Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Hydrothermal Method of CoxNi100−x NPs
2.3. Hemolytic Activity
2.4. Characterization of MNPs
2.5. Magnetic and Magnetothermal Measurements
3. Results and Discussion
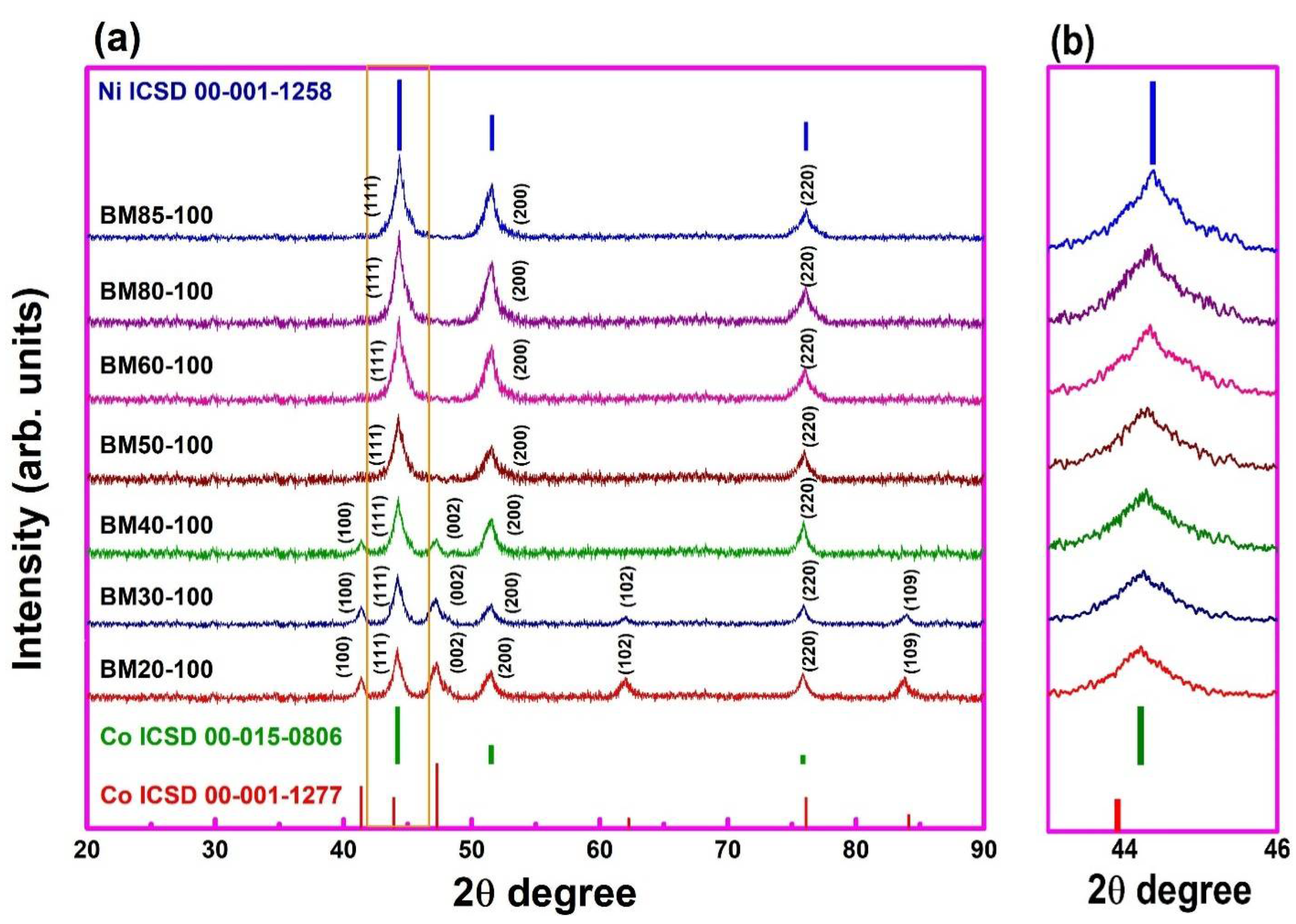
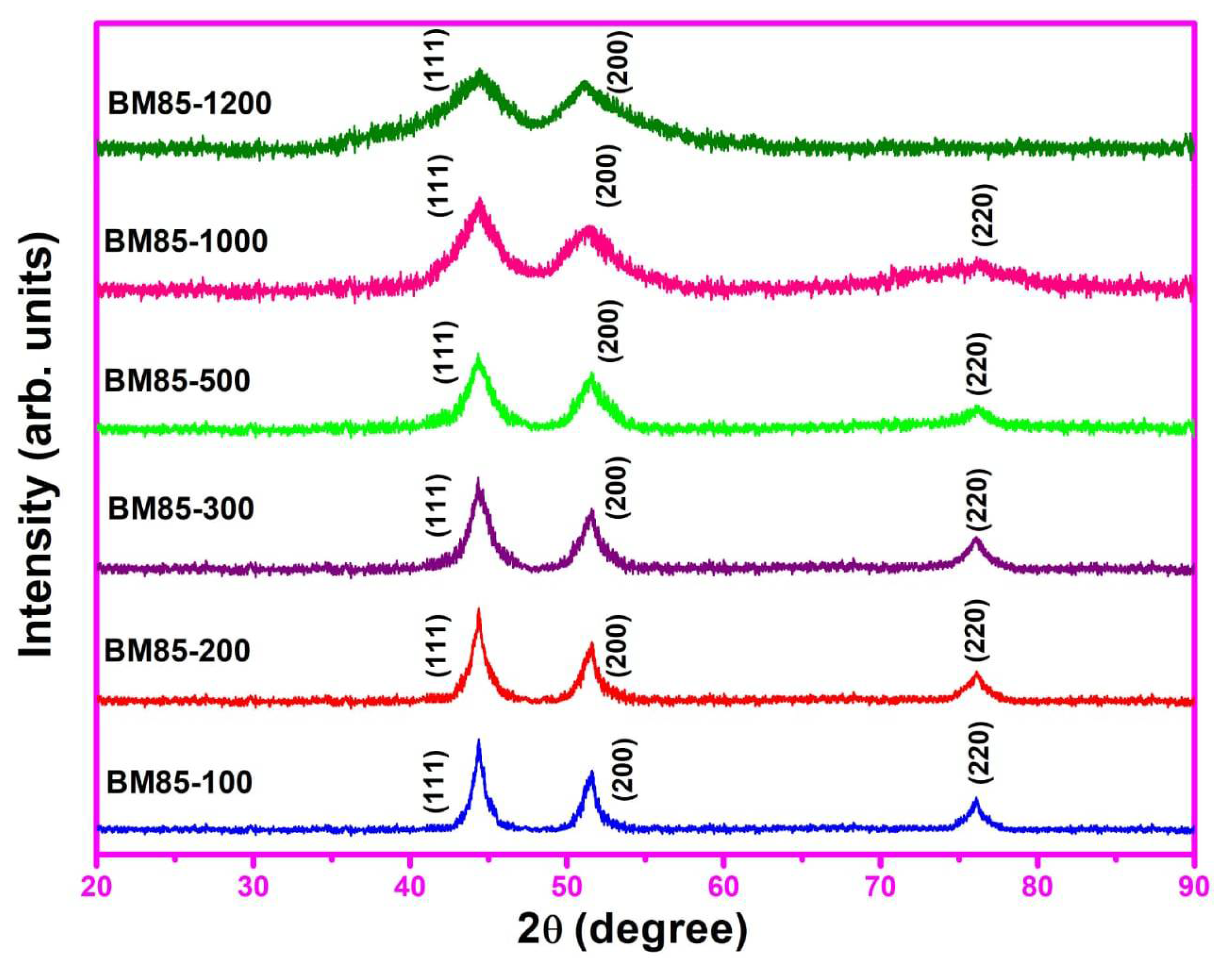
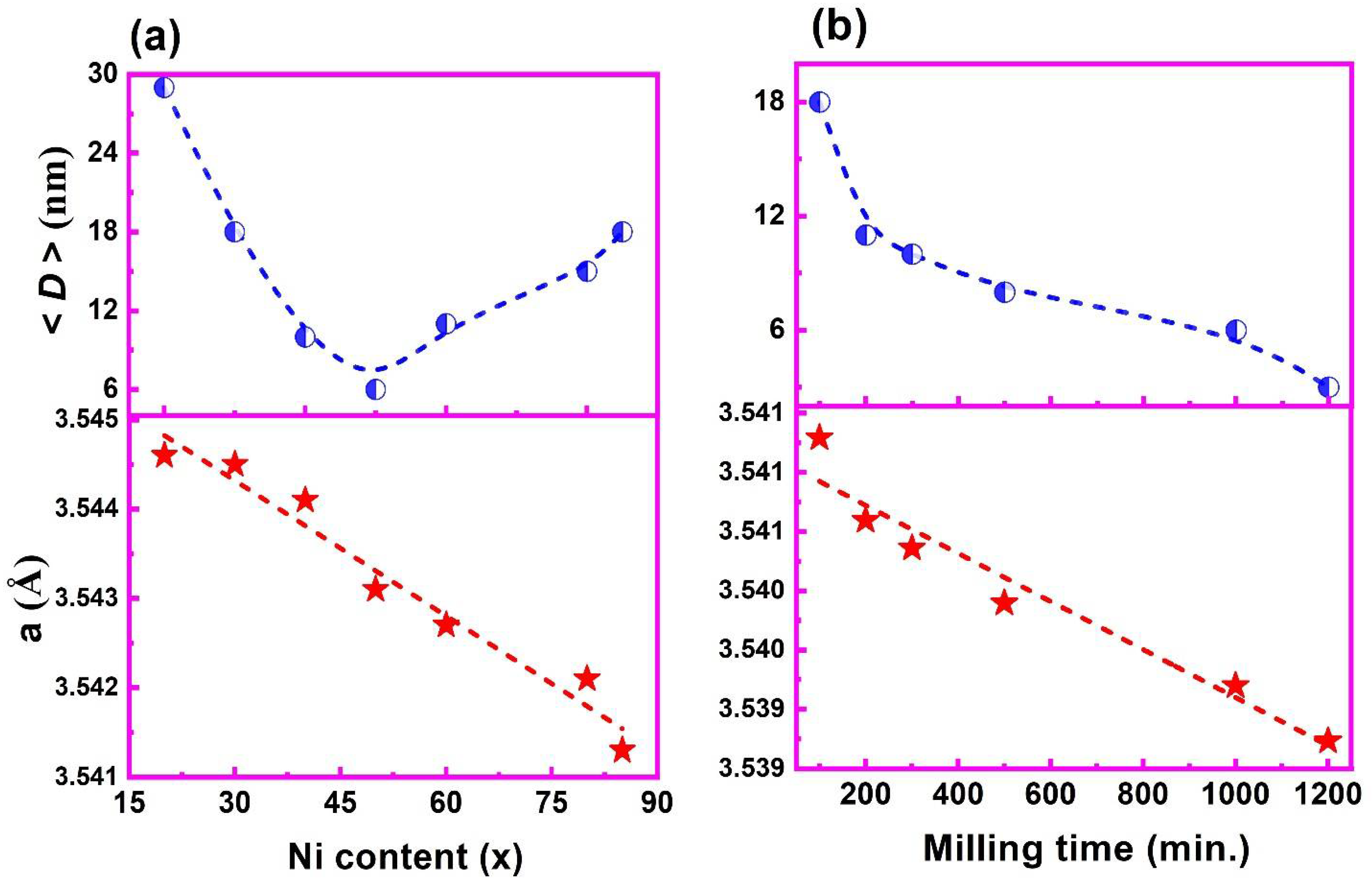
3.1. XRD Spectra Analysis
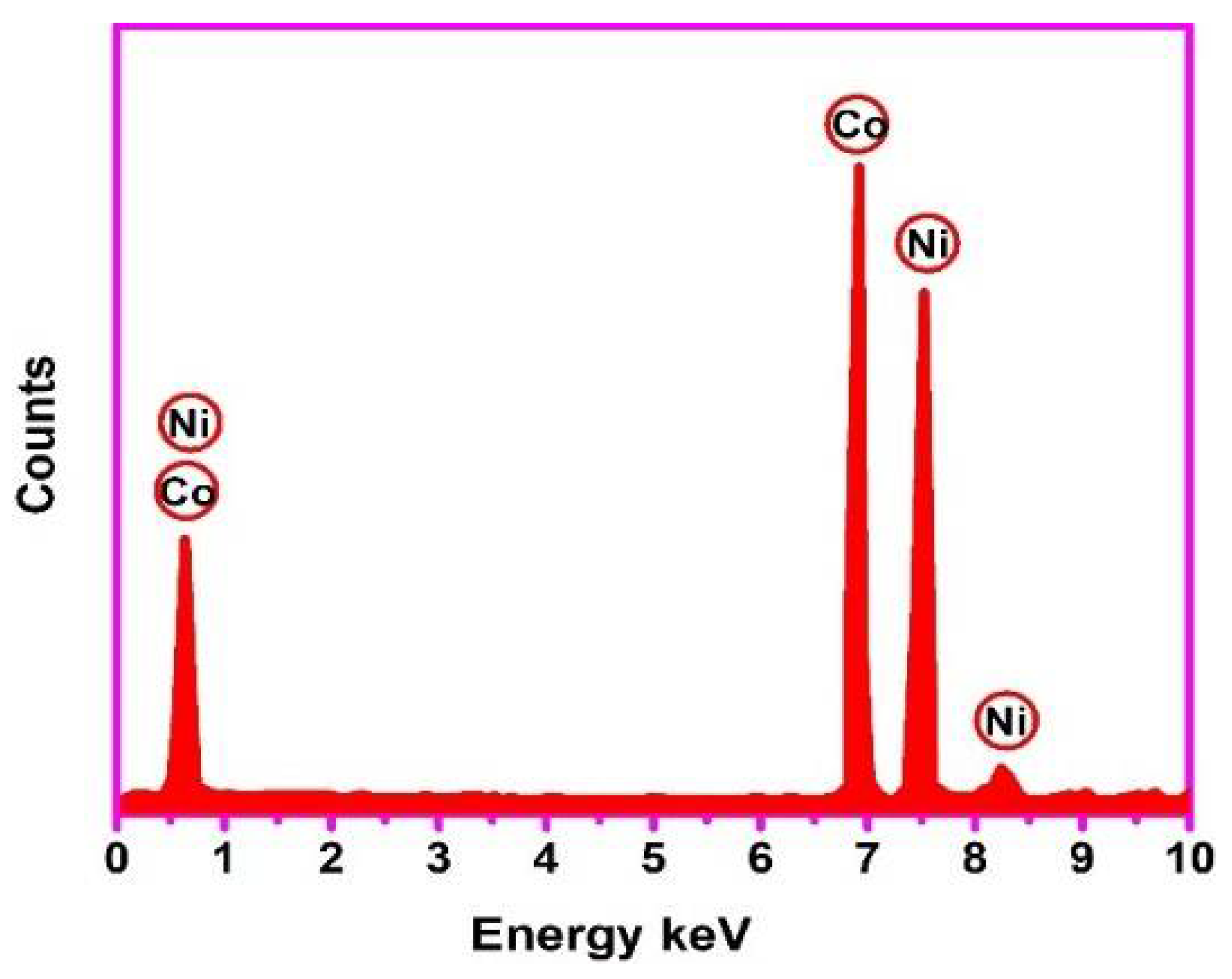
3.2. Elemental Analysis
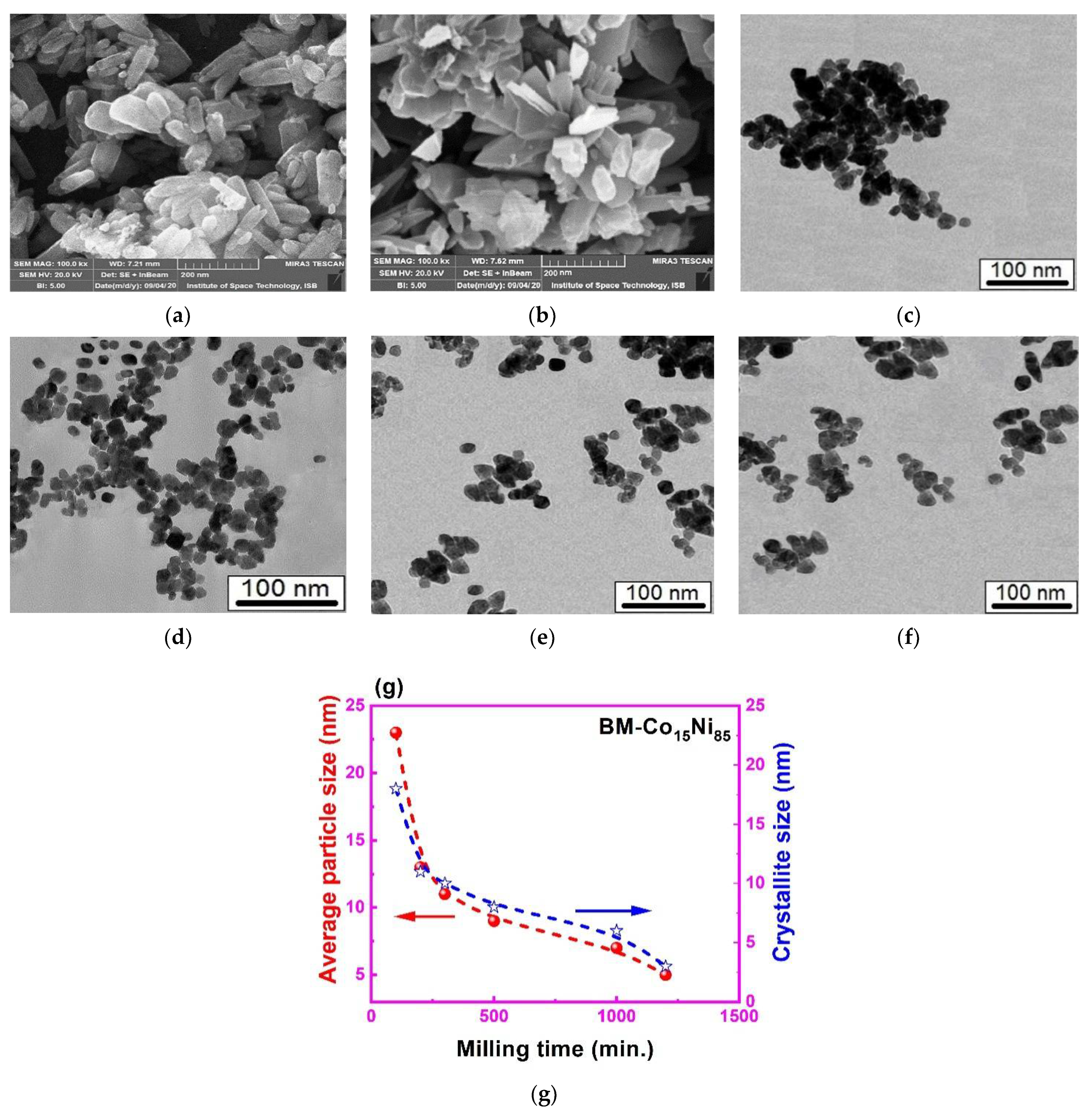
3.3. Surface Morphology
3.4. Thermo-Gravimetric Analysis (TGA)
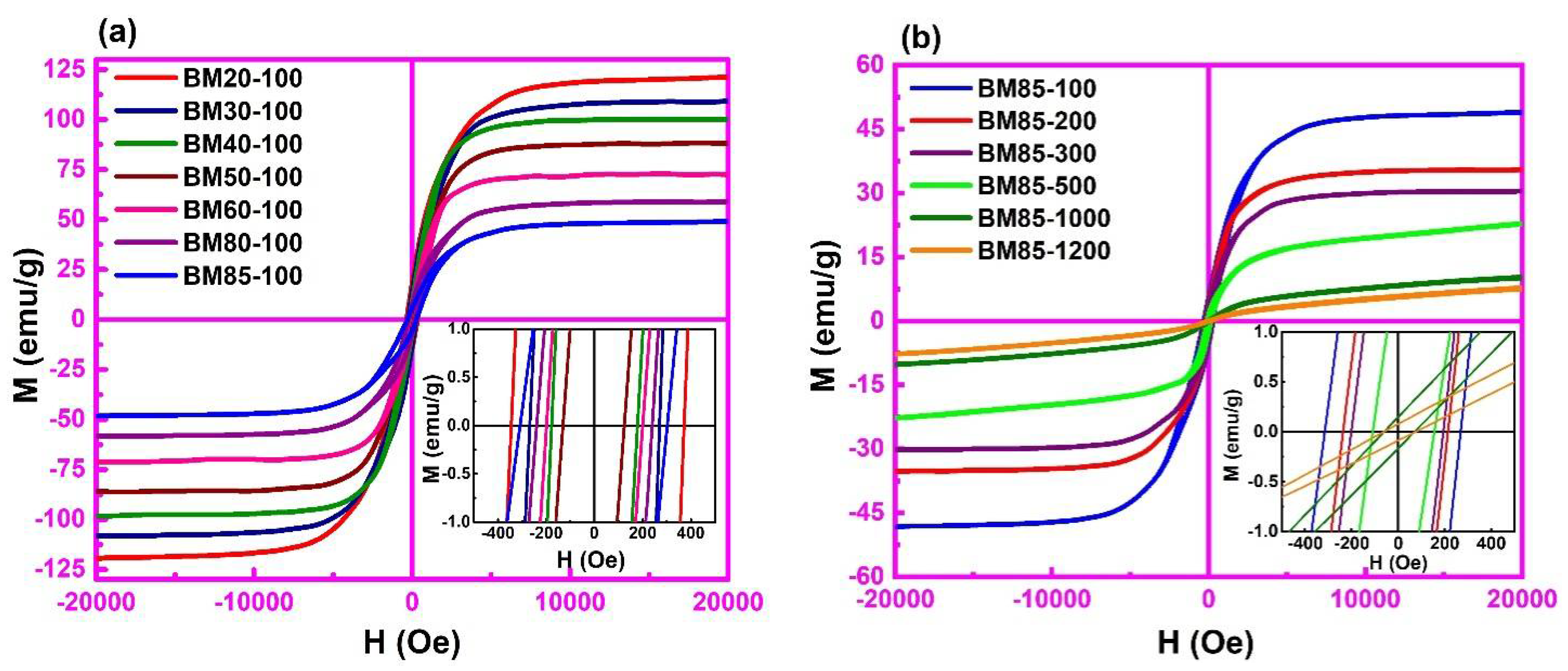
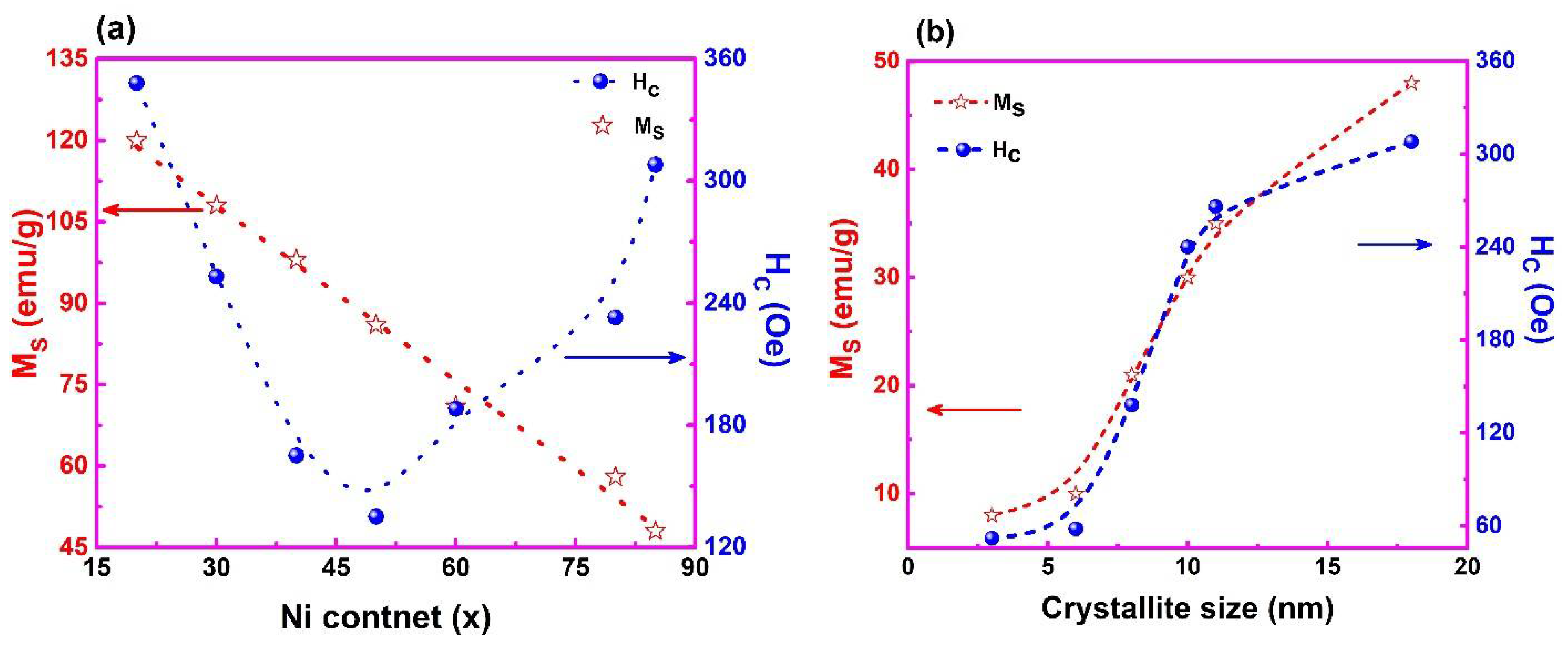
3.5. Field Dependent Magnetization
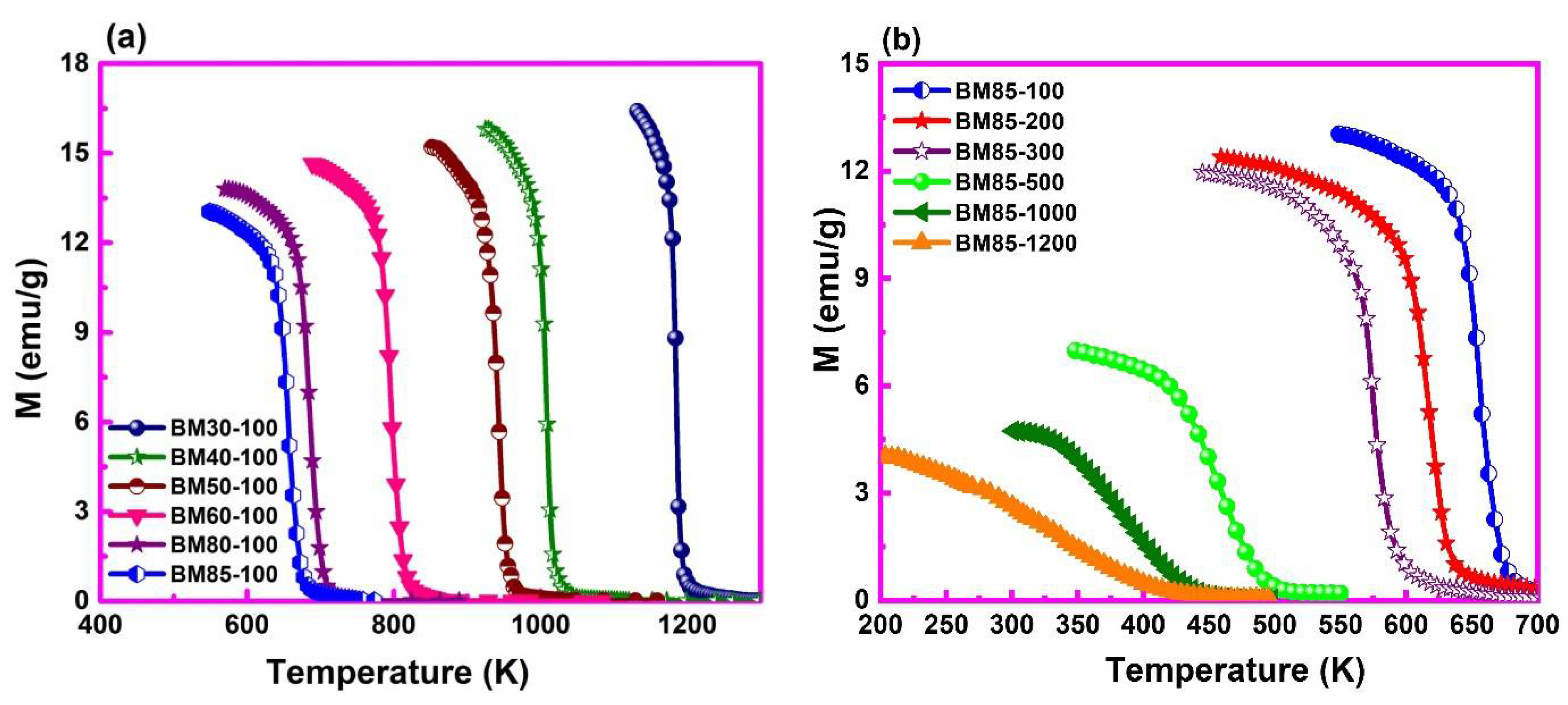
3.6. Temperature-Dependent Magnetization
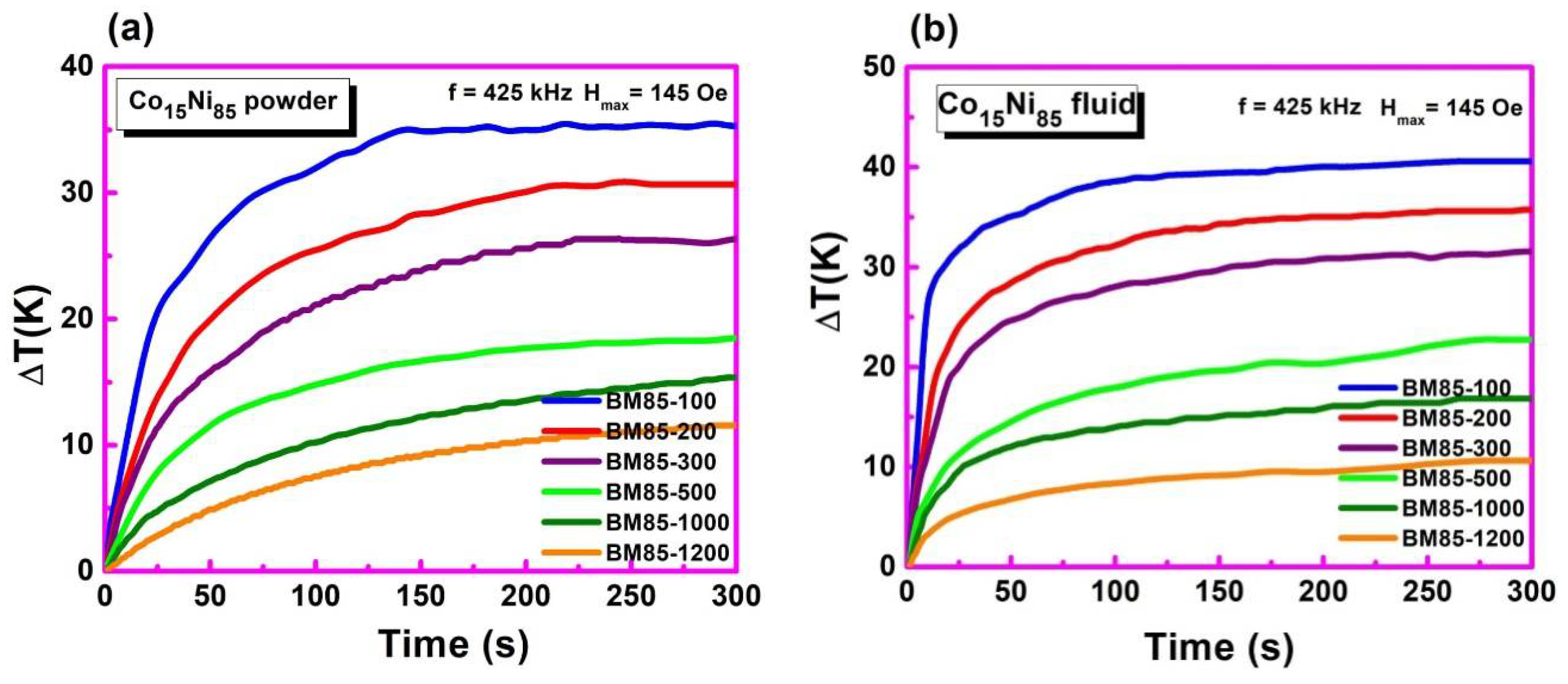
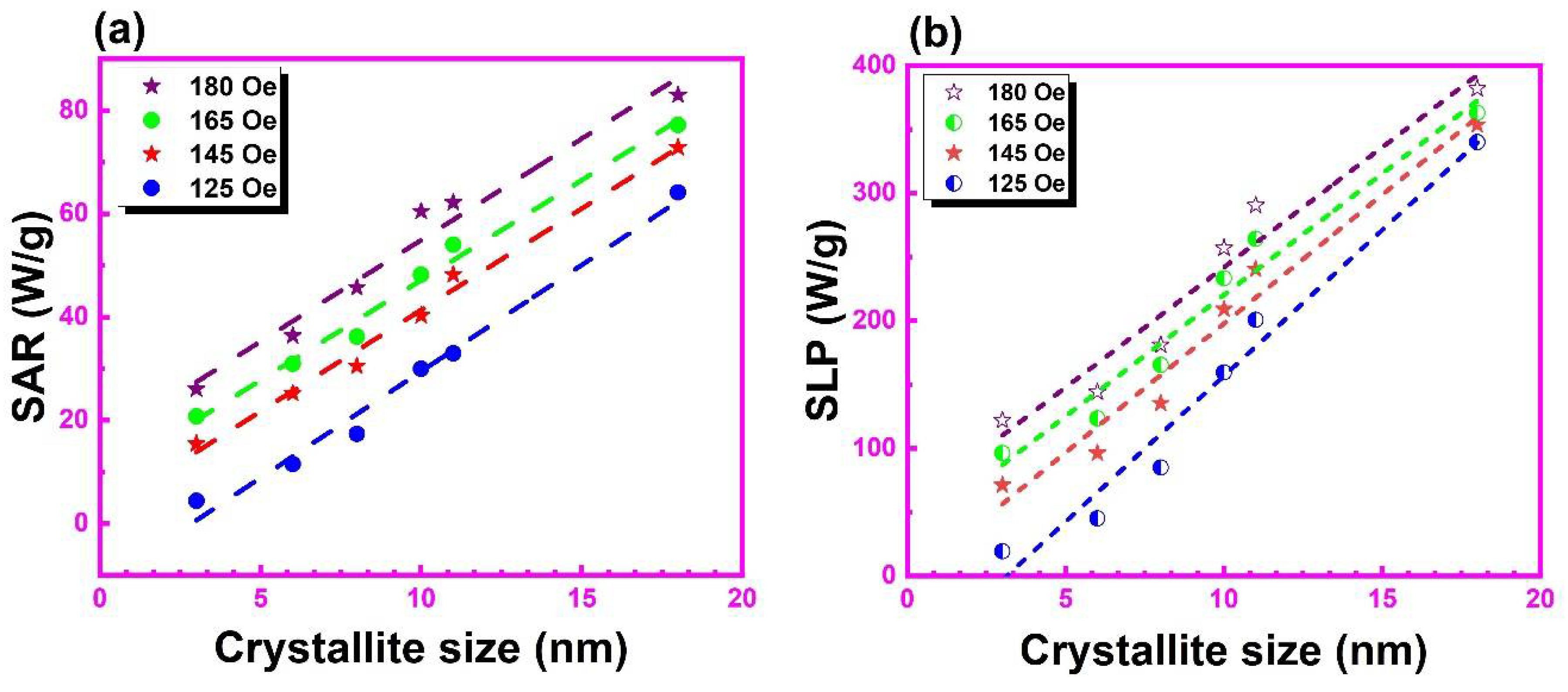
3.7. Temperature-Dependent Magnetization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, N.; He, L.; Jiang, L.; Shan, S.; Su, H. Synthesis of magnetic/pH dual responsive dextran hydrogels as stimuli-sensitive drug carriers. Carbohydr. Res. 2022, 520, 108632. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Ledari, R.; Maleki, A. Magnetic nanocatalysts utilized in the synthesis of aromatic pharmaceutical ingredients. New J. Chem. 2021, 45, 4135–4146. [Google Scholar] [CrossRef]
- Kheiri, K.; Sohrabi, N.; Mohammadi, R.; Amini-Fazl, M.S. Preparation and characterization of magnetic nanohydrogel based on chitosan for 5-fluorouracil drug delivery and kinetic study. Int. J. Biol. Macromol. 2022, 202, 191–198. [Google Scholar] [CrossRef]
- Tabasi, H.; Mosavian, M.T.H.; Darroudi, M.; Khazaei, M.; Hashemzadeh, A.; Sabouri, Z. Synthesis and characterization of amine-functionalized Fe3O4/Mesoporous Silica Nanoparticles (MSNs) as potential nanocarriers in drug delivery systems. J. Porous Mater. 2022, 1–12. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Zhang, W.; Radmanesh, M.; Mirmohammadi, S.S.; Maleki, A.; Cathcart, N.; Kitaev, V. Multi-stimuli nanocomposite therapeutic: Docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small 2020, 16, 2002733. [Google Scholar] [CrossRef] [PubMed]
- Dik, G.; Ulu, A.; Ates, B. Medicinal and Biological Application of Magnetic Alloy Nanoparticles and Their Polymer Nanocomposites. In Handbook of Magnetic Hybrid Nanoalloys and their Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–27. [Google Scholar]
- Taheri-Ledari, R.; Maleki, A. Antimicrobial therapeutic enhancement of levofloxacin via conjugation to a cell-penetrating peptide: An efficient sonochemical catalytic process. J. Pept. Sci. 2020, 26, e3277. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214. [Google Scholar] [CrossRef]
- Jordan, A. Hyperthermia classic commentary: Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia by Andreas Jordan et al., International Journal of Hyperthermia, 1993;9:51–68. Int. J. Hyperth. 2009, 25, 512–516. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38. [Google Scholar] [CrossRef]
- Čampelj, S.; Makovec, D.; Drofenik, M. Functionalization of magnetic nanoparticles with 3-aminopropyl silane. J. Magn. Magn. Mater. 2009, 321, 1346–1350. [Google Scholar] [CrossRef]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of magnetic nanoparticles to gene delivery. Int. J. Mol. Sci 2011, 12, 3705–3722. [Google Scholar] [CrossRef]
- Larumbe, S.; Gomez-Polo, C.; Pérez-Landazábal, J.; Pastor, J. Effect of a SiO2 coating on the magnetic properties of Fe3O4 nanoparticles. J. Phys. Condens. Matter. 2012, 24, 266007. [Google Scholar] [CrossRef] [PubMed]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Verde, E.; Landi, G.T.; Carrião, M.; Drummond, A.L.; Gomes, J.; Vieira, E.; Sousa, M.; Bakuzis, A.F. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv. 2012, 2, 032120. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Corrigendum to “Suitability of commercial colloids for magnetic hyperthermia”. J. Magn. Magn. Mater. 2009, 321, 3650–3651. [Google Scholar] [CrossRef]
- Baker, I.; Zeng, Q.; Li, W.; Sullivan, C.R. Heat deposition in iron oxide and iron nanoparticles for localized hyperthermia. J. Appl. Phys. 2006, 99, 08H106. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Xia, Q.-S.; Liu, X.; Xu, B.; Zhao, T.-D.; Li, H.-Y.; Chen, Z.-H.; Xiang, Q.; Geng, C.-Y.; Pan, L.; Hu, R.-L. Feasibility study of high-temperature thermoseed inductive hyperthermia in melanoma treatment. Oncol. Rep. 2011, 25, 953–962. [Google Scholar]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Gomes, J.A.; Sousa, M.H.; Bakuzis, A.F. Magnetic hyperthermia investigation of cobalt ferrite nanoparticles: Comparison between experiment, linear response theory, and dynamic hysteresis simulations. J. Appl. Phys. 2012, 111, 123902. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M. Handbook of magnetism and advanced magnetic materials. Nov. Mater. 2007, 4, 1–29. [Google Scholar]
- Nguyen, H.D.; Nguyen, T.D.; Nguyen, D.H.; Nguyen, P.T. Magnetic properties of Cr doped Fe3O4 porous nanoparticles prepared through a co-precipitation method using surfactant. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 035017. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Zhang, Y.; Wu, S.; Li, F.; Liu, J.P. Synthesis and characterization of gadolinium doped cobalt ferrite nanoparticles with enhanced adsorption capability for Congo Red. Chem. Eng. J. 2014, 250, 164–174. [Google Scholar] [CrossRef]
- Alnasir, M.H.; Khan, M.Y.; Ali, W.B.; Ansar, M.; Manzoor, S. Magnetic and hyperthermia properties of Ni1− xCux nanoparticles coated with oleic acid and silica prepared via sol–gel method. Appl. Phys. A 2019, 125, 1–13. [Google Scholar] [CrossRef]
- Alnasir, M.H.; Awan, M.; Manzoor, S.; Materials, M. Magnetic and magnetothermal studies of pure and doped gadolinium silicide nanoparticles for self-controlled hyperthermia applications. J. Magn. Magn. Mater. 2018, 449, 137–144. [Google Scholar] [CrossRef]
- Idrissi, L.; Tahiri, N.; El Bounagui, O.; Ez-Zahraouy, H. Magnetic properties of NiFe2O4 compound: Ab Initio calculation and Monte Carlo simulation. J. Supercond. Nov. Magn. 2020, 33, 1369–1375. [Google Scholar] [CrossRef]
- Dennis, C.; Jackson, A.; Borchers, J.; Ivkov, R.; Foreman, A.; Hoopes, P.; Strawbridge, R.; Pierce, Z.; Goerntiz, E.; Lau, J. The influence of magnetic and physiological behaviour on the effectiveness of iron oxide nanoparticles for hyperthermia. J. Phys. D Appl. Phys. 2008, 41, 134020. [Google Scholar] [CrossRef]
- Kawai, N.; Futakuchi, M.; Yoshida, T.; Ito, A.; Sato, S.; Naiki, T.; Honda, H.; Shirai, T.; Kohri, K. Effect of heat therapy using magnetic nanoparticles conjugated with cationic liposomes on prostate tumor in bone. Prostate 2008, 68, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Suto, M.; Hirota, Y.; Mamiya, H.; Fujita, A.; Kasuya, R.; Tohji, K.; Jeyadevan, B. Heat dissipation mechanism of magnetite nanoparticles in magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1493–1496. [Google Scholar] [CrossRef]
- Lévy, M.; Wilhelm, C.; Siaugue, J.-M.; Horner, O.; Bacri, J.-C.; Gazeau, F. Magnetically induced hyperthermia: Size-dependent heating power of γ-Fe2O3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 204133. [Google Scholar] [CrossRef] [PubMed]
- Blonska-Tabero, A.; Bosacka, M.; Filipek, E.; Piz, M.; Kochmanski, P. High-temperature synthesis and unknown properties of M3Cr4 (PO4)6, where M=Zn or Mg and a new solid solution Zn1.5Mg1.5Cr4 (PO4)6. J. Therm. Anal. Calorim. 2020, 140, 2625–2631. [Google Scholar] [CrossRef]
- Thapa, D.; Palkar, V.; Kurup, M.; Malik, S. Properties of magnetite nanoparticles synthesized through a novel chemical route. Mater. Lett. 2004, 58, 2692–2694. [Google Scholar] [CrossRef]
- Nikiforov, V.; Koksharov, Y.A.; Polyakov, S.; Malakho, A.; Volkov, A.; Moskvina, M.; Khomutov, G.; Irkhin, V.Y. Magnetism and Verwey transition in magnetite nanoparticles in thin polymer film. J. Alloys Compd. 2013, 569, 58–61. [Google Scholar] [CrossRef]
- Hergt, R.; Andra, W.; d′Ambly, C.G.; Hilger, I.; Kaiser, W.A.; Richter, U.; Schmidt, H.-G. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans. Magn. 1998, 34, 3745–3754. [Google Scholar] [CrossRef]
- Nauman, M.; Alnasir, M.H.; Hamayun, M.A.; Wang, Y.; Shatruk, M.; Manzoor, S. Size-dependent magnetic and magnetothermal properties of gadolinium silicide nanoparticles. RSC Adv. 2020, 10, 28383–28389. [Google Scholar] [CrossRef]
- Ennassiri, N.; Tahiri, N.; El Bounagui, O.; Ez-Zahraouy, H.; Benyoussef, A. Structural, electronic, magnetic, and magnetocaloric properties in metallic antiperovskite compound Mn3GaC. Mater. Res. Bull. 2018, 98, 335–339. [Google Scholar] [CrossRef]
- Ennassiri, N.; Tahiri, N.; El Bounagui, O.; Ez-Zahraouy, H.; Benyoussef, A. Magnetic, magnetocaloric and transport properties in AlCMn3 antiperovskite compound. J. Alloys Compd. 2018, 741, 1196–1202. [Google Scholar] [CrossRef]
- Bazine, W.; Tahiri, N.; El Bounagui, O.; Ez-Zahraouy, H.; Benyoussef, A. Structural, electronic, magnetic, and magnetocaloric properties in intermetallic compound TbCu2Si2. J. Magn. Magn. Mater. 2019, 481, 72–76. [Google Scholar] [CrossRef]
- Cui, B.; Marinescu, M.; Liu, J. High magnetization Fe-Co and Fe-Ni submicron and nanosize particles by thermal decomposition and hydrogen reduction. J. Appl. Phys. 2014, 115, 17A315. [Google Scholar] [CrossRef]
- Yi, Z.; Chahine, R.; Bose, T.K. Magnetic entropy change in the Ge-rich alloys Gd-Si-Ge. IEEE Trans. Magn. 2003, 39, 3358–3360. [Google Scholar] [CrossRef]
- Pandey, S.; Quetz, A.; Aryal, A.; Dubenko, I.; Mazumdar, D.; Stadler, S.; Ali, N. Magnetic, structural and magnetocaloric properties of Ni-Si and Ni-Al thermoseeds for self-controlled hyperthermia. Int. J. Hyperth. 2017, 33, 779–784. [Google Scholar] [CrossRef]
- Ucar, H.; Ipus, J.J.; Laughlin, D.; McHenry, M. Tuning the Curie temperature in γ-FeNi nanoparticles for magnetocaloric applications by controlling the oxidation kinetics. J. Appl. Phys. 2013, 113, 17A918. [Google Scholar] [CrossRef]
- Ferk, G.; Stergar, J.; Makovec, D.; Hamler, A.; Jagličić, Z.; Drofenik, M.; Ban, I. Synthesis and characterization of Ni–Cu alloy nanoparticles with a tunable Curie temperature. J. Alloys Compd. 2015, 648, 53–58. [Google Scholar] [CrossRef]
- Mohn, P.; Wohlfarth, E. The Curie temperature of the ferromagnetic transition metals and their compounds. J. Phys. F Met. Phys. 1987, 17, 2421. [Google Scholar] [CrossRef]
- Nishizawa, T.; Ishida, K. The Co−Ni (Cobalt-Nickel) system. Bull. Alloy. Phase Diagr. 1983, 4, 390–395. [Google Scholar] [CrossRef]
- Respaud, M.; Broto, J.; Rakoto, H.; Fert, A.; Thomas, L.; Barbara, B.; Verelst, M.; Snoeck, E.; Lecante, P.; Mosset, A.J.P.R.B. Surface effects on the magnetic properties of ultrafine cobalt particles. Phys. Rev. B 1998, 57, 2925. [Google Scholar] [CrossRef]
- Zhang, Y.; Or, S.-W.; Zhang, Z. Synthesis, structure and magnetic properties of CoNi submicrospherical chains. Adv. Mater. Phys. Chem. 2011, 1, 7. [Google Scholar] [CrossRef][Green Version]
- Neamtu, J.; Verga, N. Magnetic nanoparticles for magneto-resonance imaging and targeted drug delivery. Dig. J. Nanomater. Biostruct. 2011, 6, 969–978. [Google Scholar]
- Serizawa, K.; Kawai, T.; Ohtake, M.; Futamoto, M.; Kirino, F.; Inaba, N. Magnetostriction behaviors of Ni100-xFex and Ni100-yCoy (001) single-crystal films with fcc structure under rotating magnetic fields. IEEE Trans. Magn. 2017, 53, 1–4. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Pan, L.; Khan, W.S.; Iqbal, M.Z.; Qiu, H.; Farooq, M.H.; Ellahi, M.; Guo, Z.J.C. Controlled synthesis, phase formation, growth mechanism, and magnetic properties of 3-D CoNi alloy microstructures composed of nanorods. CrystEngComm 2013, 15, 5314–5325. [Google Scholar] [CrossRef]
- Nie, D.; Xu, C.; Chen, H.; Wang, Y.; Li, J.; Liu, Y.J.M.L. Chain-like CoNi alloy microstructures fabricated by a PVP-assisted solvothermal process. Mater. Lett. 2014, 131, 306–309. [Google Scholar] [CrossRef]
- Nadhman, A.; Nazir, S.; Khan, M.I.; Ayub, A.; Muhammad, B.; Khan, M.; Shams, D.F.; Yasinzai, M. Visible-light-responsive ZnCuO nanoparticles: Benign photodynamic killers of infectious protozoans. Int. J. Nanomed. 2015, 10, 6891. [Google Scholar]
- Bensouilah, A.; Guittoum, A.; Hemmous, M.; Bouremana, A.; Rahal, B.; Yavru, C.; Öksüzoglu, R.; Kechouane, M.; Magnetism, N. Structure, Microstructure and Magnetic Properties of CoxNi100−x Powders Synthesized by Hydrothermal Method. J. Supercond. Nov. Magn. 2017, 30, 2219–2225. [Google Scholar] [CrossRef]
- Gondwal, M.; Joshi nee Pant, G. Synthesis and catalytic and biological activities of silver and copper nanoparticles using Cassia occidentalis. Int. J. Biomater. 2018, 2018, 6735426. [Google Scholar] [CrossRef]
- Kapusetti, G.; Misra, N.; Singh, V.; Kushwaha, R.; Maiti, P. Bone cement/layered double hydroxide nanocomposites as potential biomaterials for joint implant. J. Biomed. Mater. Res. Part A 2012, 100, 3363–3373. [Google Scholar] [CrossRef]
- Mattei, G.; de Julián Fernández, C.; Mazzoldi, P.; Sada, C.; De, G.; Battaglin, G.; Sangregorio, C.; Gatteschi, D. Synthesis, Structure, and Magnetic Properties of Co, Ni, and Co−Ni Alloy Nanocluster-Doped SiO2 Films by Sol-Gel Processing. Chem. Mater. 2002, 14, 3440–3447. [Google Scholar] [CrossRef]
- Hu, M.J.; Lin, B.; Yu, S.H.J.N.r. Magnetic field-induced solvothermal synthesis of one-dimensional assemblies of Ni-Co alloy microstructures. Nano Res. 2008, 1, 303–313. [Google Scholar] [CrossRef]
- Jayakumar, O.; Salunke, H.; Tyagi, A.J. Synthesis and characterization of stoichiometric NiCo nano particles dispersible in both aqueous and non aqueous media. Solid State Commun. 2009, 149, 1769–1771. [Google Scholar] [CrossRef]
- Li, H.; Jin, Z.; Song, H.; Liao, S. Synthesis of Co submicrospheres self-assembled by Co nanosheets via a complexant-assisted hydrothermal approach. J. Magn. Magn. Mater. 2010, 322, 30–35. [Google Scholar] [CrossRef]
- Willis, A.L.; Turro, N.J.; O′Brien, S. Spectroscopic characterization of the surface of iron oxide nanocrystals. Chem. Mater. 2005, 17, 5970–5975. [Google Scholar] [CrossRef]
- Roca, A.; Morales, M.; O’Grady, K.; Serna, C.J.N. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783. [Google Scholar] [CrossRef]
- Toneguzzo, P.; Viau, G.; Acher, O.; Guillet, F.; Bruneton, E.; Fievet-Vincent, F.; Fievet, F. CoNi and FeCoNi fine particles prepared by the polyol process: Physico-chemical characterization and dynamic magnetic properties. J. Mater. Sci. 2000, 35, 3767–3784. [Google Scholar] [CrossRef]
- Iqbal, Y.; Bae, H.; Rhee, I.; Hong, S. Nanotechnology. Intensive analysis of core–shell silica-coated iron-oxide nanoparticles for magnetic hyperthermia. J. Nanosci. Nanotechnol. 2016, 16, 11862–11867. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E., Jr.; Foner, S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 1996, 77, 394. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, X.; Zou, B.; Wang, Y.J. Magnetic properties of nanosized MnFe2O4 particles. J. Magn. Magn. Mater. 1998, 183, 152–156. [Google Scholar] [CrossRef]
- George, M.; John, A.M.; Nair, S.S.; Joy, P.; Anantharaman, M.R.; Materials, M. Finite size effects on the structural and magnetic properties of sol–gel synthesized NiFe2O4 powders. J. Magn. Magn. Mater. 2006, 302, 190–195. [Google Scholar] [CrossRef]
- O′handley, R.C. Modern Magnetic Materials: Principles and Applications; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Rostamnejadi, A.; Salamati, H.; Kameli, P.; Ahmadvand, H. Superparamagnetic behavior of La0.67Sr0.33MnO3 nanoparticles prepared via sol–gel method. J. Magn. Magn. Mater. 2009, 321, 3126–3131. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Hamayun, M.A.; Abramchuk, M.; Alnasir, H.; Khan, M.; Pak, C.; Lenhert, S.; Ghazanfari, L.; Shatruk, M.; Manzoor, S.; Materials, M. Magnetic and magnetothermal studies of iron boride (FeB) nanoparticles. J. Magn. Magn. Mater. 2018, 451, 407–413. [Google Scholar] [CrossRef]
- Hanini, A.; Lartigue, L.; Gavard, J.; Kacem, K.; Wilhelm, C.; Gazeau, F.; Chau, F.; Ammar, S.J.J.o.M.; Materials, M. Zinc substituted ferrite nanoparticles with Zn0.9Fe2.1O4 formula used as heating agents for in vitro hyperthermia assay on glioma cells. J. Magn. Magn. Mater. 2016, 416, 315–320. [Google Scholar] [CrossRef]
- Vasseur, S.; Duguet, E.; Portier, J.; Goglio, G.; Mornet, S.; Hadová, E.; Knížek, K.; Maryško, M.; Veverka, P.; Pollert, E. Lanthanum manganese perovskite nanoparticles as possible in vivo mediators for magnetic hyperthermia. J. Magn. Magn. Mater. 2006, 302, 315–320. [Google Scholar] [CrossRef]
- Wuang, S.C.; Neoh, K.G.; Kang, E.-T.; Pack, D.W.; Leckband, D.E. Synthesis and functionalization of polypyrrole-Fe3O4 nanoparticles for applications in biomedicine. J. Mater. Chem. 2007, 17, 3354–3362. [Google Scholar] [CrossRef]
- Bruno, P. Tight-binding approach to the orbital magnetic moment and magnetocrystalline anisotropy of transition-metal monolayers. Phys. Rev. B 1989, 39, 865. [Google Scholar] [CrossRef] [PubMed]
- Ballou, R.; Deportes, J.; Gorges, B.; Lemaire, R.; Ousset, J. Anomalous thermal variation of the bulk anisotropy in GdCo5. J. Magn. Magn. Mater. 1986, 54, 465–466. [Google Scholar] [CrossRef]
- Drzazga, Z.; Bia, K.; Borgie, W. Effective magnetic anisotropy constant for B-substituted RCo5 (R= Y, Gd) compounds. J. Magn. Magn. Mater. 1991, 101, 399–400. [Google Scholar] [CrossRef]
- Szade, J. Magnetism of (Gd1-xYx) 2In. J. Magn. Magn. Mater. 1997, 170, 228–234. [Google Scholar] [CrossRef]
- Obaidat, I.; Mohite, V.; Issa, B.; Tit, N.; Haik, Y. Predicting a major role of surface spins in the magnetic properties of ferrite nanoparticles. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2009, 44, 489–494. [Google Scholar] [CrossRef]
- Dorfbauer, F.; Evans, R.; Kirschner, M.; Chubykalo-Fesenko, O.; Chantrell, R.; Schrefl, T. Effects of surface anisotropy on the energy barrier in cobalt–silver core–shell nanoparticles. J. Magn. Magn. Mater. 2007, 316, e791–e794. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemolytic effect of polymeric nanoparticles: Role of albumin. IEEE Trans. NanoBiosci. 2011, 10, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.E.; Itri, L.M. Chemotherapy-induced anemia in adults: Incidence and treatment. J. Natl. Cancer Inst. 1999, 91, 1616–1634. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T. Hemolysis as expression of nanoparticles-induced cytotoxicity in red blood cells. BMBN 2013, 1, 7–12. [Google Scholar]
- Purohit, R.; Vallabani, N.S.; Shukla, R.K.; Kumar, A.; Singh, S. Effect of gold nanoparticle size and surface coating on human red blood cells. Bioinspired Biomim. Nanobiomater. 2016, 5, 121–131. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Akamanchi, K.G. A novel biocompatible bicephalous dianionic surfactant from oleic acid for solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2013, 105, 215–222. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.G.; Huang, L.; Han, J.T.; Zhang, X.F. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: Biocompatibility, protein adsorption and cellular uptake. J. Mater. Sci. Mater. Med. 2012, 23, 1775–1783. [Google Scholar] [CrossRef]




| Oleic-Acid-Coated Co15Ni85 MNPs | Optical Density | Hemolysis (%) |
|---|---|---|
| BM-200 | 0.0652 | 4 |
| BM-300 | 0.0681 | 4.4 |
| BM-500 | 0.0724 | 4.9 |
| As prepared (uncoated) | 0.2453 | 28 |
| Positive control | 0.7805 | 100 |
| Negative control | 0.0356 | 0 |
| Sample | EDX | ICP | ||
|---|---|---|---|---|
| Co (at.%) | Ni (at.%) | Co (at.%) | Ni (at.%) | |
| BM50-100 | 50.26 | 49.74 | 49.86 | 50.14 |
| BM60-100 | 41.59 | 58.41 | 40.49 | 59.51 |
| BM80-100 | 21.58 | 78.42 | 20.68 | 79.32 |
| BM85-100 Co15Ni85 | 16.57 | 83.43 | 15.77 15.12 | 84.23 84.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Nasir, M.H.; Siddique, S.; Aisida, S.O.; Altowairqi, Y.; Fadhali, M.M.; Shariq, M.; Khan, M.S.; Qamar, M.A.; Shahid, T.; Shahzad, M.I.; et al. Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia. Coatings 2022, 12, 1272. https://doi.org/10.3390/coatings12091272
Al Nasir MH, Siddique S, Aisida SO, Altowairqi Y, Fadhali MM, Shariq M, Khan MS, Qamar MA, Shahid T, Shahzad MI, et al. Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia. Coatings. 2022; 12(9):1272. https://doi.org/10.3390/coatings12091272
Chicago/Turabian StyleAl Nasir, Muhammad Hisham, Shumaila Siddique, Samson O. Aisida, Yasir Altowairqi, Mohammed M. Fadhali, Mohammad Shariq, M. Shakir Khan, Muhammad Azam Qamar, Tauseef Shahid, Muhammad Imran Shahzad, and et al. 2022. "Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia" Coatings 12, no. 9: 1272. https://doi.org/10.3390/coatings12091272
APA StyleAl Nasir, M. H., Siddique, S., Aisida, S. O., Altowairqi, Y., Fadhali, M. M., Shariq, M., Khan, M. S., Qamar, M. A., Shahid, T., Shahzad, M. I., & Ali, S. K. (2022). Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia. Coatings, 12(9), 1272. https://doi.org/10.3390/coatings12091272






