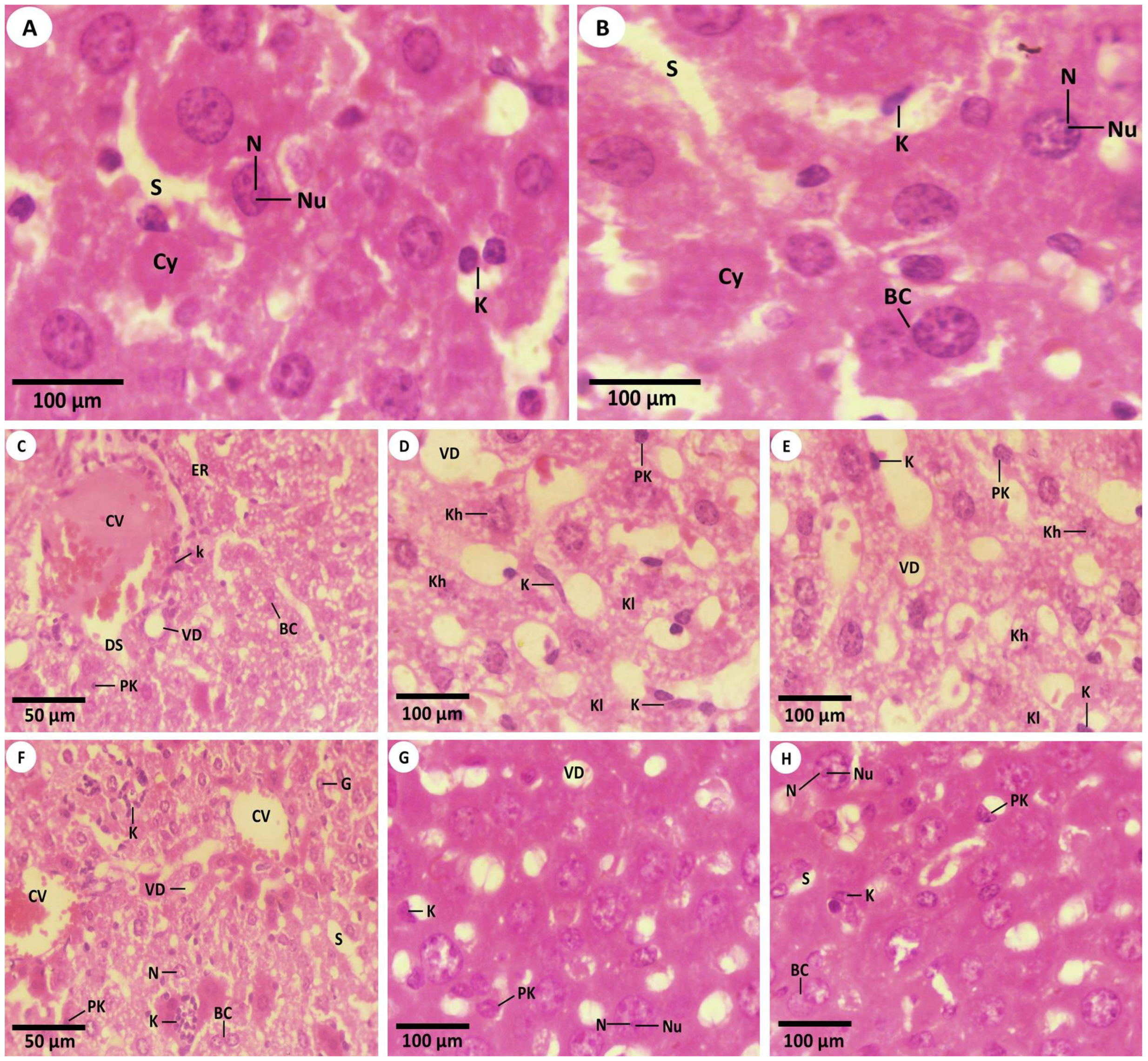
2.5.1. Examination of Liver Tissues via a Light Microscope
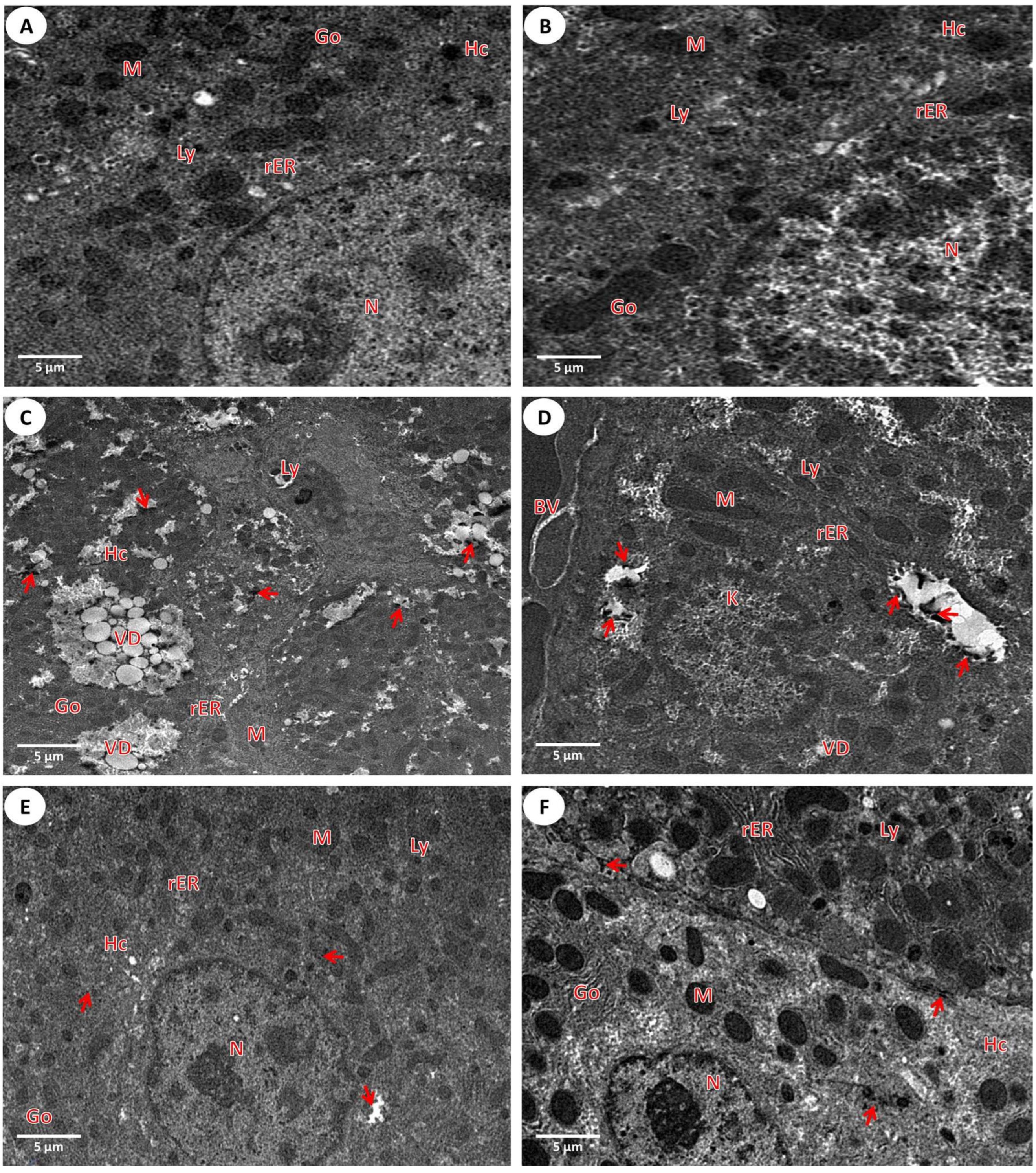
2.5.2. Detection of Liver Tissues Using the Transmission Electron Microscope
2.6. Histochemical Examinations
2.7. Apoptotic Evaluations
2.8. Morphometric Quantifications
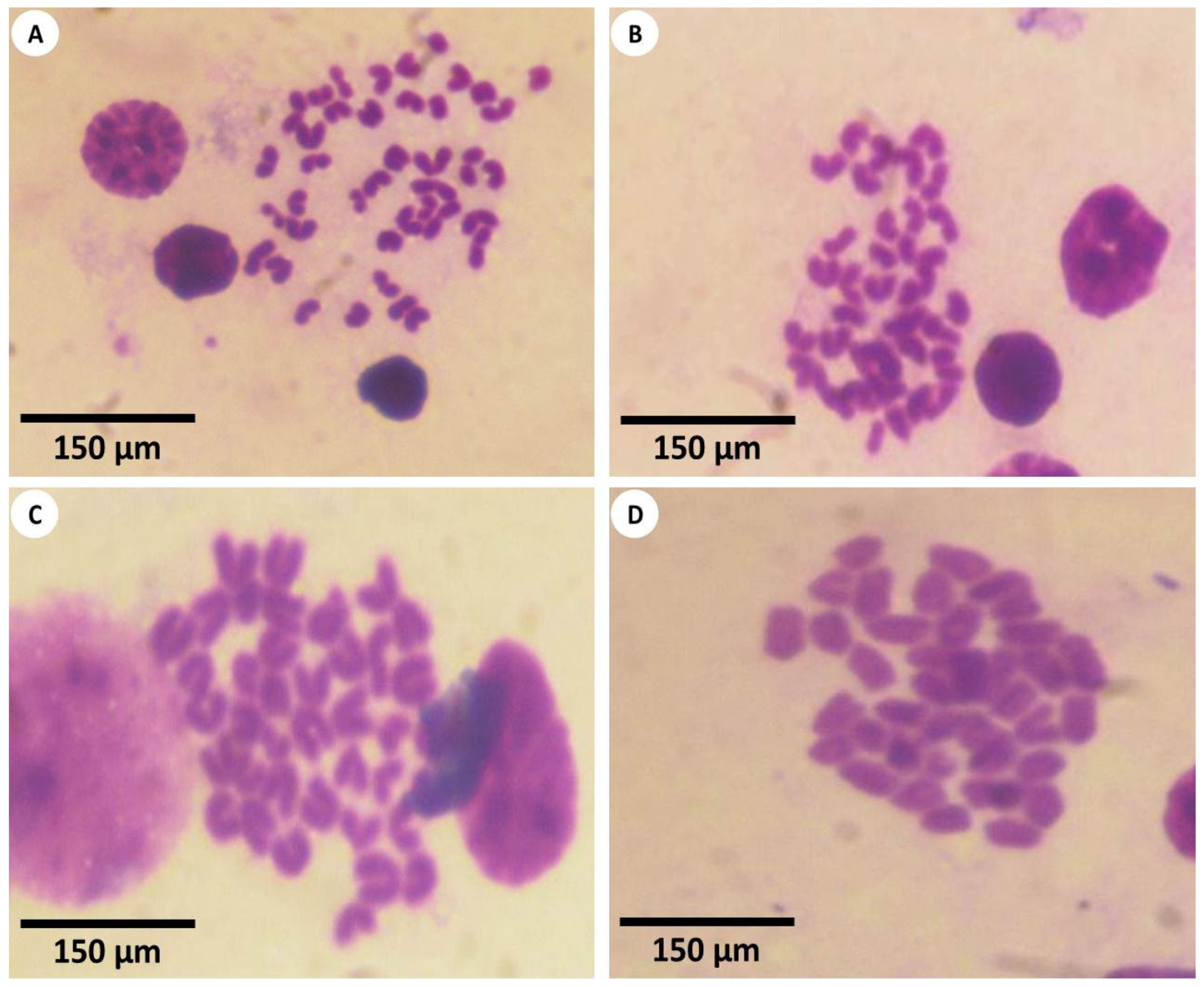
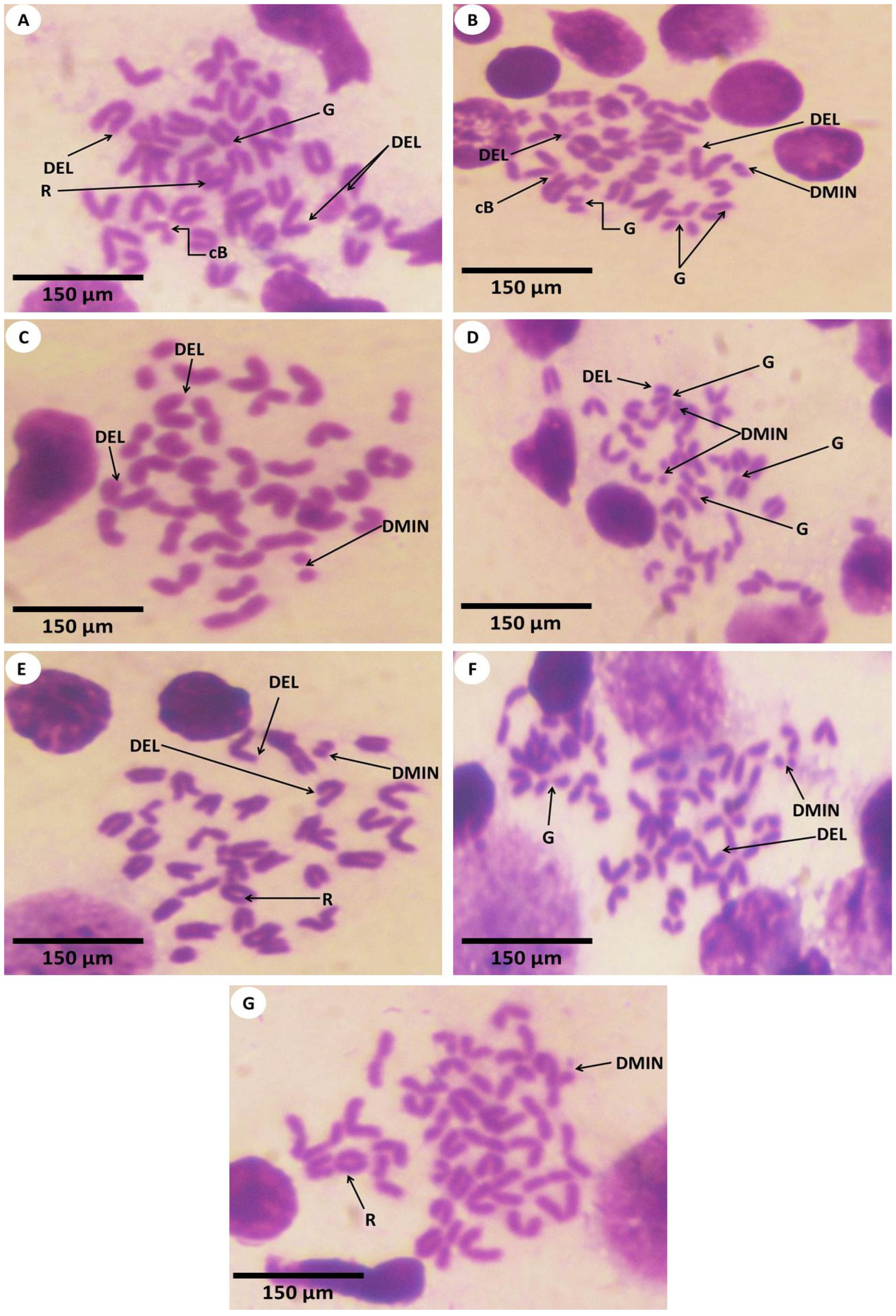
2.9. Chromosomal Aberration Analysis
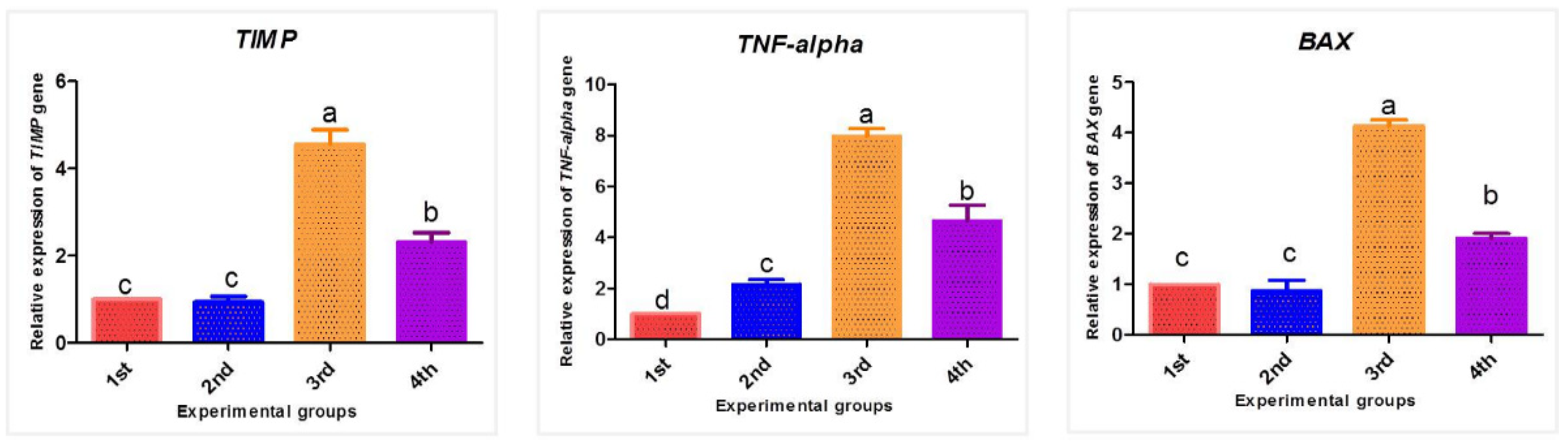
2.10. Relative Transcription of the TIMP-1, TNF-α, and BAX Genes Using Quantitative Real-Time PCR
2.10.1. Extraction of RNA and Reverse Transcription
2.10.2. Quantitative Real-Time PCR
2.11. Biochemical Assay
2.12. Statistical Analysis
3. Results
3.1. Characterization of α-Fe2O3 Nanoparticles (Fe2O3-NPs)
3.2. Histopathological Investigations
3.2.1. Studying the H&E Sections by Light Microscopy (LM)
3.2.2. Investigation of the Liver Ultrastructure by a Transmission Microscope
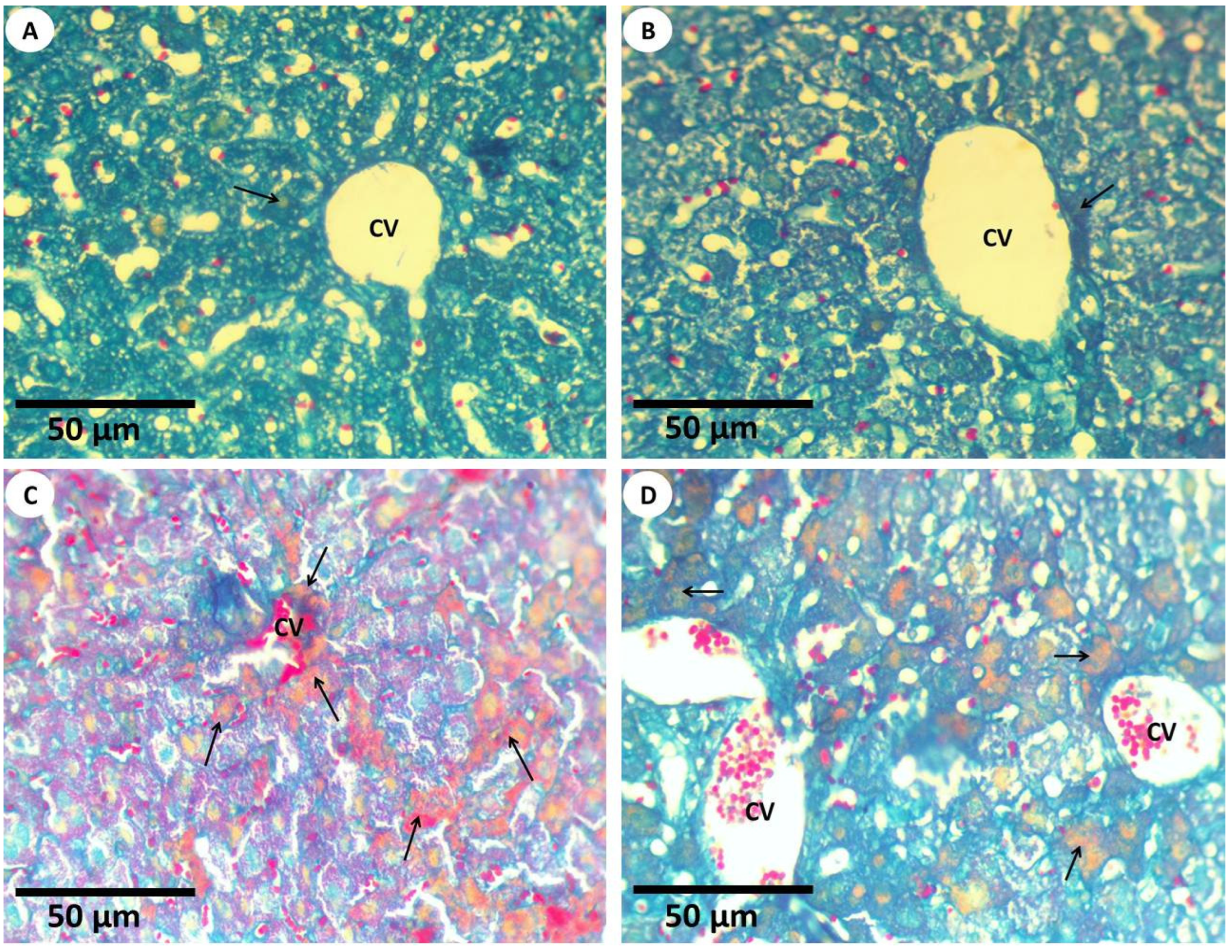
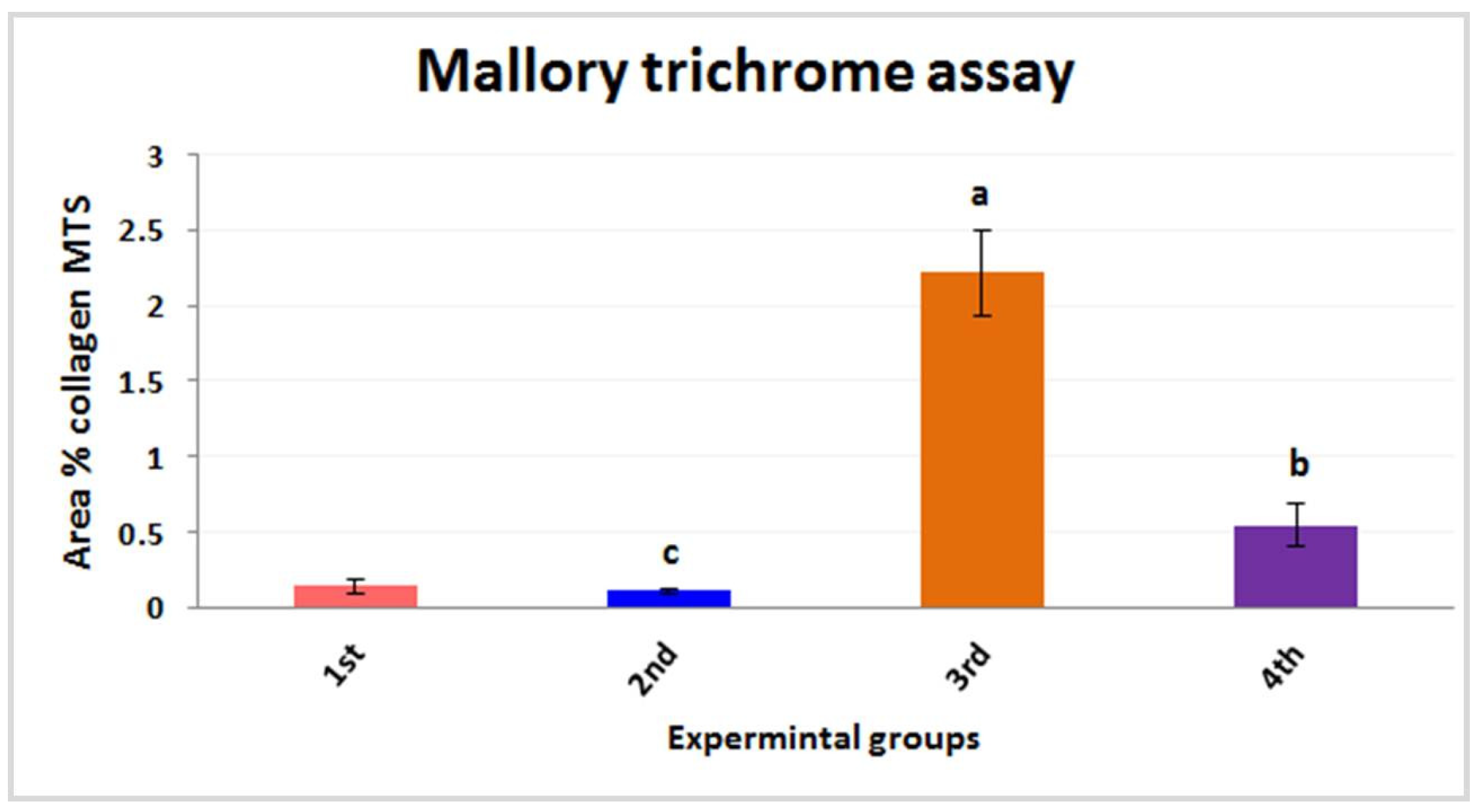
3.2.3. Histochemical Investigations and Morphometric Quantification
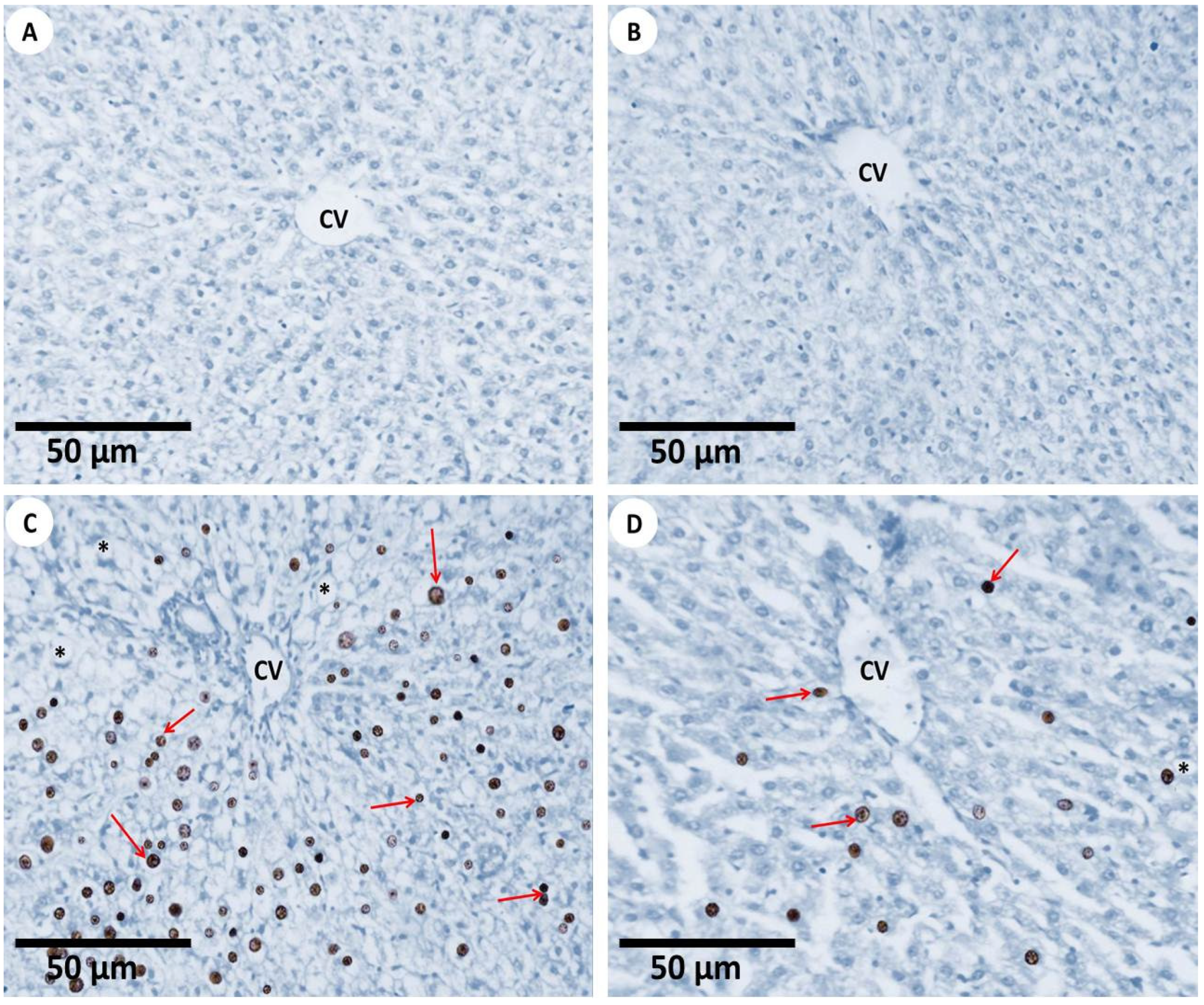
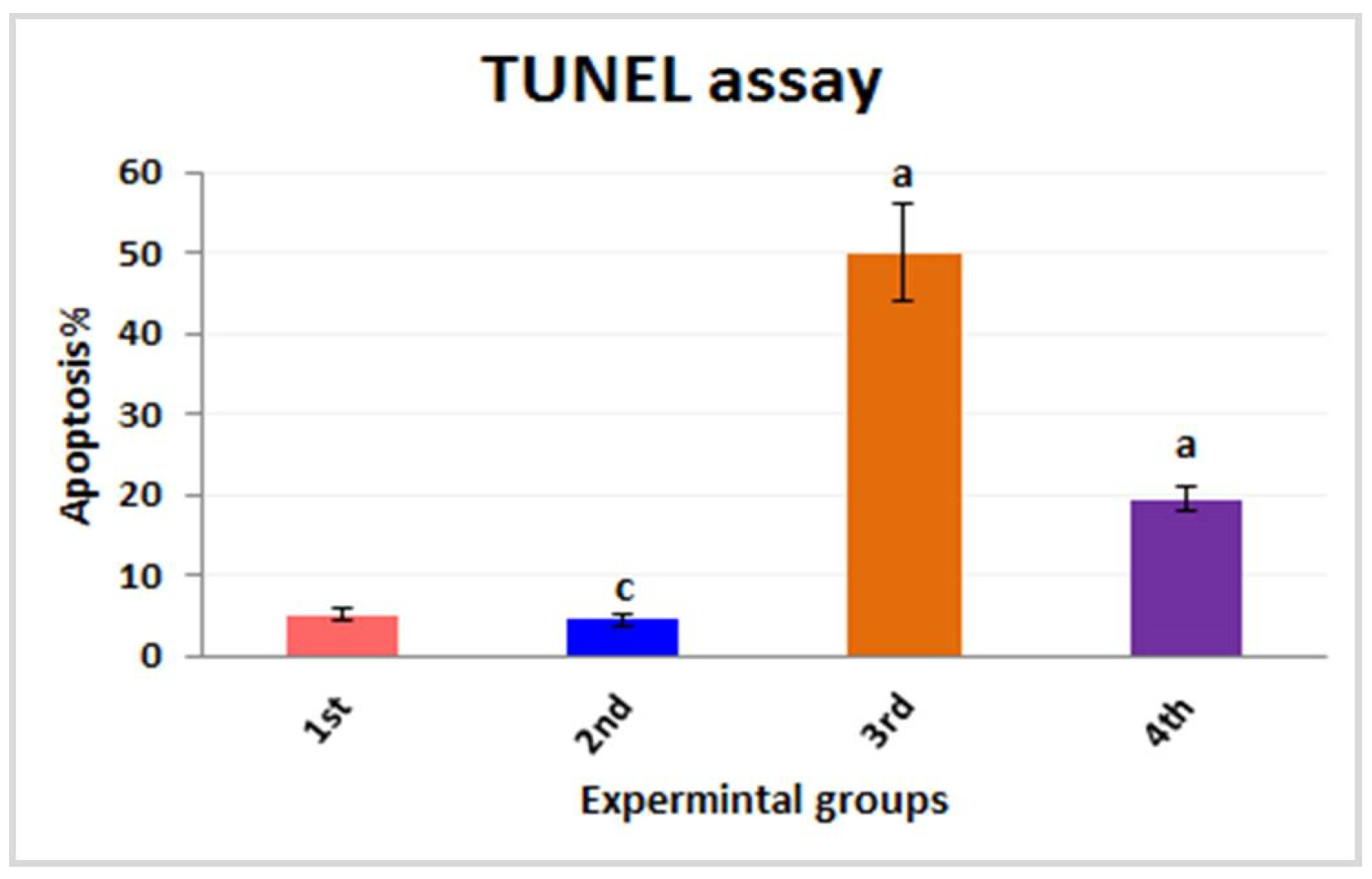
3.2.4. TUNEL Assay for Apoptosis
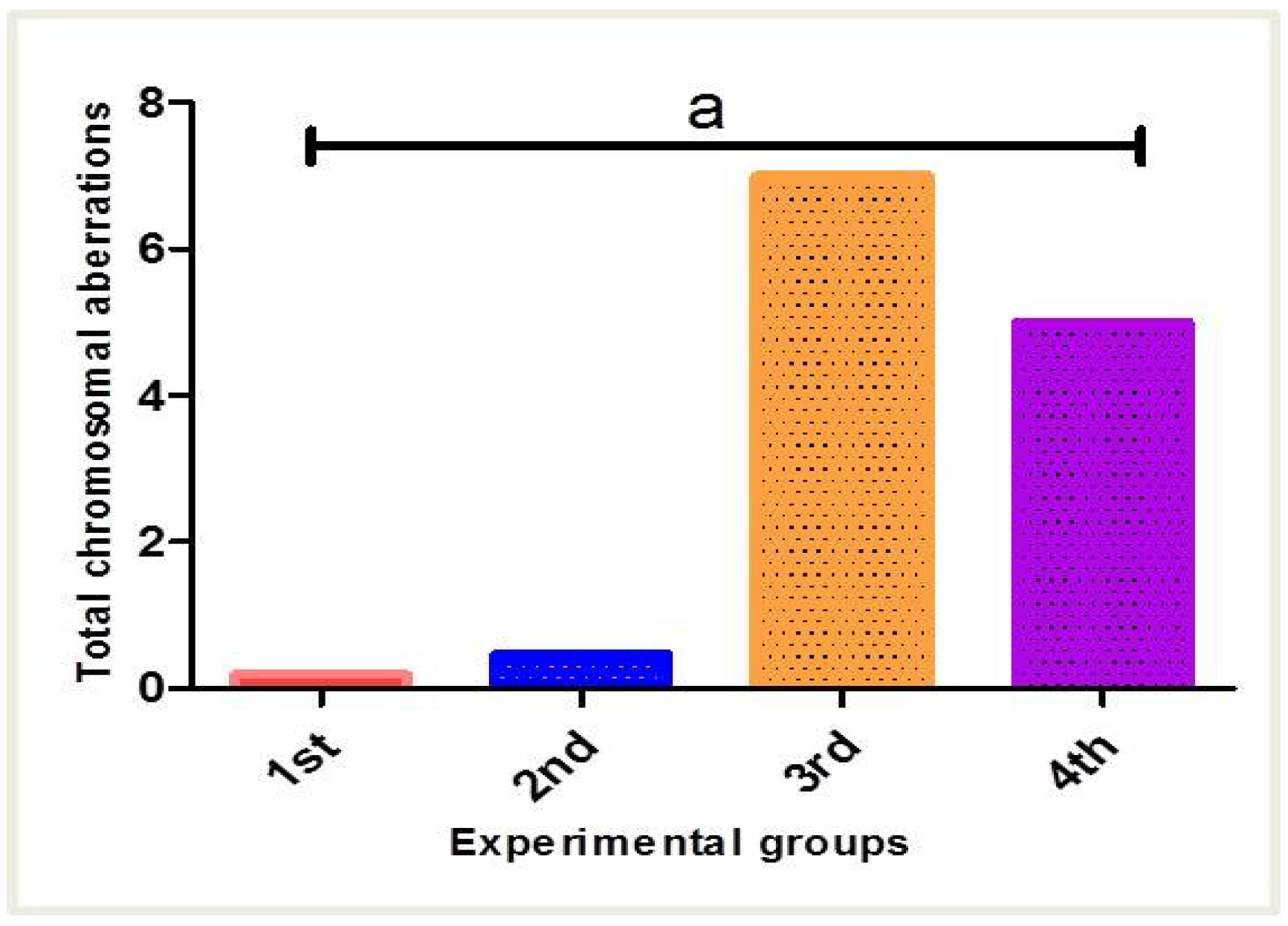
3.3. Estimation of Chromosomal Abnormalities
3.4. Effect of PPE and/or Fe2O3-NPs on mRNA Expression Patterns of TIMP-1, TNF-α, and BAX Genes
3.5. Iron Ion Metal Parameter
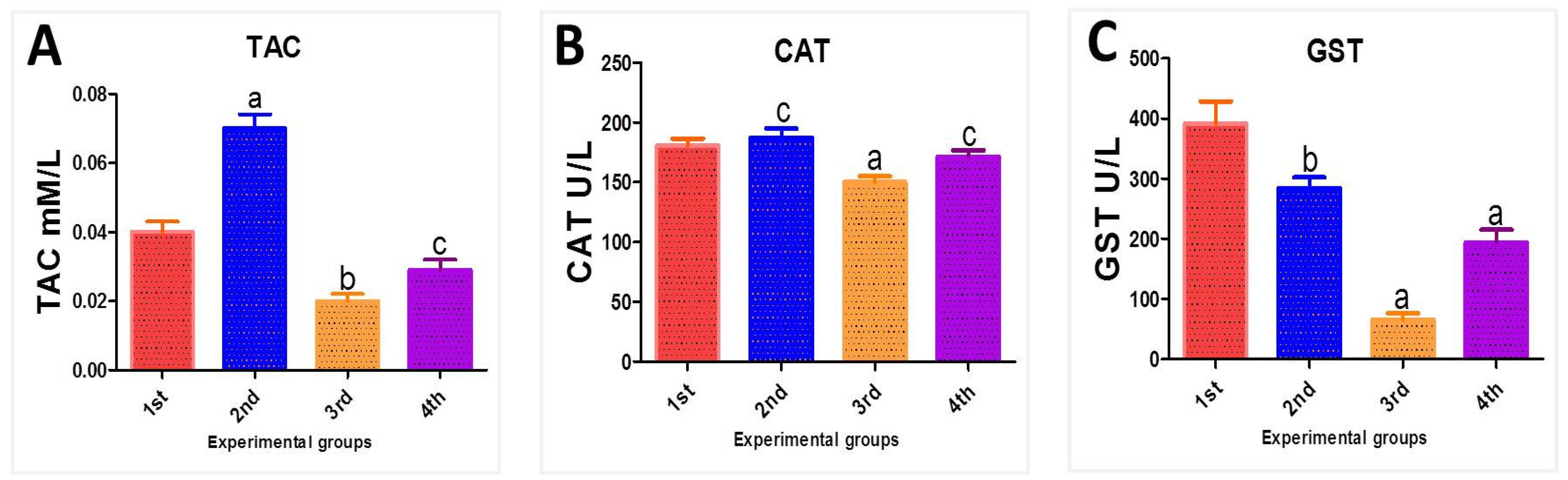
3.6. Biochemical Oxidative Stress Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gholami, M.; Zare-Hoseinabadi, A.; Mohammadi, M.; Taghizadeh, S.; Behbahani, A.B.; Amani, A.M.; Solghar, R.A. Preparation of ZnxFe3−xO4 chitosan nanoparticles as an adsorbent for methyl orange and phenol. J. Environ. Treat. Tech. 2019, 7, 245–249. [Google Scholar]
- Mahmoudi, M.; Milani, A.S.; Stroeve, P. Synthesis, surface architecture and biological response of superparamagnetic iron oxide nanoparticles for application in drug delivery: A review. Int. J. Biomed. Nanosci. Nanotechnol. 2010, 1, 164–201. [Google Scholar] [CrossRef]
- Ilani, M.; Alaee, S.; Khodabandeh, Z.; Jamhiri, I.; Owjfard, M. Effect of titanium dioxide nanoparticles on the expression of apoptotic markers in mouse blastocysts. Toxicol. Environ. Chem. 2018, 100, 228–234. [Google Scholar] [CrossRef]
- Van Rooy, I.; Cakir-Tascioglu, S.; Hennink, W.E.; Storm, G.; Schiffelers, R.M.; Mastrobattista, E. In Vivo Methods to Study Uptake of Nanoparticles into the Brain. Pharm. Res. 2010, 28, 456–471. [Google Scholar] [CrossRef]
- Baratli, Y.; Charles, A.-L.; Wolff, V.; Ben Tahar, L.; Smiri, L.; Bouitbir, J.; Zoll, J.; Sakly, M.; Auger, C.; Vogel, T.; et al. Age Modulates Fe3O4Nanoparticles Liver Toxicity: Dose-Dependent Decrease in Mitochondrial Respiratory Chain Complexes Activities and Coupling in Middle-Aged as Compared to Young Rats. BioMed Res. Int. 2014, 2014, 474081. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Pan, B.; Qiu, H.; Pan, B.; Nie, G.; Xiao, L.; Lv, L.; Zhang, W.; Zhang, Q.; Zheng, S. Highly efficient removal of heavy metals by polymer-supported nanosized hydrated Fe(III) oxides: Behavior and XPS study. Water Res. 2010, 44, 815–824. [Google Scholar] [CrossRef]
- Sadeghi, L.; Babadi, V.Y.; Espanani, H.R. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Lek. Listy 2015, 116, 373–378. [Google Scholar] [CrossRef]
- Parivar, K.; Fard, F.M.; Bayat, M.; Alavian, S.M.; Motavaf, M. Evaluation of Iron Oxide Nanoparticles Toxicity on Liver Cells of BALB/c Rats. Iran. Red Crescent Med. J. 2016, 18, e28939. [Google Scholar] [CrossRef]
- Blankenberg, F.G.; Strauss, H.W. Will imaging of apoptosis play a role in clinical care? A tale of mice and men. Apoptosis 2001, 6, 117–123. [Google Scholar] [CrossRef]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim. Biophys. Acta 2013, 1832, 876–883. [Google Scholar] [CrossRef]
- Nobili, V.; Parkes, J.; Bottazzo, G.; Marcellini, M.; Cross, R.; Newman, D.; Vizzutti, F.; Pinzani, M.; Rosenberg, W.M. Performance of ELF Serum Markers in Predicting Fibrosis Stage in Pediatric Non-Alcoholic Fatty Liver Disease. Gastroenterology 2009, 136, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-I.; Do, S.-H.; Jeong, D.-H.; Hong, I.-H.; Park, J.-K.; Ran, K.-M.; Yang, H.-J.; Yuan, D.-W.; Kim, S.-B.; Cha, M.-S.; et al. Kinetics of MMP-1 and MMP-3 produced by mast cells and macrophages in liver fibrogenesis of rat. Anticancer Res. 2006, 26, 3517–3526. [Google Scholar] [PubMed]
- Cawthorn, W.P.; Sethi, J.K. TNF-α and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jiang, J.; Jing, Y.; Liu, W.; Yang, X.; Hou, X.; Gao, L.; Wei, L. The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- de Castro Barbosa, M.L.; Fumian, M.M.; De-Miranda, P.A.L.; Barreiro, E.J.; Lima, L.M. Therapeutic approaches for tumor necrosis factor inhibition. Braz. J. Pharm. Sci. 2011, 47, 427–446. [Google Scholar] [CrossRef]
- Fargion, S.; Valenti, L.; Dongiovanni, P.; Scaccabarozzi, A.; Fracanzani, A.L.; Taioli, E.; Mattioli, M.; Sampietro, M.; Fiorelli, G. Tumor necrosis factor α promoter polymorphisms influence the phenotypic expression of hereditary hemochromatosis. Blood 2001, 97, 3707–3712. [Google Scholar] [CrossRef]
- Wullaert, A.; Van, L.G.; Heyninck, K.; Beyaert, R. Hepatic Tumor Necrosis Factor Signaling and Nuclear Factor-κB: Effects on Liver Homeostasis and Beyond. Endocr. Rev. 2007, 28, 365–386. [Google Scholar] [CrossRef]
- Tomita, K.; Tamiya, G.; Ando, S.; Ohsumi, K.; Chiyo, T.; Mizutani, A.; Kitamura, N.; Toda, K.; Kaneko, T.; Horie, Y.; et al. Tumour necrosis factor signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 2006, 55, 415–424. [Google Scholar] [CrossRef]
- Delbridge, A.R.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Guicciardi, M.E.; Gores, G.J. Hepatocyte Death: A Clear and Present Danger. Physiol. Rev. 2010, 90, 1165–1194. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef]
- Puneeth, H.R.; Chandra, S.S.P. A review on potential therapeutic properties of Pomegranate (Punica granatum L.). Plant Sci. Today 2020, 7, 9. [Google Scholar] [CrossRef]
- Sohrab, G.; Angoorani, P.; Tohidi, M.; Tabibi, H.; Kimiagar, M.; Nasrollahzadeh, J. Pomegranate (Punica granatum) juice decreases lipid peroxidation, but has no effect on plasma advanced glycated end-products in adults with type 2 diabetes: A randomized doubleblind clinical trial. Food Nutr. Res. 2015, 59, 28551. [Google Scholar]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Hosny, N.M.; Al-Hussaini, A.S.; Nowesser, N.; Zoromba, M. Polyanthranilic acid: Effect of inclusion of some transition metal ions and use of the doped polymer in synthesizing a-Fe2O3 nanoparticles via thermal decomposition rout. J. Therm. Anal. Calorim. 2016, 124, 287–293. [Google Scholar] [CrossRef]
- Faddladdeen, K.A.; Ojaimi, A.A. Protective Effect of Pomegranate (Punica granatum) Extract against Diabetic Changes in Adult Male Rat Liver: Histological Study. J. Microsc. Ultrastruct. 2020, 7, 165–170. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Kumar, S.; Rajamani, P. Iron oxide nanoparticle-induced hematopoietic and immunological response in rats. RSC Adv. 2020, 10, 35753–35764. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: London, UK, 2008. [Google Scholar]
- Almaaty, A.A.; Basma; Hendam, M.; Althobaiti, F.; Fayad, E.; El-Aziz, Y.M.A. Evaluation of the hepatoprotective and antioxidant effects of Tegillarca granosa flesh body extract against potassium bromide toxicity via targeting the histomorphometry, chromosomal and expressions of TGF-β1, VEGF and COX-2 genes in rats. Biocell 2022, 46, 219–234. [Google Scholar] [CrossRef]
- Dawson, B.; Trapp, R. Basic and Clinical Biostatistics; McGraw-Hill: Monterey, CA, USA, 2004. [Google Scholar]
- Singh, P.; Sankhla, V. In situ protective effect of curcumin on cadmium chloride induced genotoxicity in bone marrow chromosomes of Swiss albino mice. J. Cell Mol. Biol. 2010, 8, 57–64. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Akit, H.; Collins, C.; Fahri, F.; Hung, A.; D’Souza, D.; Leury, B.; Dunshea, F. Dietary Lecithin Decreases Skeletal Muscle COL1A1 and COL3A1 Gene Expression in Finisher Gilts. Animals 2016, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, J.; Li, Y.; Li, J. Margatoxin mitigates CCl4-induced hepatic fibrosis in mice via macrophage polarization, cytokine secretion and STAT signaling. Int. J. Mol. Med. 2020, 45, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Al Doghaither, H.; Elmorsy, E.; Al-Ghafari, A.; Ghulam, J. Roles of oxidative stress, apoptosis, and inflammation in metal-induced dysfunction of beta pancreatic cells isolated from CD1 mice. Saudi J. Biol. Sci. 2021, 28, 651–663. [Google Scholar] [CrossRef]
- Ghatei, N.; Nabavi, A.S.; Toosi, M.H.B.; Azimian, H.; Homayoun, M.; Targhi, R.G.; Haghir, H. Evaluation of bax, bcl-2, p21 and p53 genes expression variations on cerebellum of BALB/c mice before and after birth under mobile phone radiation exposure. Iran. J. Basic Med. Sci. 2017, 20, 1037–1043. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Aitkulov, A.; Jelbuldina, M.; Issatayeva, A.; Beisenova, A.; Molardi, C.; Saccomandi, P.; Blanc, W.; Inglezakis, V.; Tosi, D. Distributed 2D temperature sensing during nanoparticles assisted laser ablation by means of high-scattering fiber sensors. Sci. Rep. 2020, 10, 12593. [Google Scholar] [CrossRef]
- Xie, J.; Huang, J.; Li, X.; Sun, S.; Chen, X. Iron Oxide Nanoparticle Platform for Biomedical Applications. Curr. Med. Chem. 2009, 16, 1278–1294. [Google Scholar] [CrossRef]
- Ward, J.; Guthrie, J.A.; Wilson, D.; Arnold, P.; Lodge, J.P.; Toogood, G.J.; Wyatt, J.I.; Robinson, P.J. Colorectal Hepatic Metastases: Detection with SPIO-enhanced Breath-hold MR Imaging—Comparison of Optimized Sequences. Radiology 2003, 228, 709–718. [Google Scholar] [CrossRef]
- Voinov, M.A.; Pagán, J.O.S.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-Mediated Production of Hydroxyl Radicals as a Mechanism of Iron Oxide Nanoparticle Biotoxicity. J. Am. Chem. Soc. 2011, 133, 35–41. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Kilic, G.; Costa, C.; Fernandez-Bertolez, N.; Pasaro, E.; Teixeira, J.P.; Laffon, B. Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2014, 56, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, D.J.; Yun, W.S.; Park, J.-E.; Choi, J.S.; Key, J.; Seo, Y.J. Endocytic trafficking of polymeric clustered superparamagnetic iron oxide nanoparticles in mesenchymal stem cells. J. Control. Release 2020, 326, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Deng, C.; Peng, Y.; Zheng, J.; Wei, L.; Shi, Y.; Gong, Z.; Hu, G. Dynamic Changes in MMP1 and TIMP1 in the Antifibrotic Process of Dahuang Zhechong Pill in Rats with Liver Fibrosis. Open Chem. 2019, 17, 346–356. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef]
- Kamel, H.R.; AL-Taee, A.A. Iron oxide nanoparticles induced histological alteration and fetal skeletal abnormalities in the embryo of Albino rats. Med.-Leg. Update 2020, 20, 911–915. [Google Scholar] [CrossRef]
- Philippe, M.A.; Ruddell, R.G.; Ramm, G.A. Role of iron in hepatic fibrosis: One piece in the puzzle. World J. Gastroenterol. 2007, 13, 4746–4754. [Google Scholar] [CrossRef]
- Prabhakar, P.V.; Reddy, U.A.; Singh, S.P.; Balasubramanyam, A.; Rahman, M.F.; Kumari, S.I.; Agawane, S.B.; Murty, U.S.N.; Grover, P.; Mahboob, M. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J. Appl. Toxicol. 2012, 32, 436–445. [Google Scholar] [CrossRef]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef]
- Ni, H.-M.; Bockus, A.; Boggess, N.; Jaeschke, H.; Ding, W.-X. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 2012, 55, 222–232. [Google Scholar] [CrossRef]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Huerta-Gertrudis, B.; García-Cruz, M.E.; Ramírez-Morales, A.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Hepatoprotective effect of acetonic and methanolic extracts of Heterotheca inuloides against CCl4-induced toxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kotsafti, A.; Farinati, F.; Cardin, R.; Burra, P.; Bortolami, M. Bax Inhibitor-1 down-regulation in the progression of chronic liver diseases. BMC Gastroenterol. 2010, 10, 35. [Google Scholar] [CrossRef]
- Wei, X.-L.; Fang, R.-T.; Yang, Y.-H.; Bi, X.-Y.; Ren, G.-X.; Luo, A.-L.; Zhao, M.; Zang, W.-J. Protective effects of extracts from Pomegranate peels and seeds on liver fibrosis induced by carbon tetrachloride in rats. BMC Complement. Altern. Med. 2015, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape Anisotropic Iron Oxide-Based Magnetic Nanoparticles: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef] [PubMed]
- Karnakar, R.Y.; Saritha, C.-H.; Sridhar, Y.; Shankaraiah, P. Naringenin prevents the zinc oxide nanoparticles induced toxicity in Swiss albino mice. J. Pharmacol. Clin. Toxicol. 2014, 2, 1021. [Google Scholar]
- Khorsandi, L.; Orazizadeh, M.; Mansori, E.; Fakhredini, F. Glycyrrhizic acid attenuated lipid peroxidation induced by titanium dioxide nanoparticles in rat liver. Bratisl. Lek. Listy 2015, 116, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.; Chauhan, L.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef]
- Azim, S.A.A.; Darwish, H.A.; Rizk, M.Z.; Ali, S.A.; Kadry, M.O. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: Possible role of some antioxidants. Exp. Toxicol. Pathol. 2015, 67, 305–314. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Sundararajan, R.; Ahamed, K.N.; Kumar, V.; Mukherjee, K.; Bandyopadhyay, A.; Mukherjee, P.K. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J. Ethnopharmacol. 2007, 109, 41–47. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. A case study—Regulation and functional mechanisms of cancer cells and control its activity using plants and their derivatives. J. Pharm. Res. 2013, 6, 884–892. [Google Scholar] [CrossRef][Green Version]
- Parveen, N.; Umar, A.; Gupta, S.; Singh, S.; Shadab, G.G.H.A. Amelioration of Iron Induced Clastogenicity and DNA Damage in Wistar Rats by Thymoquinone. Sci. Adv. Mater. 2014, 6, 933–945. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.; Alam, J.; Khan, M.A.M.; Ali, D.; Alarafi, S. Iron Oxide Nanoparticle-induced Oxidative Stress and Genotoxicity in Human Skin Epithelial and Lung Epithelial Cell Lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Parveen, N.; Ahmad, F.; Wani, A.L.; Afrin, S.; Rahman, Y.; Jameel, S.; Khan, Y.A.; Siddique, H.; Tabish, M.; et al. Evaluation of DNA interaction, genotoxicity and oxidative stress induced by iron oxide nanoparticles both in vitro and in vivo: Attenuation by thymoquinone. Sci. Rep. 2019, 9, 6912. [Google Scholar] [CrossRef]
- De Minicis, S.; Seki, E.; Uchinami, H.; Kluwe, J.; Zhang, Y.; Brenner, D.; Schwabe, R.F. Gene Expression Profiles During Hepatic Stellate Cell Activation in Culture and In Vivo. Gastroenterology 2007, 132, 1937–1946. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Pietsch, D.; Bantel, H.; Manns, M.P.; Brand, K.; Bahr, M.J. The Enhanced Liver Fibrosis (ELF) score: Normal values, influence factors and proposed cut-off values. J. Hepatol. 2013, 59, 236–242. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis—A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef]
- Wroblewski, R.; Armaka, M.; Kondylis, V.; Pasparakis, M.; Walczak, H.; Mittrücker, H.; Schramm, C.; Lohse, A.W.; Kollias, G.; Ehlken, H. Opposing role of tumor necrosis factor receptor 1 signaling in T cell–mediated hepatitis and bacterial infection in mice. Hepatology 2016, 64, 508–521. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell Death and Cell Death Responses in Liver Disease: Mechanisms and Clinical Relevance. Gastroenterology 2014, 147, 765–783. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Tari, S.B.; Koohpar, Z.K.; Falahati, M. The role of iron oxide nanoparticles and magnetic field on apoptosis and Bax gene expression in rat hippocampus after ischemic reperfusion. J. Babol. Univ. Med. Sci. 2018, 20, 42–48. [Google Scholar]
- Singh, D.; Cho, W.C.; Upadhyay, G. Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview. Front. Physiol. 2016, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, U.S.; Meena, R.; Rajamani, P. Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J. Appl. Toxicol. 2017, 37, 1232–1244. [Google Scholar] [CrossRef]
- Könczöl, M.; Ebeling, S.; Goldenberg, E.; Treude, F.; Gminski, R.; Gieré, R.; Grobéty, B.; Rothen-Rutishauser, B.; Merfort, I.; Mersch-Sundermann, V. Cytotoxicity and Genotoxicity of Size-Fractionated Iron Oxide (Magnetite) in A549 Human Lung Epithelial Cells: Role of ROS, JNK, and NF-κB. Chem. Res. Toxicol. 2011, 24, 1460–1475. [Google Scholar] [CrossRef]
- Babadi, Y.V.; Najafi, Y.; Najafi, A.; Gholami, H.; Zarji, B.E.M.; Jalal, G.J.; Amraie, E.; Shirband, A. Evaluation of iron oxide nanoparticles effects on tissue and enzymes of liver in rats. J. Pharm. Biomed. Sci. 2012, 23, 1–4. [Google Scholar]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef]
- Kazemipour, N.; Nazifi, S.; Poor, M.H.H.; Esmailnezhad, Z.; Najafabadi, R.E.; Esmaeili, A. Hepatotoxicity and nephrotoxicity of quercetin, iron oxide nanoparticles, and quercetin conjugated with nanoparticles in rats. Comp. Clin. Pathol. 2018, 27, 1621–1628. [Google Scholar] [CrossRef]
- Sonmez, E.; Aydin, E.; Turkez, H.; Özbek, E.; Togar, B.; Meral, K.; Çetin, D.; Cacciatore, I.; Di Stefano, A. Cytotoxicity and genotoxicity of iron oxide nanoparticles: An in vitro biosafety study. Arch. Biol. Sci. 2016, 68, 41–50. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Alhader, M.S. Iron Oxide Nanoparticles Induce Oxidative Stress, DNA Damage, and Caspase Activation in the Human Breast Cancer Cell Line. Biol. Trace Elem. Res. 2014, 159, 416–424. [Google Scholar] [CrossRef]
- El-Sayeda, A.A.; Husseinb, M.M.; Solimana, A.H. Naringenin and hesperidin ameliorate iron oxide nanoparticles toxicity in rat liver. Arab. J. Med. Sci. 2018, 1, 26–30. [Google Scholar] [CrossRef]
- Bagheri, S.; Khorramabadi, R.M.; Assadollahi, V.; Khosravi, P.; Venol, A.C.; Veiskerami, S.; Ahmadvand, H. The effects of pomegranate peel extract on the gene expressions of antioxidant enzymes in a rat model of alloxan-induced diabetes. Arch. Physiol. Biochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Aniagu, S.O.; Nwinyi, F.C.; Akumka, D.D.; Ajoku, G.A.; Dzarma, S.; Izebe, K.S.; Ditse, M.; Nwaneri, P.E.C.; Wambebe, C.; Gamaniel, K. Toxicity studies in rats fed nature cure bitters. Afr. J. Biotech. 2005, 4, 72–78. [Google Scholar]
- Bhandary, B.S.K.; Sharmila, K.P.; Kumari, N.S.; Bhat, V. Acute and subacute toxicity study of the ethanol extracts of punica granatum (linn) whole fruit and seeds and synthetic ellagic acid in Swiss albino mice. Asian J. Pharm. Clin. Res. 2013, 6, 192–198. [Google Scholar]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the toxicity of Punica granatum L. (Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef]
- Ahmed, E.; Arshad, M.; Khan, M.Z.; Amjad, M.S.; Sadaf, H.M.; Riaz, I.; Sabir, S.; Ahmad, N.; Sabaoo. Secondary metabolites and their multidimensional prospective in plant life. J. Pharmacogn. Phytochem. 2017, 6, 205–214. [Google Scholar]
- Faria, A.; Calhau, C. The Bioactivity of Pomegranate: Impact on Health and Disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef]
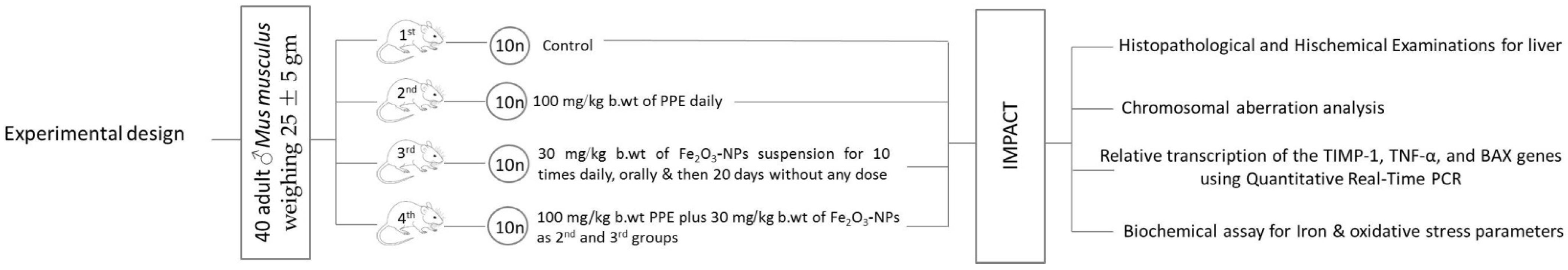

| Experimental Groups | Design/Dose |
|---|---|
| 1st Group | Ten mice received physiological saline solution only. |
| 2nd Group | Ten mice were administered with 100 mg/kg b. wt. of PPE daily by a stomach feeding tube for 4 weeks. |
| 3rd Group | Ten mice were intoxicated by 30 mg/kg b. wt. of Fe2O3-NPs suspension for 10 doses daily by a stomach feeding tube, followed by 20 days without receiving 30 mg/kg b. wt. of Fe2O3-NPs |
| 4th Group | Ten mice were given 100 mg/kg b. wt. PPE plus 30 mg/kg b. wt. of Fe2O3-NPs, as described for the second and third groups. |
| Target Gene | Primer Sequences | NCBI Gen Bank Accession No. | References |
|---|---|---|---|
| TIMP-1 | F: 5-CCCAGAAATCAACGAGA-3 R: 5-TGGGACTTGTGGGCATA-3 | NM_011593.2 | [35] |
| TNF-α | F: 5-GACAGTGACCTGGACTGTGG-3 R: 5-TGAGACAGAGGCAACCTGAC-3 | NM_001278601.1 | [36] |
| BAX | F: 5-CTACAGGGTTTCATCCAG-3 R: 5-CCAGTTCATCTCCAATTCG-3 | XM_011250780.4 | [37] |
| GAPDH | F: 5-GAGAAACCTGCCAAGTATG-3 R:5-GGAGTTGCTGTTGAAGTC-3 | XM_036165840.1 | [38] |
| Chromosomal Abnormalities | ||||||
|---|---|---|---|---|---|---|
| Chromatid Gap | Chromatid Break | Deletion | Ring Chromosome | Double Minute Chromosome | ||
| Experimental groups | Control | 4.60 | 5.50 | 3.60 | 7.10 | 6.50 |
| PPE | 6.40 | 5.50 | 7.40 | 3.90 | 4.50 | |
| Fe2O3-NPs | 13.00 | 16.00 | 18.00 | 18.00 | 18.00 | |
| PPE plus Fe2O3-NPs | 18.00 | 15.00 | 13.00 | 13.00 | 13.00 | |
| Chi-Square | 16.489 | 15.350 | 17.498 | 16.982 | 17.502 | |
| p-value | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Aziz, Y.M.; Hendam, B.M.; Al-Salmi, F.A.; Qahl, S.H.; Althubaiti, E.H.; Elsaid, F.G.; Shati, A.A.; Hosny, N.M.; Fayad, E.; Abu Almaaty, A.H. Ameliorative Effect of Pomegranate Peel Extract (PPE) on Hepatotoxicity Prompted by Iron Oxide Nanoparticles (Fe2O3-NPs) in Mice. Nanomaterials 2022, 12, 3074. https://doi.org/10.3390/nano12173074
Abd El-Aziz YM, Hendam BM, Al-Salmi FA, Qahl SH, Althubaiti EH, Elsaid FG, Shati AA, Hosny NM, Fayad E, Abu Almaaty AH. Ameliorative Effect of Pomegranate Peel Extract (PPE) on Hepatotoxicity Prompted by Iron Oxide Nanoparticles (Fe2O3-NPs) in Mice. Nanomaterials. 2022; 12(17):3074. https://doi.org/10.3390/nano12173074
Chicago/Turabian StyleAbd El-Aziz, Yasmin M., Basma M. Hendam, Fawziah A. Al-Salmi, Safa H. Qahl, Eman H. Althubaiti, Fahmy G. Elsaid, Ali A. Shati, Nasser M. Hosny, Eman Fayad, and Ali H. Abu Almaaty. 2022. "Ameliorative Effect of Pomegranate Peel Extract (PPE) on Hepatotoxicity Prompted by Iron Oxide Nanoparticles (Fe2O3-NPs) in Mice" Nanomaterials 12, no. 17: 3074. https://doi.org/10.3390/nano12173074
APA StyleAbd El-Aziz, Y. M., Hendam, B. M., Al-Salmi, F. A., Qahl, S. H., Althubaiti, E. H., Elsaid, F. G., Shati, A. A., Hosny, N. M., Fayad, E., & Abu Almaaty, A. H. (2022). Ameliorative Effect of Pomegranate Peel Extract (PPE) on Hepatotoxicity Prompted by Iron Oxide Nanoparticles (Fe2O3-NPs) in Mice. Nanomaterials, 12(17), 3074. https://doi.org/10.3390/nano12173074







