Quartz Crystal Microbalance-Based Aptasensors for Medical Diagnosis
Abstract
:1. Introduction
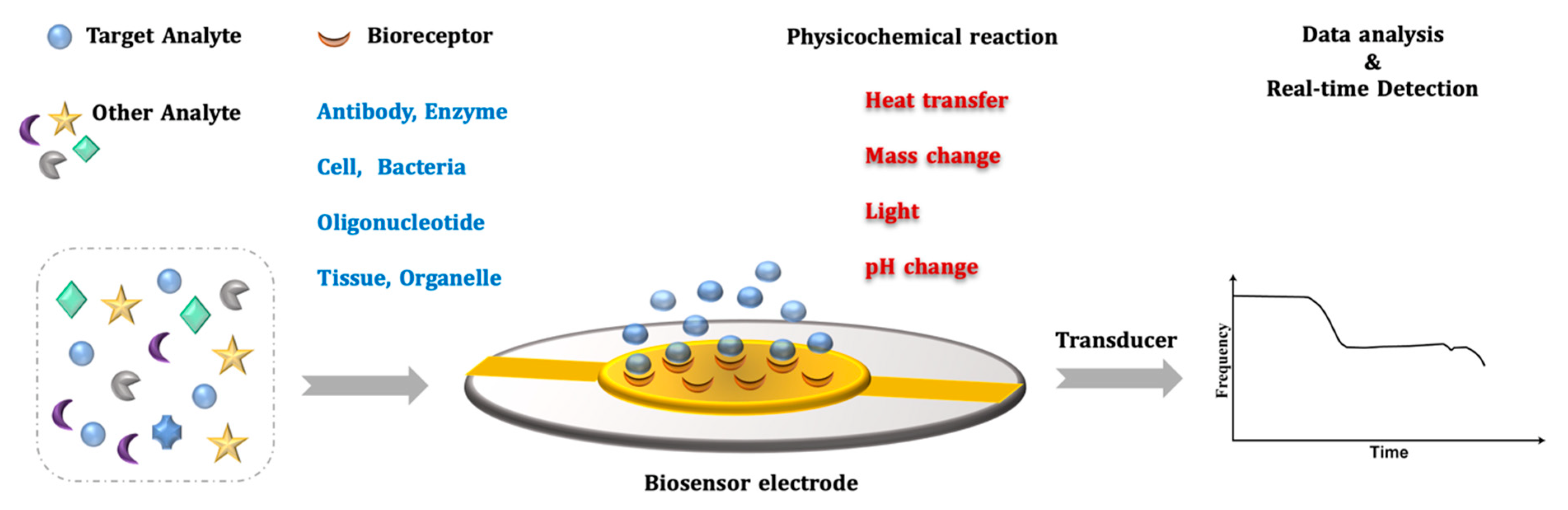
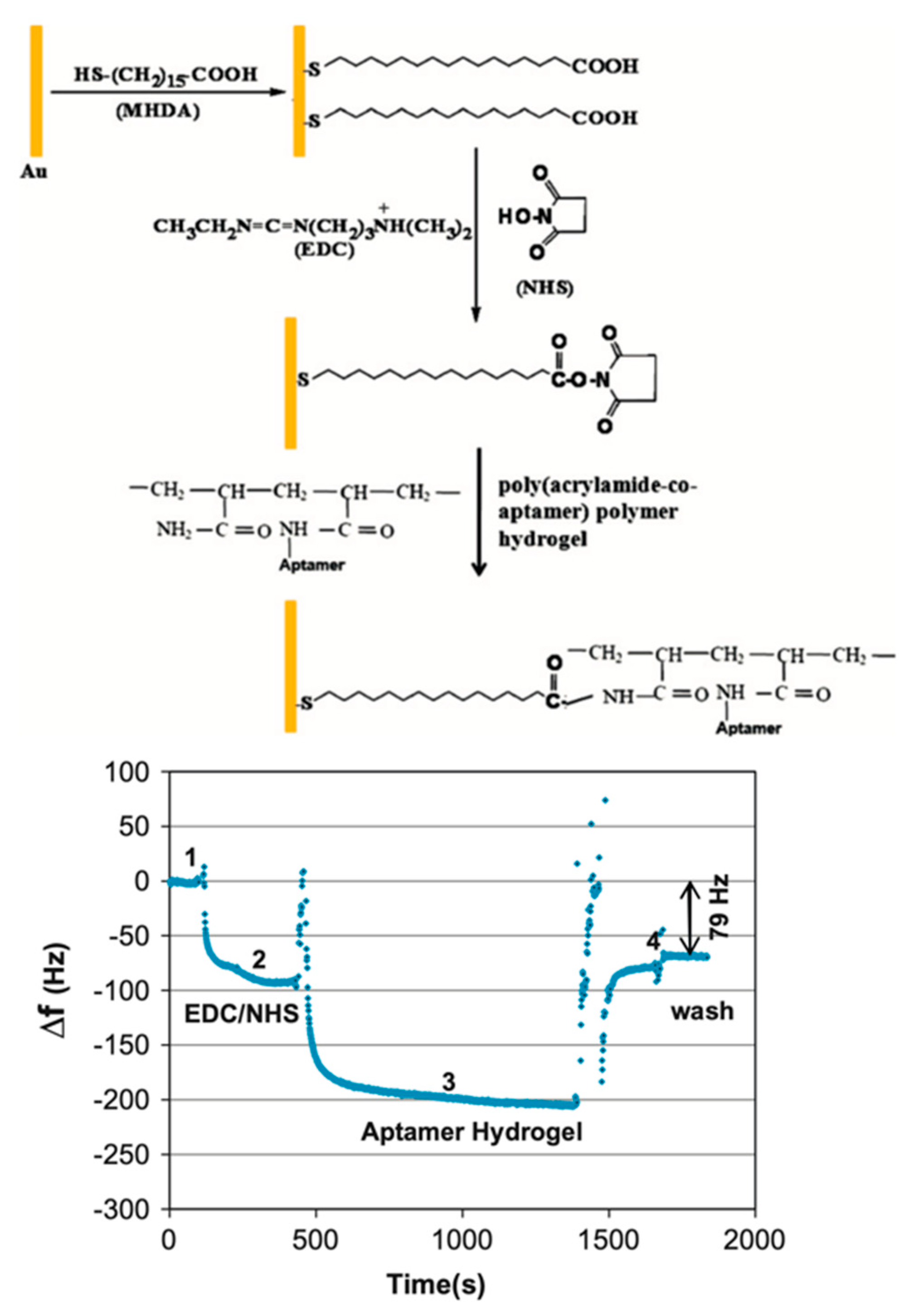
2. QCM Biosensor

3. Application of QCM Aptasensors for Medical Diagnostics
3.1. Viruses
3.2. Bacteria
3.3. Proteins
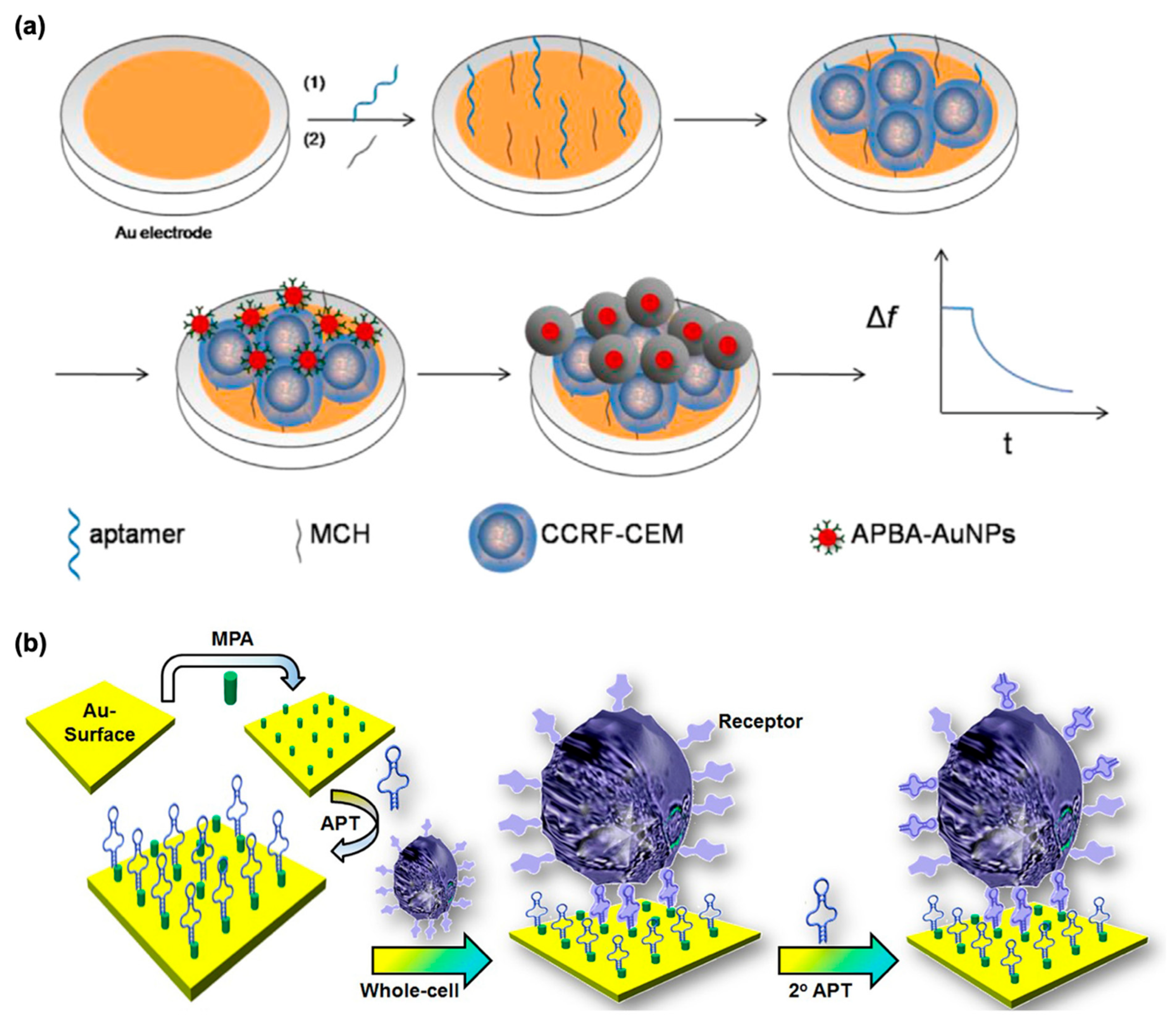
3.4. Cells
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Khedri, M.; Ramezani, M.; Rafatpanah, H.; Abnous, K. Detection of food-born allergens with aptamer-based biosensors. TrAC-Trends Anal. Chem. 2018, 103, 126–136. [Google Scholar] [CrossRef]
- Sharma, A.; Dulta, K.; Nagraik, R.; Dua, K.; Singh, S.K.; Chellappan, D.K.; Kumar, D.; Shin, D.S. Potentialities of aptasensors in cancer diagnosis. Mater. Lett. 2022, 308, 131240. [Google Scholar] [CrossRef]
- Kudłak, B.; Wieczerzak, M. Aptamer based tools for environmental and therapeutic monitoring: A review of developments, applications, future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 816–867. [Google Scholar] [CrossRef]
- Khan, N.I.; Song, E. Lab-on-a-chip systems for aptamer-based biosensing. Micromachines 2020, 11, 220. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Huang, Q.; Tong, T.; Zhou, Y.; Li, Z.; Bai, Q.; Liang, H.; Chen, L. Recent advances on aptamer-based biosensors for detection of pathogenic bacteria. World J. Microbiol. Biotechnol. 2021, 37, 45. [Google Scholar] [CrossRef]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, Y.; Yong, W.; Chu, X.; Wang, D. Aptamer and Its Potential Applications for Food Safety. Crit. Rev. Food Sci. Nutr. 2014, 54, 1548–1561. [Google Scholar] [CrossRef]
- Burbulis, I.; Yamaguchi, K.; Yu, R.; Resnekov, O.; Brent, R. Quantifying small numbers of antibodies with a “near-universal” protein-DNA chimera. Nat. Methods 2007, 4, 1011–1013. [Google Scholar] [CrossRef]
- Li, Y.K.; Li, W.T.; Liu, X.; Yang, T.; Chen, M.L.; Wang, J.H. Functionalized magnetic composites based on the aptamer serve as novel bio-adsorbent for the separation and preconcentration of trace lead. Talanta 2019, 203, 210–219. [Google Scholar] [CrossRef]
- Ji, D.; Wang, H.; Ge, J.; Zhang, L.; Li, J.; Bai, D.; Chen, J.; Li, Z. Label-free and rapid detection of ATP based on structure switching of aptamers. Anal. Biochem. 2017, 526, 22–28. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q. Competitive horseradish peroxidase-linked aptamer assay for sensitive detection of Aflatoxin B1. Talanta 2018, 179, 344–349. [Google Scholar] [CrossRef]
- Hermann, T.; Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef]
- Wu, J.; He, T.; Guo, P.; Cai, F.; Zhao, C. An electrochemical sense array based on aptamer and biotin-avidin system for the selective detection of glucagon-like peptide-1. Clin. Lab. 2019, 65, 10–11. [Google Scholar] [CrossRef]
- Idili, A.; Gerson, J.; Parolo, C.; Kippin, T.; Plaxco, K.W. An electrochemical aptamer-based sensor for the rapid and convenient measurement of l-tryptophan. Anal. Bioanal. Chem. 2019, 411, 4629–4635. [Google Scholar] [CrossRef]
- Senturk, H.; Eksin, E.; Işık, Ö.; İlaslan, Z.; Mısırlı, F.; Erdem, A. Impedimetric aptasensor for lysozyme detection based on carbon nanofibres enriched screen-printed electrodes. Electrochim. Acta 2021, 377, 138078. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, N.; Wu, C.; Liang, Y.; Jiang, B.; Yang, K.; Liang, Z.; Zhang, L.; Zhang, Y. Aptamer functionalized hydrophilic polymer monolith with gold nanoparticles modification for the sensitive detection of human α-thrombin. Talanta 2016, 154, 555–559. [Google Scholar] [CrossRef]
- Liu, X.; Deng, K.; Wang, H.; Li, C.; Zhang, S.; Huang, H. Aptamer based ratiometric electrochemical sensing of 17β-estradiol using an electrode modified with gold nanoparticles, thionine, and multiwalled carbon nanotubes. Microchim. Acta 2019, 186, 347. [Google Scholar] [CrossRef]
- Janik, M.; Brzozowska, E.; Czyszczoń, P.; Celebańska, A.; Koba, M.; Gamian, A.; Bock, W.J.; Śmietana, M. Optical fiber aptasensor for label-free bacteria detection in small volumes. Sens. Actuators B Chem. 2021, 330, 129316. [Google Scholar] [CrossRef]
- Sun, D.; Lu, J.; Zhang, L.; Chen, Z. Aptamer-based electrochemical cytosensors for tumor cell detection in cancer diagnosis: A review. Anal. Chim. Acta 2019, 1082, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Poturnayová, A.; Buríková, M.; Bízik, J.; Hianik, T. DNA Aptamers in the Detection of Leukemia Cells by the Thickness Shear Mode Acoustics Method. ChemPhysChem 2019, 20, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Bagheryan, Z.; Raoof, J.B.; Golabi, M.; Turner, A.P.F.; Beni, V. Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample. Biosens. Bioelectron. 2016, 80, 566–573. [Google Scholar] [CrossRef]
- Koyun, S.; Akgönüllü, S.; Yavuz, H.; Erdem, A.; Denizli, A. Surface plasmon resonance aptasensor for detection of human activated protein C. Talanta 2019, 194, 528–533. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Koyun, S.; Yavuz, H.; Erdem, A.; Denizli, A. Preparation of Surface Plasmon Resonance Aptasensor for Human Activated Protein C Sensing. In Methods in Molecular Biology; Humana: New York, NY, USA, 2022; pp. 37–56. ISBN 9781071618035. [Google Scholar]
- Kim, S.M.; Kim, J.; Noh, S.; Sohn, H.; Lee, T. Recent Development of Aptasensor for Influenza Virus Detection. Biochip J. 2020, 14, 327–339. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Ozalp, V.C.; Oztekin, M.; Arica, M.Y. Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor. Talanta 2019, 200, 263–271. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, X.; Shao, X.; Jiang, C.; Ning, L. Recent advances in optical aptasensor technology for amplification strategies in cancer diagnostics. Anal. Bioanal. Chem. 2020, 412, 6691–6705. [Google Scholar] [CrossRef]
- Ali, M.H.; Elsherbiny, M.E.; Emara, M. Updates on aptamer research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef]
- Liu, L.S.; Wang, F.; Ge, Y.; Lo, P.K. Recent Developments in Aptasensors for Diagnostic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9329–9358. [Google Scholar] [CrossRef]
- Keiser, G.; Xiong, F.; Cui, Y.; Shum, P.P. Review of diverse optical fibers used in biomedical research and clinical practice. J. Biomed. Opt. 2014, 19, 080902. [Google Scholar] [CrossRef]
- Heller, A. Amperometric biosensors. Curr. Opin. Biotechnol. 1996, 7, 50–54. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and applications of thermal biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Koncki, R. Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta 2007, 599, 7–15. [Google Scholar] [CrossRef]
- Wang, Y.; Irudayaraj, J. A SERS DNAzyme biosensor for lead ion detection. Chem. Commun. 2011, 47, 4394–4396. [Google Scholar] [CrossRef]
- Panda, A.; Pukhrambam, P.D.; Keiser, G. Performance analysis of graphene-based surface plasmon resonance biosensor for blood glucose and gas detection. Appl. Phys. A Mater. Sci. Process. 2020, 126, 153. [Google Scholar] [CrossRef]
- Mao, K.; Yang, Z.; Li, J.; Zhou, X.; Li, X.; Hu, J. A novel colorimetric biosensor based on non-aggregated Au@Ag core–shell nanoparticles for methamphetamine and cocaine detection. Talanta 2017, 175, 338–346. [Google Scholar] [CrossRef]
- Jafari, S.; Faridbod, F.; Norouzi, P.; Dezfuli, A.S.; Ajloo, D.; Mohammadipanah, F.; Ganjali, M.R. Detection of Aeromonas hydrophila DNA oligonucleotide sequence using a biosensor design based on Ceria nanoparticles decorated reduced graphene oxide and Fast Fourier transform square wave voltammetry. Anal. Chim. Acta 2015, 895, 80–88. [Google Scholar] [CrossRef]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. Development of Gold Nanoparticles Decorated Molecularly Imprinted–Based Plasmonic Sensor for the Detection of Aflatoxin M1 in Milk Samples. Chemosensors 2021, 9, 363. [Google Scholar] [CrossRef]
- Liu, N.; Xiang, X.; Sun, M.; Li, P.; Qin, H.; Liu, H.; Zhou, Y.; Wang, L.; Wu, L.; Zhu, J. Flexible hydrogel non-enzymatic QCM sensor for continuous glucose monitoring. Biosens. Bioelectron. X 2022, 10, 100110. [Google Scholar] [CrossRef]
- Culebras, M.; López, A.M.; Gómez, C.M.; Cantarero, A. Thermal sensor based on a polymer nanofilm. Sens. Actuators A Phys. 2016, 239, 161–165. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, C.; Chau, Y.; Lee, Y.K. The synergy of chemical immobilization and electrical orientation of T4 bacteriophage on a micro electrochemical sensor for low-level viable bacteria detection via Differential Pulse Voltammetry. Biosens. Bioelectron. 2020, 151, 111914. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ren, P.; Huang, L.; Ouyang, Z.; Wang, Z.; Kong, X.; Li, T.; Yin, Y.; Wu, Y.; He, Q. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface plasmon resonance for cell-based clinical diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Corrie, S.R.; Clark, H.A. In Vivo Biosensing: Progress and Perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Yu, K.; Lu, G.; Chen, J. Highly sensitive protein sensor based on thermally-reduced graphene oxide field-effect transistor. Nano Res. 2011, 4, 921–930. [Google Scholar] [CrossRef]
- Ghosh, S.; Khan, N.I.; Tsavalas, J.G.; Song, E. Selective detection of lysozyme biomarker utilizing large area chemical vapor deposition-grown graphene-based field-effect transistor. Front. Bioeng. Biotechnol. 2018, 6, 29. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-Free Biosensors Based on Aptamer-Modified Graphene Field-Effect. JACS Commun. 2010, 132, 18012–18013. [Google Scholar] [CrossRef]
- Sönmezler, M.; Özgür, E.; Yavuz, H.; Denizli, A. Quartz crystal microbalance based histidine sensor. Artif. Cells Nanomed. Biotechnol. 2019, 47, 221–227. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, T.; Siang, W.; Wei, C. Quartz crystal microbalance-based biosensors as rapid diagnostic devices for infectious diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Lim, Y.C.; Kouzani, A.Z.; Duan, W. Aptasensors: A review. J. Biomed. Nanotechnol. 2010, 6, 93–105. [Google Scholar] [CrossRef]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef]
- Yan, S.R.; Foroughi, M.M.; Safaei, M.; Jahani, S.; Ebrahimpour, N.; Borhani, F.; Rezaei Zade Baravati, N.; Aramesh-Boroujeni, Z.; Foong, L.K. A review: Recent advances in ultrasensitive and highly specific recognition aptasensors with various detection strategies. Int. J. Biol. Macromol. 2020, 155, 184–207. [Google Scholar] [CrossRef]
- Wadhera, T.; Kakkar, D.; Wadhwa, G.; Raj, B. Recent Advances and Progress in Development of the Field Effect Transistor Biosensor: A Review. J. Electron. Mater. 2019, 48, 7635–7646. [Google Scholar] [CrossRef]
- Saftics, A.; Kurunczi, S.; Peter, B.; Szekacs, I.; Ramsden, J.J.; Horvath, R. Data evaluation for surface-sensitive label-free methods to obtain real-time kinetic and structural information of thin films: A practical review with related software packages. Adv. Colloid Interface Sci. 2021, 294, 102431. [Google Scholar] [CrossRef]
- Saftics, A.; Prósz, G.A.; Türk, B.; Peter, B.; Kurunczi, S.; Horvath, R. In situ viscoelastic properties and chain conformations of heavily hydrated carboxymethyl dextran layers: A comparative study using OWLS and QCM-I chips coated with waveguide material. Sci. Rep. 2018, 8, 11840. [Google Scholar] [CrossRef]
- Orgovan, N.; Kovacs, B.; Farkas, E.; Szabó, B.; Zaytseva, N.; Fang, Y.; Horvath, R. Bulk and surface sensitivity of a resonant waveguide grating imager. Appl. Phys. Lett. 2014, 104, 083506. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhang, L.; Liu, B.; Bai, P.; Wang, C.; Xu, J.; Wang, H. Highly Selective Gas Sensor Based on Hydrophobic Silica Decorated with Trimethoxyoctadecylsilane. ACS Appl. Mater. Interfaces 2021, 13, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ellis, J.E.; Howard, B.H.; Ohodnicki, P.R. Centimeter-Scale Pillared-Layer Metal−Organic Framework Thin Films Mediated by Hydroxy Double Salt Intermediates for CO2 Sensor Applications. ACS Appl. Mater. Interfaces 2021, 13, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Akgönüllü, S.; Özgür, E.; Denizli, A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors 2022, 10, 106. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Sadowska, M.; Paulina, Z. Applicability of QCM-D for Quantitative Measurements of Nano- and Microparticle Deposition Kinetics: Theoretical Modeling and Experiments. Anal. Chem. 2020, 92, 15087–15095. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Sadowska, M. Hydrodynamic Solvent Coupling E ff ects in Quartz Crystal Microbalance Measurements of Nanoparticle Deposition Kinetics. Anal. Chem. 2020, 92, 3896–3903. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, L.; Liu, Y.; Harms, H.; Wick, L.Y. Effects of Electrokinetic Phenomena on Bacterial Deposition Monitored by Quartz Crystal Microbalance with Dissipation Monitoring. Environ. Sci. Technol. 2020, 54, 14036–14045. [Google Scholar] [CrossRef]
- Sunar, M. Piezoelectric Materials. Compr. Energy Syst. 2018, 2–5, 696–719. [Google Scholar] [CrossRef]
- Son, J.; Ji, S.; Kim, S.; Kim, S.; Kim, S.K.; Song, W.; Lee, S.S.; Lim, J.; An, K.; Myung, S. GC-like Graphene-Coated Quartz Crystal Microbalance Sensor with Microcolumns. ACS Appl. Mater. Interfaces 2021, 13, 4703–4710. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, K.; Luo, Z.; Xue, Z.; Gao, H.; Cao, Z.; Cheng, J.; Liu, C.; Zhang, L. Construction of Self-Assembled Polyelectrolyte/Cationic Microgel Multilayers and Their Interaction with Anionic Dyes Using Quartz Crystal. ACS Omega 2021, 6, 5764–5774. [Google Scholar] [CrossRef]
- Chinwangso, P.; Lee, H.J.; Lee, T.R. Self-Assembled Monolayers Generated from Unsymmetrical Partially Fluorinated Spiroalkanedithiols. Langmuir 2015, 31, 13341–13349. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. Nanoporous MIL-101(Cr) as a sensing layer coated on a quartz crystal microbalance (QCM) nanosensor to detect volatile organic compounds (VOCs). RSC Adv. 2019, 9, 24460–24470. [Google Scholar] [CrossRef]
- Latif, U.; Can, S.; Hayden, O.; Grillberger, P.; Dickert, F.L. Sauerbrey and anti-Sauerbrey behavioral studies in QCM sensors—Detection of bioanalytes. Sens. Actuators B Chem. 2013, 176, 825–830. [Google Scholar] [CrossRef]
- Lee, D.; Yoo, M.; Seo, H.; Tak, Y.; Kim, W.G.; Yong, K.; Rhee, S.W.; Jeon, S. Enhanced mass sensitivity of ZnO nanorod-grown quartz crystal microbalances. Sens. Actuators B Chem. 2009, 135, 444–448. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Quartz-Crystal Microbalance (QCM) for Public Health: An Overview of Its Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Lucklum, R.; Behling, C.; Hauptmann, P. Role of mass accumulation and viscoelastic film properties for the response of acoustic-wave-based chemical sensors. Anal. Chem. 1999, 71, 2488–2496. [Google Scholar] [CrossRef]
- Migoń, D.; Wasilewski, T.; Suchy, D. Application of QCM in Peptide and Protein-Based Drug Product Development. Molecules 2020, 25, 3950. [Google Scholar] [CrossRef]
- Arnau, A. A review of interface electronic systems for AT-cut quartz crystal microbalance applications in liquids. Sensors 2008, 8, 370–411. [Google Scholar] [CrossRef]
- Lucklum, R.; Eichelbaum, F. Interface Circuits for QCM Sensors. In Springer Series on Chemical Sensors and Biosensors Methods and Applications; Wolfbeis, O.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–47. [Google Scholar]
- Trajcheva, A.; Politakos, N.; Pérez, B.T.; Joseph, Y.; Blazevska Gilev, J.; Tomovska, R. QCM nanocomposite gas sensors–Expanding the application of waterborne polymer composites based on graphene nanoribbon. Polymer 2021, 213, 123335. [Google Scholar] [CrossRef]
- Fauzi, F.; Rianjanu, A.; Santoso, I.; Triyana, K. Gas and humidity sensing with quartz crystal microbalance (QCM) coated with graphene-based materials–A mini review. Sens. Actuators A Phys. 2021, 330, 112837. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Lvova, L.B. A perspective on recent advances in piezoelectric chemical sensors for environmental monitoring and foodstuffs analysis. Chemosensors 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Livingston, J.L.; Spear, N.J.; Jennings, G.K. PH-Responsive Copolymer Films Prepared by Surface-Initiated Polymerization and Simple Modification. Langmuir 2020, 36, 715–722. [Google Scholar] [CrossRef]
- Rydzek, G.; Polavarapu, P.; Rios, C.; Tisserant, J.N.; Voegel, J.C.; Senger, B.; Lavalle, P.; Frisch, B.; Schaaf, P.; Boulmedais, F.; et al. Morphogen-driven self-construction of covalent films built from polyelectrolytes and homobifunctional spacers: Buildup and pH response. Soft Matter 2012, 8, 10336–10343. [Google Scholar] [CrossRef]
- Cuddy, M.F.; Poda, A.R.; Brantley, L.N. Determination of isoelectric points and the role of pH for common quartz crystal microbalance sensors. ACS Appl. Mater. Interfaces 2013, 5, 3514–3518. [Google Scholar] [CrossRef]
- Burda, I. Advanced Impedance Spectroscopy for QCM Sensor in Liquid Medium. Sensors 2022, 22, 2337. [Google Scholar] [CrossRef]
- Ding, X.; Chen, X.; Chen, X.; Zhao, X.; Li, N. A QCM humidity sensor based on fullerene/graphene oxide nanocomposites with high quality factor. Sens. Actuators B Chem. 2018, 266, 534–542. [Google Scholar] [CrossRef]
- Easley, A.D.; Ma, T.; Eneh, C.I.; Yun, J.; Thakur, R.M.; Lutkenhaus, J.L. A practical guide to quartz crystal microbalance with dissipation monitoring of thin polymer films. J. Polym. Sci. 2022, 60, 1090–1107. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B. Piezoelectric Sensors; Steinem, C., Janshoff, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Uddenberg, H. (Ed.) Q-Sense. In Q-Sense E4 Operator Manual; Q-Sense AB: Gothensburg, Sweden, 2009. [Google Scholar]
- Rudolph, G.; Hermansson, A.; Jönsson, A.S.; Lipnizki, F. In situ real-time investigations on adsorptive membrane fouling by thermomechanical pulping process water with quartz crystal microbalance with dissipation monitoring (QCM-D). Sep. Purif. Technol. 2021, 254, 117578. [Google Scholar] [CrossRef]
- Schmode, P.; Savva, A.; Kahl, R.; Ohayon, D.; Meichsner, F.; Dolynchuk, O.; Thurn-Albrecht, T.; Inal, S.; Thelakkat, M. The Key Role of Side Chain Linkage in Structure Formation and Mixed Conduction of Ethylene Glycol Substituted Polythiophenes. ACS Appl. Mater. Interfaces 2020, 12, 13029–13039. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hedayati, P.; Ghatkesar, M.K.; Sun, W.; Onay, H.; Groenendijk, D.; van Wunnik, J.; Sudhölter, E.J.R. Reducing anionic surfactant adsorption using polyacrylate as sacrificial agent investigated by QCM-D. J. Colloid Interface Sci. 2021, 585, 1–11. [Google Scholar] [CrossRef]
- You, F.; Shi, Q.-H. In situ investigation of lysozyme adsorption into polyelectrolyte brushes by quartz crystal microbalance with dissipation. Chin. J. Chem. Eng. 2021, 48, 106–115. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, C.; Zhang, Y.; Kong, H.; An, R.; Li, S.; Liu, W.; Ji, Q. Chiral Recognition on Bare Gold Surfaces by Quartz Crystal Microbalance. Angew. Chem.-Int. Ed. 2021, 60, 25028–25033. [Google Scholar] [CrossRef]
- Meléndrez, D.; Hampitak, P.; Jowitt, T.; Iliut, M.; Vijayaraghavan, A. Development of an open-source thermally stabilized quartz crystal microbalance instrument for biomolecule-substrate binding assays on gold and graphene. Anal. Chim. Acta 2021, 1156, 338329. [Google Scholar] [CrossRef]
- Lee, M.H.; Thomas, J.L.; Tseng, H.Y.; Lin, W.C.; Liu, B.D.; Lin, H.Y. Sensing of digestive proteins in saliva with a molecularly imprinted poly(ethylene-co-vinyl alcohol) thin film coated quartz crystal microbalance sensor. ACS Appl. Mater. Interfaces 2011, 3, 3064–3071. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Wang, Y.; Wang, S.; Wang, J. Hollow molecularly imprinted polymer based quartz crystal microbalance sensor for rapid detection of methimazole in food samples. Food Chem. 2020, 309, 125787. [Google Scholar] [CrossRef]
- Li, B.; Hu, X.; Zhang, Q.; Peng, X.; Xiang, Y. Improved piezoelectricity of polylactide using vitamin B2 for poling-free mechanical and acoustic nanogenerators. J. Mater. Sci. 2021, 56, 902–912. [Google Scholar] [CrossRef]
- Li, D.-M.; Zhang, S.-Y.; Wan, J.-Y.; Zhang, W.-Q.; Yan, Y.-L.; Tang, X.-H.; Zheng, S.-R.; Cai, S.; Zhang, W.-G. A new hydrazone-linked covalent organic framework for Fe(Ⅲ) detection by fluorescence and QCM technologies. CrystEngComm 2021, 23, 3594–3601. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Juan-Borrás, M.; March, C.; Arnau, A.; Escriche, I.; Montoya, Á.; Jiménez, Y. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCM) immunosensor for pesticide detection in honey. Food Control 2018, 92, 1–6. [Google Scholar] [CrossRef]
- Su, L.; Zou, L.; Fong, C.C.; Wong, W.L.; Wei, F.; Wong, K.Y.; Wu, R.S.S.; Yang, M. Detection of cancer biomarkers by piezoelectric biosensor using PZT ceramic resonator as the transducer. Biosens. Bioelectron. 2013, 46, 155–161. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Jiménez, Y.; Montoya, Á.; Juan-Borrás, M.; Pascual, N.; Arnau, A.; Escriche, I. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCMD) Immunosensor for detection of sulfathiazole in honey. Food Control 2020, 115, 107296. [Google Scholar] [CrossRef]
- Buchatip, S.; Ananthanawat, C.; Sithigorngul, P.; Sangvanich, P.; Rengpipat, S.; Hoven, V.P. Detection of the shrimp pathogenic bacteria, Vibrio harveyi, by a quartz crystal microbalance-specific antibody based sensor. Sens. Actuators B Chem. 2010, 145, 259–264. [Google Scholar] [CrossRef]
- Biemmi, E.; Darga, A.; Stock, N.; Bein, T. Direct growth of Cu3(BTC)2(H2O)3 · xH2O thin films on modified QCM-gold electrodes-Water sorption isotherms. Microporous Mesoporous Mater. 2008, 114, 380–386. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Chen, F.; Jiang, T.; Wang, H.; Slavik, M.; Wei, H.; Li, Y. QCM-based aptamer selection and detection of Salmonella typhimurium. Food Chem. 2017, 221, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Akgönüllü, S.; Denizli, A. Piezoelectric biosensors for virus detection. In Biosensors for Virus Detection; IOP Publishing: Bristol, UK, 2021; pp. 4–18. [Google Scholar] [CrossRef]
- Fulgione, A.; Cimafonte, M.; Della Ventura, B.; Iannaccone, M.; Ambrosino, C.; Capuano, F.; Proroga, Y.T.R.; Velotta, R.; Capparelli, R. QCM-based immunosensor for rapid detection of Salmonella Typhimurium in food. Sci. Rep. 2018, 8, 16137. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Kevser, A.; Yavuz, H.; Denizli, A. Quartz crystal microbalance biosensor for label-free MDA MB 231 cancer cell detection via notch-4 receptor. Talanta 2019, 204, 840–845. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, S.; Liu, D.; Wen, S.; Lin, Q. Antiproliferative drug-loaded multi-functionalized intraocular lens for reducing posterior capsular opacification. J. Biomater. Sci. Polym. Ed. 2021, 32, 735–748. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, L. Uniform Mass Sensitivity Distribution of Elliptically Designed Electrodes Based on a Quartz Crystal Microbalance. ACS Omega 2021, 6, 32917–32924. [Google Scholar] [CrossRef]
- Xu, L.; Wang, R.; Kelso, L.C.; Ying, Y.; Li, Y. A target-responsive and size-dependent hydrogel aptasensor embedded with QD fluorescent reporters for rapid detection of avian influenza virus H5N1. Sens. Actuators B Chem. 2016, 234, 98–108. [Google Scholar] [CrossRef]
- Lum, J.; Wang, R.; Hargis, B.; Tung, S.; Bottje, W.; Lu, H.; Li, Y. An impedance aptasensor with microfluidic chips for specific detection of H5N1 avian influenza virus. Sensors 2015, 15, 18565–18578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Li, Y. Hydrogel based QCM aptasensor for detection of avian influenza virus. Biosens. Bioelectron. 2013, 42, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, L.; Callaway, Z.T.; Lu, H.; Huang, T.J.; Li, Y. A nanowell-based QCM aptasensor for rapid and sensitive detection of avian influenza virus. Sens. Actuators B Chem. 2017, 240, 934–940. [Google Scholar] [CrossRef]
- Brockman, L.; Wang, R.; Lum, J.; Li, Y. QCM Aptasensor for Rapid and Specific Detection of Avian Influenza Virus. Open J. Appl. Biosens. 2013, 02, 97–103. [Google Scholar] [CrossRef]
- Giamblanco, N.; Conoci, S.; Russo, D.; Marletta, G. Single-step label-free hepatitis B virus detection by a piezoelectric biosensor. RSC Adv. 2015, 5, 38152–38158. [Google Scholar] [CrossRef]
- Skládal, P.; Dos Santos Riccardi, C.; Yamanaka, H.; Da Costa, P.I. Piezoelectric biosensors for real-time monitoring of hybridization and detection of hepatitis C virus. J. Virol. Methods 2004, 117, 145–151. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Z.; Yang, D.; Xie, S.; Jiang, Z.; Niessner, R.; Haisch, C.; Zhou, H.; Sun, P. Bacteria Detection: From Powerful SERS to Its Advanced Compatible Techniques. Adv. Sci. 2020, 7, 2001739. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- CDC. Multistate outbreak of human Salmonella typhimurium infections linked to live poultry in backyard flocks; CDC: Atlanta, GA, USA, 2013.
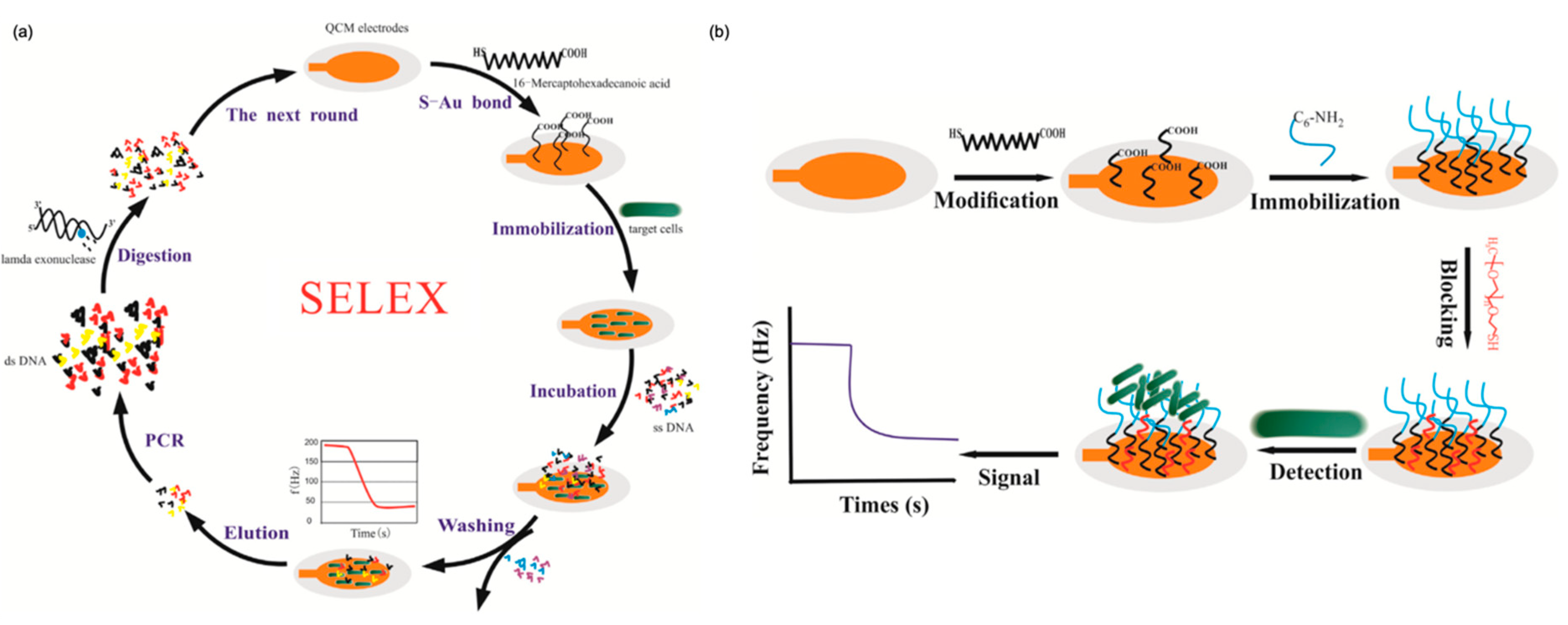
- Yu, X.; Chen, F.; Wang, R.; Li, Y. Whole-bacterium SELEX of DNA aptamers for rapid detection of E.coli O157:H7 using a QCM sensor. J. Biotechnol. 2018, 266, 39–49. [Google Scholar] [CrossRef]
- Wen, J.T.; Ho, C.M.; Lillehoj, P.B. Coffee ring aptasensor for rapid protein detection. Langmuir 2013, 29, 8440–8446. [Google Scholar] [CrossRef]
- Jaberi, N.; Soleimani, A.; Pashirzad, M.; Abdeahad, H.; Mohammadi, F.; Khoshakhlagh, M.; Khazaei, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Role of thrombin in the pathogenesis of atherosclerosis. J. Cell. Biochem. 2019, 120, 4757–4765. [Google Scholar] [CrossRef]
- ten Cate, H.; Hemker, H.C. Thrombin Generation and Atherothrombosis: What Does the Evidence Indicate? J. Am. Heart Assoc. 2016, 5, e003553. [Google Scholar] [CrossRef]
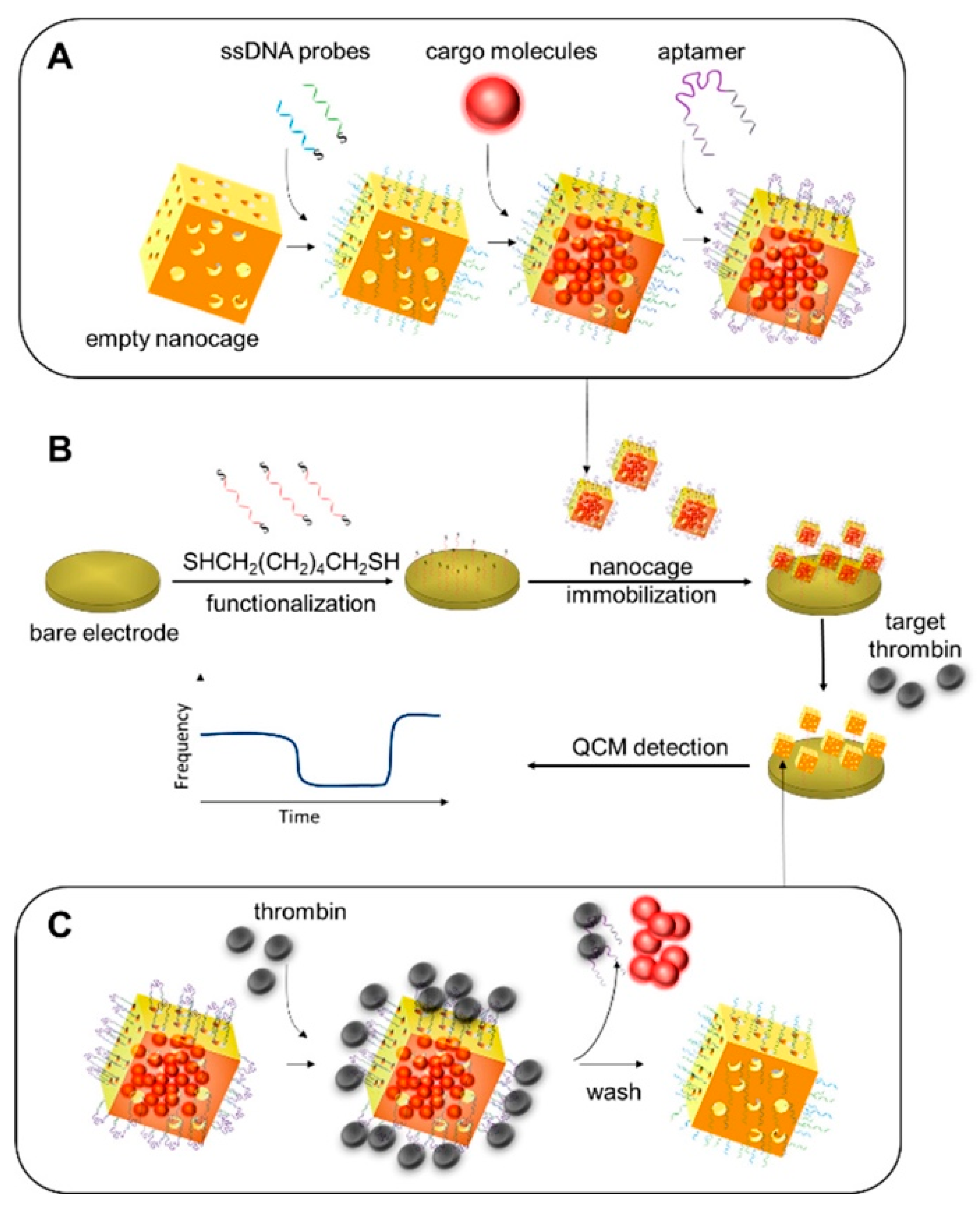
- Xi, X.; Niyonshuti, I.I.; Yu, N.; Yao, L.; Fu, Y.; Chen, J.; Li, Y. Label-Free Quartz Crystal Microbalance Biosensor Based on Aptamer-Capped Gold Nanocages Loaded with Polyamidoamine for Thrombin Detection. ACS Appl. Nano Mater. 2021, 4, 10047–10054. [Google Scholar] [CrossRef]
- Hianik, T.; Ostatná, V.; Zajacová, Z.; Stoikova, E.; Evtugyn, G. Detection of aptamer-protein interactions using QCM and electrochemical indicator methods. Bioorg. Med. Chem. Lett. 2005, 15, 291–295. [Google Scholar] [CrossRef]
- Iijima, M.; Yamada, Y.; Nakano, H.; Nakayama, T.; Kuroda, S. Bio-nanocapsules for oriented immobilization of DNA aptamers on aptasensors. Analyst 2022, 147, 489–495. [Google Scholar] [CrossRef]
- Deng, Y.; Yue, X.; Hu, H.; Zhou, X. A new analytical experimental setup combining quartz crystal microbalance with surface enhancement Raman spectroscopy and its application in determination of thrombin. Microchem. J. 2017, 132, 385–390. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Luzi, E.; Mascini, M. Aptamer-based biosensors for the detection of HIV-1 Tat protein. Bioelectrochemistry 2005, 67, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Minunni, M.; Tombelli, S.; Gullotto, A.; Luzi, E.; Mascini, M. Development of biosensors with aptamers as bio-recognition element: The case of HIV-1 Tat protein. Biosens. Bioelectron. 2004, 20, 1149–1156. [Google Scholar] [CrossRef]
- Yao, C.; Qi, Y.; Zhao, Y.; Xiang, Y.; Chen, Q.; Fu, W. Aptamer-based piezoelectric quartz crystal microbalance biosensor array for the quantification of IgE. Biosens. Bioelectron. 2009, 24, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Hayden, O.; Dickert, F.L. Selective microorganism detection with cell surface imprinted polymers. Adv. Mater. 2001, 13, 1480–1483. [Google Scholar] [CrossRef]
- Yilmaz, E.; Majidi, D.; Ozgur, E.; Denizli, A. Whole cell imprinting based Escherichia coli sensors: A study for SPR and QCM. Sens. Actuators B Chem. 2015, 209, 714–721. [Google Scholar] [CrossRef]
- Nur, Y.; Gaffar, S.; Hartati, Y.W.; Subroto, T. Applications of electrochemical biosensor of aptamers-based (APTASENSOR) for the detection of leukemia biomarker. Sens. Bio-Sens. Res. 2021, 32, 100416. [Google Scholar] [CrossRef]
- Acheson, E.D. Cancer statistics, 2012. Br. Med. J. 1976, 1, 394. [Google Scholar] [CrossRef]
- Ghossein, R.A.; Bhattacharya, S. Molecular detection and characterisation of circulating tumour cells and micrometastases in solid tumours. Eur. J. Cancer 2000, 36, 1681–1694. [Google Scholar] [CrossRef]
- Peters, J.M.; Ansari, M.Q. Multiparameter flow cytometry in the diagnosis and management of acute leukemia. Arch. Pathol. Lab. Med. 2011, 135, 44–54. [Google Scholar] [CrossRef]
- Misra, R.; Sahoo, S.K. Coformulation of doxorubicin and curcumin in poly(d, l-lactide-co- glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol. Pharm. 2011, 8, 852–866. [Google Scholar] [CrossRef]
- Shan, W.; Pan, Y.; Fang, H.; Guo, M.; Nie, Z. An aptamer-based quartz crystal microbalance biosensor for sensitive and selective detection of leukemia cells using silver-enhanced gold nanoparticle label. Talanta 2014, 126, 130–135. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A.; Wiechec, E.; Turner, A.P.F.; Tiwari, A. Ultrasensitive detection of human liver hepatocellular carcinoma cells using a label-free aptasensor. Anal. Chem. 2014, 86, 4956–4960. [Google Scholar] [CrossRef]
- Jiang, X.; Kim, K.; Zhang, S.; Johnson, J.; Salazar, G. High-temperature piezoelectric sensing. Sensors 2013, 14, 144–169. [Google Scholar] [CrossRef]
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric viral diagnostics: QCM based biosensors for early detection of viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Ming, T.; Cheng, Y.; Xing, Y.; Luo, J.; Mao, G.; Liu, J.; Sun, S.; Kong, F.; Jin, H.; Cai, X. Electrochemical Microfluidic Paper-Based Aptasensor Platform Based on a Biotin-Streptavidin System for Label-Free Detection of Biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 46317–46324. [Google Scholar] [CrossRef]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1558. [Google Scholar] [CrossRef]
- Chauhan, R.; Solanki, P.R.; Singh, J.; Mukherjee, I.; Basu, T.; Malhotra, B.D. A novel electrochemical piezoelectric label free immunosensor for aflatoxin B1 detection in groundnut. Food Control 2015, 52, 60–70. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef]
- Wang, L. Metal-organic frameworks for QCM-based gas sensors: A review. Sens. Actuators A Phys. 2020, 307, 111984. [Google Scholar] [CrossRef]


| Method and Materials | Advantages | Challenges | Ref. |
|---|---|---|---|
| Aptamer | label-free detection, specific recognition, online, rapid, highly sensitive analysis, simple to functionalize, non-aggregating, very stable in dehydrated form, more resistant to thermal degradation | anchoring to the surface of QCM electrode, low reproducibility, costly | [143,144,145] |
| Antibody | selective affinity to target molecules, sensitive assays, reproducible results, | substantial decrease in bioactivity owing to the denaturation and random orientation, costly production | [146] |
| Molecular imprinting polymer (MIP) | high selectivity to template molecule, long-term storage stability, potential re-usability, cheap | creates wide cavities, template molecule may covalently bound to the polymer, difficult target removal | [147] |
| Metal-organic frameworks (MOFs) | high sensitivity to target, low power consumption, easy modification | Large-scale manufacturing, improved selectivity, enhancing reproducibility, miniaturized manufacturing methods | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgönüllü, S.; Özgür, E.; Denizli, A. Quartz Crystal Microbalance-Based Aptasensors for Medical Diagnosis. Micromachines 2022, 13, 1441. https://doi.org/10.3390/mi13091441
Akgönüllü S, Özgür E, Denizli A. Quartz Crystal Microbalance-Based Aptasensors for Medical Diagnosis. Micromachines. 2022; 13(9):1441. https://doi.org/10.3390/mi13091441
Chicago/Turabian StyleAkgönüllü, Semra, Erdoğan Özgür, and Adil Denizli. 2022. "Quartz Crystal Microbalance-Based Aptasensors for Medical Diagnosis" Micromachines 13, no. 9: 1441. https://doi.org/10.3390/mi13091441
APA StyleAkgönüllü, S., Özgür, E., & Denizli, A. (2022). Quartz Crystal Microbalance-Based Aptasensors for Medical Diagnosis. Micromachines, 13(9), 1441. https://doi.org/10.3390/mi13091441









