1. Introduction
Grasslands, besides being the predominant forage source for grazing animals, also provide critical human goods (products from cattle, sheep, goats, and camels) and ecosystem services (climate change regulation, wildlife habitat, genetic resources, erosion control, water provision, air purification, and cultural and amenity services), thus are considered essential to sustain human societies [
1]. However, all the socioeconomic, environmental, and ecological functions provided by grasslands are being progressively placed under severe threat or even being lost due to a long-term degradation process [
2].
Despite the causes of grasslands degradation being multifactorial, [
3] reported that, in a global scale, climate variation is associated with approximately 45.5% of the degradation processes in grassland areas, whereas human activities accounted for around 40.1% of this degradation. According to [
1,
2,
3], overgrazing by livestock, land abandonment, extensive clearing for crop production, a heightened fire frequency, and inappropriate management and soil conservation practices that lead to soil erosion and woody plant encroachment, are included in the human activities that play critical roles in the degradation process. In this way, in a future scenario of climate change, equating human activities will be a decisive step towards the establishment of sustainable management practices.
In order to revert the negative impacts of human activities on grasslands degradation, progress can only be made through deeper knowledge of the specific context of grasslands at a regional or landscape scale [
2]. A progressive and planned sustainable intensification process is considered an important way to enhance grasslands productivity at the same time as contributing to reduced greenhouse gases emissions (GHG), preventing soil degradation [
4], preserving ecosystem diversity, functioning and resilience [
5], and also ensuring food security [
6]. Consequently, the current scenario of pasture-based livestock production systems has highlighted the need to adopt more conscious agronomic and management practices.
These strategies are considered particularly effective for pasture-based livestock systems in which tropical (warm-season) perennial grasses are the basis of the forage supply. The relevance of warm-season perennial grass for sustaining ruminant production in tropical and subtropical regions is well recognized worldwide [
7,
8,
9]. Among the more important genus are included
Urochloa (Syn.
Brachiaria),
Cynodon,
Megathyrsus (Syn.
Panicum) and
Pennisetum [
10,
11,
12]. These grasses are considered highly productive species, being associated with high growth rates, efficient nutrient extraction capability, and high water and N-use efficiencies [
8]; most of them expressing adaptability to warm and cold conditions from tropical to subtropical regions [
11], and also offering genetic variability for drought tolerance and against several diseases [
7].
However, to obtain the expected benefits from warm-season perennial grasses, literature reveals that the ongoing intensification process for pasture-based livestock systems should be initially constructed to ensure two key factors: adjustments on grazing management targets [
1,
4,
13], considering the limits of plasticity of grasses under grazing, and more precise fertilization practices, with particular emphasis on nitrogen (N) fertilization [
14,
15]. An important point to be highlighted is that these two factors need to be considered in conjunction [
16,
17], once the negative effects from deficiencies on one practice will probably overcome the possible benefits obtained from another.
It is well known that N is a key nutrient for sustaining growth and persistency of warm-season perennial grasses, and N fertilization is important for pasture intensification [
18]. However, more than the definition of the annual amount of N fertilizers to be applied, an effective fertilization strategy must consider the possible variations in the plant’s demand along successive regrowth cycles, which only can be obtained when considering the plant’s requirements integrated with the impacts of in-season and between-season variations in climatic conditions on the pasture’s response.
For most tropical perennial grasses, evidence indicates that there are no benefits to applying more than 50 kg ha
−1 of N per application or after each regrowth cycle [
19,
20]. However [
21], suggested that a N fertilization protocol using variable rates along the growing season is highly effective to sustain pasture growth using fewer N fertilizer amounts. Authors observed that N fertilization rates required for Mavuno grass pastures (
Urochloa hybrid) to sustain 90% of the maximum forage accumulation varied along the regrowth cycles of the growing season. This is in line with the recommendations of [
15] and [
14], which argued that fertilization strategies based on the principles of the synchronization of N demand with N supply ensure high yields and maintain soil health.
Despite it not being a simple task to estimate and monitor the transformations and losses of N in grazed pastures, given the complexity of soil N cycling [
15], reinforced that improved N fertilization management is an urgent need to increase profitability and N use efficiency but also to reduce N losses to the environment, ensuring environmental benefits, without any negative impacts on pasture growth, nutritive value and animal daily gains. The challenge ahead is to identify the actual demand of the different tropical perennial grasses currently used under grazing, the possible variations between regrowth cycles along the growing season, and to integrate the in-season and between-season climatic variations with more flexible N fertilization strategies.
Based on this background, the aims of this paper are to discuss the current N fertilization protocols applied in tropical environments and to examine the possible gaps in knowledge for the definition of more precise N fertilization strategies in pasture-based livestock systems where tropical perennial grasses are the basis of the forage supply.
2. Current N Fertilization Strategies for Tropical Perennial Grasses
Even with a wide body of information available on the yield responses of warm-season perennial grasses to N fertilization, current fertilization strategies are relatively far from following the principles of best management practices (BMP), which should combine the 4R of nutrient stewardship: right source, right rate, right place, and right time [
22,
23]. Although source and place can be considered factors already well defined for established pastures [
18,
22,
24], there is an enormous gap in how much to apply (rate) and when to apply (time).
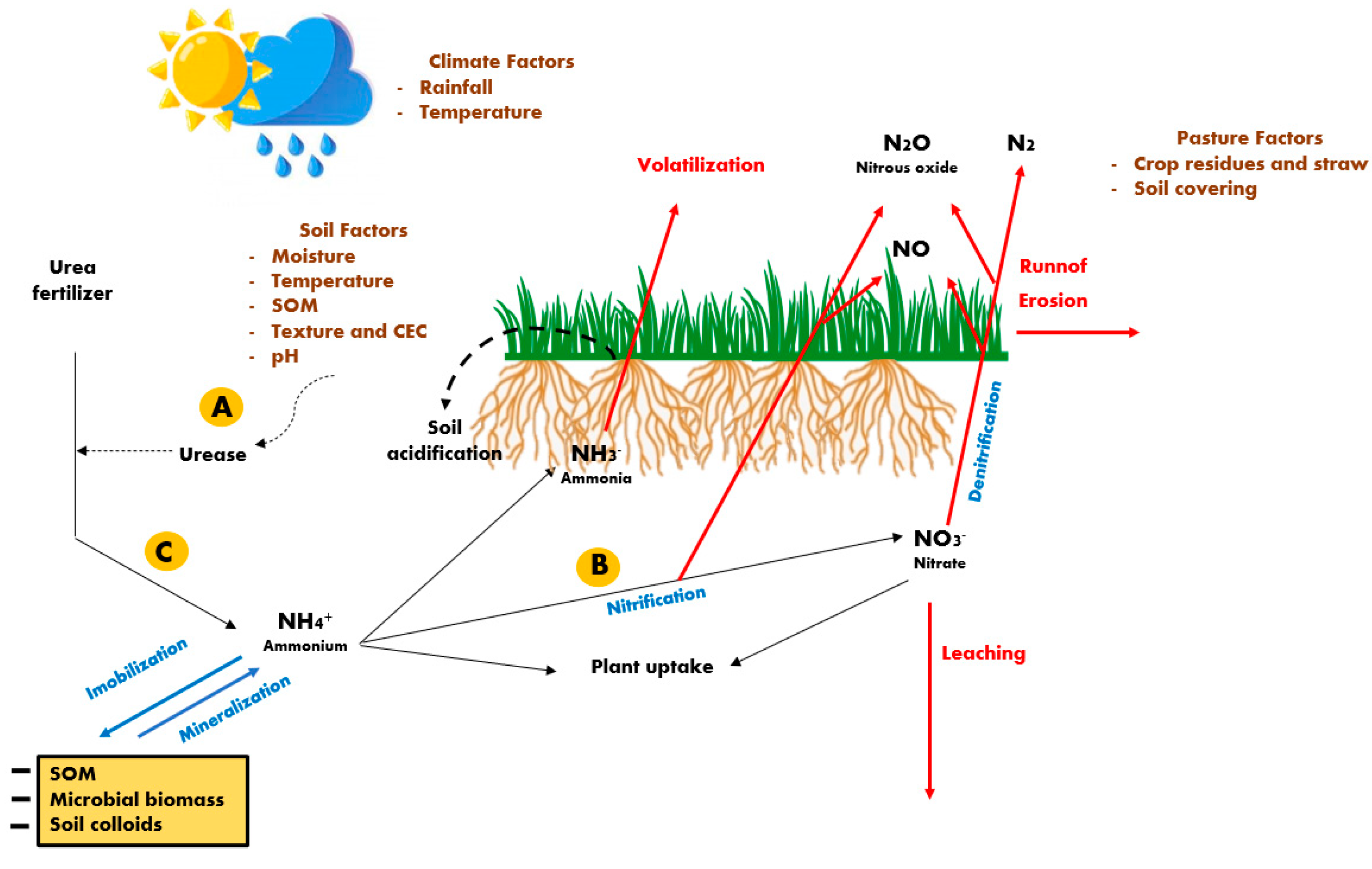
Surface application without incorporation is the commonly used method in established pastures, being considered the most practiced procedure in large areas [
22] despite the risks of high N losses when using urea [
24]. When urea is applied to soils, its breakdown by urease enzyme forms ammonium (NH
4+) and carbamate ions, whose process is followed by a second step where the rapid decomposition of the carbamate ion produces NH
4+ and bicarbonate [
25]. The NH
4+ can be assimilated (immobilized) by soil microorganisms and plants, but soil colloids also exert a strong adsorption effect on NH
4+ up to saturation [
26].
During urea hydrolysis, the increased soil pH around the urea granules favors the production of ammonia (NH
3), which can be volatilized from the soil surface [
24], whereas a part of the volatilized NH
3 may be deposited on the soil surface and potentially contribute to soil acidification or N enrichment of the N limited ecosystem [
25]. The increased losses of N through NH
3 volatilization, which can reach more than 40% of the urea-N applied in tropical soils [
24,
27], are favored by a high soil pH, temperature and soil moisture content, and low soil organic matter (SOM) and cation exchange capacity (CEC), as well as by the presence crop residues and high amounts of straw (
Figure 1).
The NH
4+ formed during urea hydrolysis that is not volatilized may subsequently be subjected to nitrification, where it is oxidized to nitrate (NO
3−), which also results in soil acidification and the production of nitrous oxide (N
2O) [
25,
26,
28]. Both the NH
4+ and NO
3− are subjected to plant uptake, despite the preference for each normally differing between plant species. Nevertheless, NO
3− is subjected to losses through leaching to the groundwater during events with large amounts of rainfall, particularly on sandy soils [
9,
29]. The NO
3− is also subjected to denitrification, causing minor N losses through the production of molecular nitrogen (N
2) and NO, which are released into the atmosphere [
24,
26].
There are options that could reduce N losses, particularly from urea, such as using synthetic nitrification inhibitors, urease inhibitors, polymer-coated or controlled-release fertilizers, which are called enhanced efficiency N fertilizers [
28,
30,
31], but the results are quite variable in terms of pasture yield, NH
3, and N
2O soil emissions in tropical pastures. Other practices not considered in this paper are those that could minimize N losses from fertilizers, such as irrigation and mechanical incorporation, despite being part of so-called best management practices [
24], as these practices are, in the same aspect, restrictive and not frequently used in most pasture-based livestock systems [
15].
Research on the effectiveness of enhanced efficiency N fertilizers (EFF’s) on tropical perennial grasses has been mainly focused on their potential impact for decreasing NH
3 and N
2O soil emissions [
24,
30,
31]. In [
32,
33], it was argued that well-managed tropical perennial grasses express an abundant root system, particularly between 0 and 40 cm depth, which is associated with high root renewal rates and a very branched root architecture. According to [
34] these traits result in high efficiency of N uptake, quickly depleting the NO
3- from the root zone. Moreover, tropical perennial grasses also express intensive tillering renewal rates, allowing for cycles of new root development, assisting nutrient uptake. These are the probable reasons why the focus of using EFF’s in tropical pastures had been on NH
3 and N
2O soil emissions.
The urease inhibitors act on biological processes that temporarily block soil ureases (
Figure 1, in “A”), preventing or delaying urea hydrolysis, and then, exert effects mainly on reducing NH
3 volatilization [
24,
35]. Considering the different soil types, representative of the Brazilian Amazonia Biome, at 100% of field capacity under laboratory conditions, [
35] evaluated different urease inhibitors on NH
3 losses from urea. The authors reported that cumulative N losses by NH
3 volatilization after 16 days from N application (80 kg N ha
−1 per application) were consistently lower for all soil types when using urea plus N-(n-butyl) thiophosphoric triamide compared with conventional urea and with a benzimidazole-type urease inhibitor and a benzoylthiourea-type urease inhibitor. However, [
24] argued that the urease inhibitor persistence in the soil directly affects its effectiveness in controlling NH
3 losses and reported that its efficacy is significantly lowered in acidic and moist soils under warm climates, typical of tropical conditions.
The nitrification inhibitors, such as dicyandiamide, nitrapyrin, and 3,4-dimethylpyrazole phosphate (DMPP), act to decelerate the rate of soil nitrification (
Figure 1, in “B”) by deactivating the enzymes involved in autotrophic nitrification (from ammonia-oxidizing archaea and ammonia-oxidizing bacteria) [
36]. The action of the nitrification inhibitors in combination with urea delays the oxidation of NH
4+ to NO
3−, allowing NH
4+ to persist longer in the soil [
25,
26] and reducing the subsequent N leaching as well as decreasing the N
2O produced into the soil [
36]. In soils with different textures (sand, loam, and clay) using ammonium sulphate as the N source plus a nitrification inhibitor (dicyandiamide, DCD), [
37] observed low effectiveness of the nitrification inhibitor only on sandy soils, suggesting that the low clay and SOM contents had already limited the nitrification process.
In field conditions, [
38] reported no differences in cumulative NH
3 volatilization and N
2O soil emissions between pastures receiving urea (~40 kg N ha
−1 per application) or urea plus the nitrification inhibitor nitrapyrin in a
Cynodon nlemfuensis pasture in Costa Rica. However, a strong relationship was observed between rainfall events and water-filled pore space with N
2O emissions, which suggested that N
2O could be produced by other ways not affected by the nitrification inhibitor, such as heterotrophic denitrification in anaerobic microsites in the soil profile. Moreover, the authors highlighted that the site conditions, including high soil organic C content, low pH and high rainfall intensity may contribute to the decrease in effectiveness of nitrification inhibitors.
Similar results were reported in a ryegrass (
Lolium multiflorum) and predominantly setaria (
Setaria sphacelata) and kikuyu (
Pennisetum clandestinum) pasture in Australia, where a nitrification inhibitor (3,4-dimethylpyrazole phosphate, DMPP) did not reduce N
2O emissions when using the equivalent of 57 kg N ha
−1 per application [
39].
Slow-release polymer coatings act to delay the urea hydrolysis (
Figure 1, in “C”) and the subsequent nitrification by limiting its solubility [
26]. Both [
40] and [
24], reviewing several results from the literature, reported that using EFF’s has only a modest impact on grain yield or biomass but their major benefits would be in potentially decreasing soil N losses and increasing N use efficiency. However, inconsistent results are frequently reported [
36], and may be attributed to the fact that soil type [
35], soil C content and pH [
38], and current soil moisture and temperature [
24] interfere with their effectiveness, being frequently higher in subtropical compared with tropical conditions [
41].
From a sustainable perspective, other factors also need to be considered in the decision-making processes for using EFF’s, including their higher costs compared with conventional urea [
40]. However, the possibility of their strategic use, depending on climatic conditions and grass species, may play a significant role in reducing costs and lowering N losses. The authors of [
24] argued that using EFF’s as a tool to decrease N losses and improve N use efficiency becomes more attractive in some scenarios, such as urea application over crop residues or for specific grazing cycles within the growing season, especially when higher N rates are required, and applications are associated with periods of high temperatures and soil moisture. Despite those technologies appearing to be promising for many agricultural systems, specific research on tropical perennial pastures is still lacking.
Regarding the N source, there is a consensus that N from ammonium nitrate or ammonium sulfate fertilizers are less prone to losses, particularly through NH3 volatilization, compared with urea. However, recent evidence has suggested that the N rate per application can also be a key factor when defining a N fertilization protocol using urea for tropical pastures.
The authors of [
18] observed that N losses from NH
3 volatilization in Marandu palisadegrass (
Urochloa brizantha ‘Marandu’) pastures in the Brazilian Cerrado biome is significantly reduced when using ammonium nitrate or ammonium sulfate as the N source when compared with urea. However, when considering annual N amounts equivalent to 90, 180 or 270 kg ha
−1 using urea, the %N applied lost as NH
3 decreased from 13.9%, 24.4% and 44.6% to 6.3%, 10.9% and 24.1%, respectively, when the doses were split into three applications of 30, 60 and 90 kg N ha
−1 compared with a single application.
In Marandu palisadegrass pastures in a moderate degradation stage in the Brazilian Amazonia biome, [
20] observed that N
2O emissions were higher when using 80 kg N ha
−1 as urea per application, whereas non-significant differences were observed in N
2O emissions between urea and ammonium sulfate when using the equivalent of 40 kg N ha
−1 per application. In this sense, the first emerging point is that significant N losses from conventional urea could be prevented by strategically using lower N rates per application.
In most parts of the tropical regions in Brazil, the main GHG emissions from soils under pastures are strongly affected by seasonal temperatures and rainfall [
27,
42]. Two major periods are known: the wet season which, depending on the region, extends from September/October to April/May and concentrates from 70 to 90% of the total annual rainfall with average temperatures varying from 22 °C to 32 °C; and a dry season, normally characterized by a strong soil water deficit, a shortage of rainfall, and irregular distribution of precipitation events, with average temperatures rarely reaching less than 18 °C [
43,
44]. Pasture responses are highly reduced during the dry season, where only 20 to 30% of the annual yield is obtained, and N fertilization is predominantly applied along the regrowth cycles of the wet season, called the growing season [
17,
45].
The current fertilization practices recommend a wide range on annual rates, varying from 50 kg N ha
−1 up to 500 kg N ha
−1 for non-irrigated pastures [
46,
47,
48]. Despite it being recommended that annual fertilization amounts follow an installment protocol, the N rate per application aiming at strategic fertilization remains uncertain. In the Brazilian Cerrado, linear responses in daily herbage accumulation and stocking rates in Mombaça guineagrass (
Panicum maximum ‘Mombaça’ Syn.
Megathyrsus maximum) pastures were observed with increasing annual N amounts from 100 to 300 kg N ha
−1, whereas using 200 kg N ha
−1 resulted in the best net return and cost-benefit ratio, even considering a pessimistic scenario with a 25% reduction in productivity [
45].
The installment protocol of the above-mentioned research considered four applications along the regrowth cycles of the growing season, using fixed N rates equivalent to 25, 50 and 75 kg N ha−1, respectively, for the annual amounts of 100, 200 and 300 kg N ha−1, suggesting that the N rate applied at each instalment was the main driver of the increased herbage accumulation instead of the annual amounts of N fertilizers applied.
In Marandu palisadegrass under a moderate degradation stage, [
19] reported that maximum yield was obtained when using 75 kg N ha
−1 during the regrowth cycles of the first growing season of pasture recuperation, but only 50 kg N ha
−1 per application was required after each regrowth cycle during the second year. In well managed Marandu palisadegrass pastures under continuous stocking [
49], observed that three installments of 30 kg N ha
−1 would be enough to produce a high forage yield with high quality. On the other hand, the authors suggested that if the grazing system aims to achieve higher animal productivity, a moderate N fertilization should be considered, using a fertilization protocol that considers installments of up to 60 kg N ha
−1.
For most tropical perennial grasses, evidence indicates that there are no benefits to applying more than 50 kg ha
−1 of N per application [
19,
20,
45], regardless of the grazing method (continuous or intermittent stocking). Moreover, recent research results have consistently showed that when pastures are maintained within an adequate range of grazing management, the annual N amounts required for sustaining their optimum responses are much lower than the traditional recommendations, for example, 300 kg N ha
−1 year
−1 [
21,
50] in most of the tropical regions of Brazil or around 400 kg N ha
−1 year
−1 in South Africa [
51].
Despite being recognized that the yield potential of tropical perennial grasses, considering adequate strategies for grazing management and soil fertility, is largely related to the genotype and environmental factors, the question remains, in which scenario could using moderate N rates improve nitrogen use efficiency and decrease soil N losses?
In a meta-analysis of the N losses from tropical field studies, [
29] revealed that all forms of loss increased with increasing N inputs, but environmental and management factors strongly controlled the gaseous N losses. The N rate was a strong predictor for gaseous N losses, where a change from 50 to 150 kg N ha
−1 year
−1 would lead to a 30%, 66% and 74% increase in mean N
2O, NO and NH
3 losses, respectively [
29].
Moreover, croplands have been recognized as the main source for NO
3− production, with NO
3− loss fluxes accounting for 6.7–19.0% of applied N in individual cropping years [
52], becoming a potential source of contamination for water resources. Reviewing several research results, [
52] reported that the average annual N-NO
3− leaching losses were highly variable between countries, from 29.0 to 63.3 kg ha
−1 in China, and 15.0 to 45.0 kg ha
−1 in Sweden and Norway. The authors of [
29] showed slightly lower rates of NO
3− leaching in tropical regions compared with temperate ones and reported that an increase from 50 to 150 kg ha
−1 year
−1 of N in tropical croplands would lead to a 30% increase in mean NO
3− leaching losses (12.52–16.30 kg N-NO
3− ha
−1 year
−1).
The authors of [
33] reported that nitrate moved down the soil profile, being leached below the rooting zone, in pastures of
Chloris gayana ‘Katambora’,
Digitaria eriantha ‘Premier’ and in an annual
Sorghum hybrid ‘Sweet Jumbo’ receiving three applications of 100 kg N ha
−1, which did not occur when the pastures received one or two applications of 50 kg N ha
−1. In pastures of
Urochloa plantaginea receiving four applications of 50 or 100 kg N ha
−1 during the wet season [
32], observed a progressive accumulation of NO
3− in the first 40 cm of the soil profile only when pastures received the highest N rate. We were not able to find recent research results estimating the magnitude of NO
3− annually lost by leaching in tropical perennial grasses, despite some studies suggesting very low NO
3− leaching rates in permanent grasslands.
Soil texture and water inputs influencing drainage were important controls on NO
3− leaching [
29,
34]. Once NO
3− electrostatic adsorption is lower than of other anions in the solid–liquid interface of the topsoil [
32], losses are favored when the amount of NO
3− accumulated surpass the plant’s uptake potential, particularly if followed by a high drainage volume and on light-textured soils (sandy soils) [
52]. In this way, recent literature [
29,
34,
52] has suggested that when longer drought periods are interspersed with short extreme rainfall events, such as during the beginning of the growing season, or during times of potential high drainage, delaying N application or strategically using lower N fertilizer rates could reduce the leachable NO
3−.
Research in [
29] also highlighted that different tropical regions and grass species exert important impacts on N losses, and some of these particularities reveal great opportunities for sustainable intensification using moderate fertilization rates with low N losses for grassland systems based on tropical perennial grasses.
For example, [
53] argued that the mechanism known as biological nitrification inhibition (BNI) expressed by some tropical grasses, such as the genus
Urochloa (formerly
Brachiaria) and
Megathyrsus (formerly
Panicum), could be considered a regulatory ecosystem service that mitigates the N
2O losses, once the release of inhibiting substances through the root exudates suppresses the nitrification process [
41]. In this way, associating opportunities for strategically using low N rates per application on pastures composed by plant species exhibiting BNI mechanisms may contribute to eco-efficient livestock production [
53].
Moreover, as previously described, the high tillering and root renewal rates commonly observed in well-managed tropical perennial grasses play a significant mitigation effect on NO
3− leaching [
32,
33,
34]. Despite these mitigation effects appearing to be dependent upon N fertilization rates, the results reveal that losses of N in the form of NO
3− can also be modulated by the fertilization rates per application [
32,
33].
An effective fertilization strategy must consider possible variations in the plant’s demand and potential responses along successive regrowth cycles, which can only be obtained through an improved knowledge of pasture responses, the specificity of the different grass species, in terms of ecophysiological and nutritional requirements, and an integrated view of in-season and between-season variations on climatic conditions and their potential impacts on soil N release [
54].
Following this rationale, the second emerging point for defining best N fertilization strategies on tropical perennial pastures requires an integrated approach, which should answer the following questions: Is there some specific regrowth cycle or month, within the growing season, where low N rates would be enough to sustain a maximum pasture response? At specific regrowth cycles or months where pastures require higher N rates, are there some soil traits that can act to minimize N losses?
The authors of [
29] discussed that some tropical soil orders, such as Oxisols and some Ultisols, can have net anion exchange capacity that decreases NO
3− leaching, tending to express lower N
2O emission factors than IPCC estimates. This integrated information could guide the identification of specific areas for the intensification of tropical regions to prioritize [
29] or specific periods within the growing season where the application of higher N rates would be associated with improved pasture responses [
21] and minimum N losses [
18].
In this way, it seems a natural consequence that the traditional N fertilization recommendations should move from a flat N rate per regrowth cycle or month to a variable and strategic protocol [
15,
55,
56], and then, annual amounts are the result of the prevalent growing conditions in a given year and for a given location. From this point of view, the presently non answered questions about how much to apply (rate) and when to apply (time) may be more easily clarified.
3. Moving to a New Approach on N Fertilization for Tropical Perennial Grasses
The efforts for defining modern N fertilization protocols have increased in the last years, revealing promising opportunities for reducing total N fertilizer amounts and losses without any negative impacts on pasture yield [
15]. Nevertheless, traditional fertilization practices based on general recommendations, which consider instalments using flat rates, still prevail in research with tropical perennial grasses.
A great part of this is related to the lack of information on basic processes contributing to the build-up of soil N in different soil types and under variable climatic conditions in tropical environments, which drive the fate of N into mineralization and immobilization processes. In addition, poor knowledge about N requirements for tropical perennial grasses, such as critical N ranges and sufficiency indexes, as well as the inexistence of integrated approaches considering how in-season and between-season variations on soil parameters and pasture growth dynamics modulate N requirements in tropical perennial grasses, make it difficult to identify opportunities for intervention [
22].
This reveals an enormous gap in knowledge that prevents the definition of N fertilization rates required to attain these indexes for the different tropical perennial grasses as well as the possible variations along successive regrowth cycles of the growing season. Sustainable approaches can be defined by combining the identification of specific regrowth cycles where high N rates are required by plants with the use of EEF’s but also by using grass species with the potential for biological nitrification inhibition.
The beginning of the wet season is characterized by returning favorable growth conditions when precipitation events are more evenly distributed [
22]. In this period, in well managed monospecific swards of tropical perennial grasses, tiller population undergo to a strong renewal process, characterized by intense tiller mortality, predominantly those plants that survived from the dry season, associated with high rates of tiller appearance [
47]. As a result of this process, the profile of the population is characterized by the predominance of young tillers, which are highly responsive to soil N availability [
47,
57].
In regularly managed pastures, the process is part of a natural cycle of tiller population dynamics, and occurs regardless of the grazing method [
58,
59]. Demand for N by plants is higher during the beginning of the growing season [
56] due to the intense tiller population renewal, high proportion of young tillers, and fast leaf turnover [
47,
60]. However, with the onset of the rainy season and soil rewetting, microbial and soil fauna activity are enhanced [
61], and then, litter breakdown and decomposition may substantially contribute to N release [
62].
In [
7], the authors described that, in tropical conditions, the rapid growth and large amounts of biomass produced by tropical perennial grasses have a great influence on nutrient release. The authors argued that tropical grasses produce a higher root mass than herbaceous legumes, and because of the large and deep root system, root litter decomposition can also be an important source of C and nutrients for soil microbial activity and plant uptake [
63]. The soil N mineralization occurs when organic N from different sources, such as SOM, above and belowground litter and microbial biomass turnover, are converted into plant-available mineral forms [
62]. Despite N being rapidly recycled through the soil microbial biomass, large reserves of N can be mineralized at this period [
64].
From a global analysis, [
65] showed an average soil net N mineralization of 2.41 mg N soil kg
−1 day
−1, whereas grassland soils from South America showed values of up to 3.18 mg N soil kg
−1 day
−1. Soil net N mineralization was positively correlated with mean annual temperatures and precipitation, soil clay content, total soil organic carbon and N, microbial C and N biomass, and initial soil NH
4+ concentration, whereas it was negatively correlated with soil pH, soil and microbial biomass C:N ratio, and initial soil NO
3− concentration. Moreover, for a given soil type, the quantity of N released from different soil fractions and potentially available for plants at the beginning of the growing season is affected by litter quantity, though mainly by its quality [
66].
Chemical composition and the nature of the chemical compounds define the litter quality [
67] that, in turn, regulate the fate of the N into the mineralization and immobilization processes. Aboveground litter is built-up via natural senescence and death (from tiller and leaf tissues) and grazing-induced losses of plant material newly deposited on the soil surface, or partially decomposed-plant parts not selected and consumed by animals, or standing biomass damaged by trampling [
68].
The litter mass reflects the net balance between litter deposition and decomposition rates and, for a given grass species, varies between seasons due to the prevailing climatic conditions [
60,
66], pasture management [
69] and stocking rates [
17,
63], as well as previous fertilization practices [
67]. Litter deposition is expected to be increased on pastures subjected to high N fertilization rates, particularly when subjected to low stocking rates, light grazing intensities under continuous stocking, or in pastures maintained under long regrowth periods with intermittent stocking [
60,
67,
69].
However, in the above-mentioned situations, a high litter amount deposition is associated with low quality litter, and then, low decomposition rates. The rate of litter decomposition, in the presence of adequate soil temperatures and humidity, are primarily affected by soil fauna and microorganisms, as well as the litter chemical composition [
61], including the initial N-litter, C:N ratio and the Lignin:N ratio [
70].
In a general way, in tropical perennial grass pastures, higher aboveground litter mass is observed in the regrowth cycles of the beginning of the growing season [
60,
61] compared with the subsequent regrowth cycles. The litter mass in this period is mainly composed of old plant material accumulated during the previous dry season, and then, is affected by the previous grazing management and N fertilization rates. In [
71], the authors reported that the net and gross N mineralization rates are higher in regularly fertilized pastures, which reflects a greater supply and quality of substrates for mineralization from litter. In [
60], the authors showed that the existing litter biomass was increased by 43% in Marandu palisadegrass intermittently stocked receiving three instalments of 50 kg N ha
−1 during the growing season compared with non-fertilized pastures.
In continuously stocked Marandu palisadegrass, [
17] reported that N supply increased N-litter and reduced the litter C:N ratio, resulting in the greatest litter decomposition rates in fertilized pastures. In [
67], the authors described that the nutrients released from plant litter decomposition are less prone to losses because litter is more evenly distributed onto the soil surface.
Returning on pasture use, through regular grazing at the beginning of the growing season, also favors the input of nutrients to pasture from animal excreta, which has a high concentration of easily decomposable C and N [
70]. According to [
72], in a general way, extensive grazing systems may experience greater influence on nutrient return from litter, whereas an animal’s excreta may play a major role in nutrient cycling on intensively managed pasture-based systems.
Differences in the amount of nutrients returning to the soil via urine and feces are affected by the chemical composition of the herbage consumed by the grazing animals [
70], and then, by the grass species and grazing strategies. In [
73], it was observed that, on a daily basis, N excretion from urine and feces varied, respectively, from 143 to 180 g day
−1 and from 121 to 125 g day
−1 on well managed rotationally stocked ‘Cameroon’ elephant grass (
Pennisetum purpureum Schum.) pastures. Whereas, in continuously stocked Marandu palisadegrass pastures receiving three applications of 50 kg N ha
−1, [
74] found N excretions from urine and feces corresponding to, respectively, 98.9 and 43.4 g day
−1.
On the other hand, [
75], also in continuously stocked Marandu palisadegrass pastures, reported that N excreted via urine ranged from 67.8 to 106.6 g day
−1. The N excreted via urine represented 42–79% of the total N ingested. The variation in fecal N output was much lower than urinary N and corresponded with between 14.7 and 17.1 g N kg
−1 DM of feces. In [
72], the authors reported an average urinary N concentration during the warm season corresponding to 3.17 g kg
−1 in fertilized pastures (receiving 224 kg N ha
−1 year
−1) of bahiagrass (
Paspalum notatum Flüggé) overseeded with rye (
Secale cereale L.), with a proportion of N excreted via urine corresponding to 58.2%. Annually, N inputs from urine (48.9 kg N ha
−1 year
−1) were higher than feces (39.7 kg N ha
−1 year
−1).
Moreover, once grazing management strategies significantly impact the nutritive value [
13] and stocking rates [
72] in tropical perennial grasses, direct effects on nutrient cycling are also expected. Both [
69,
73] showed that defoliation frequency is an important management parameter for maximizing leaf mass, growth rates, nutritive value, and stocking rates, allowing for a greater total N intake as well as the highest fecal and urinary N excretions.
On the other hand, animal excretions are considered important contributors to GHG emissions with urine being considered the main source of N
2O emissions during the wet season [
27]. Once the proportion of excreta increases with increasing stocking rates, and considering that nutrients, particularly from urine, are concentrated in small areas, strategic grazing [
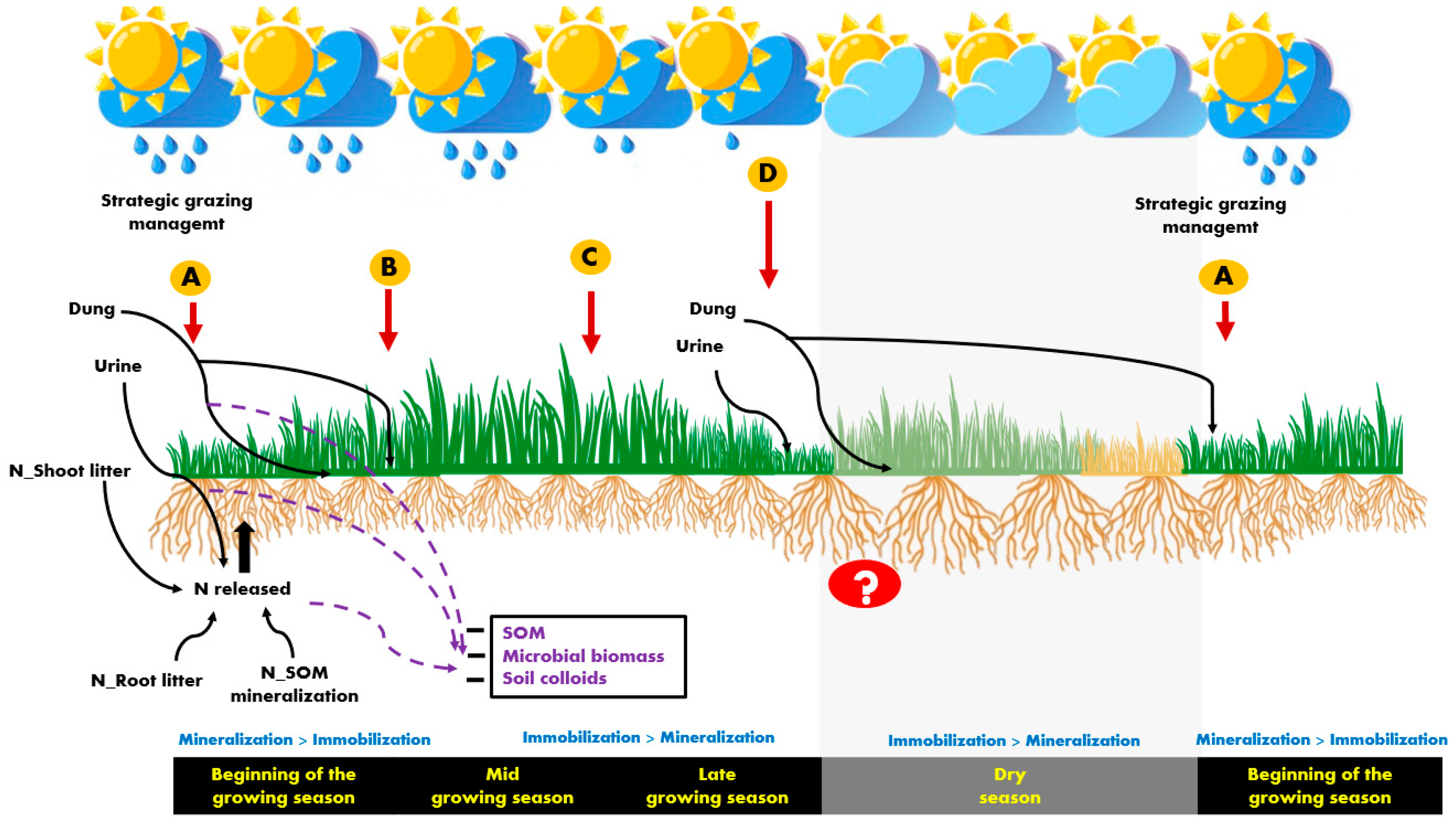
1] and N fertilization could potentially reduce negative environmental impacts and N losses and improve N release from mineralization (
Figure 2).
In this way [
67], suggested that employing a strategic short grazing period with high stocking rates could contribute to a more uniform distribution of animal excreta. This strategic management allied to the flush of nutrients from litter decomposition and N mineralization may support the N demand of plants [
56]. Both [
76] and [
56] argued that developing site and seasonal-specific N fertilization guidelines are both environmentally and economically beneficial and suggested that N inputs could be reduced without compromising soil fertility, and subsequent pasture yield, during periods in which a large build-up of mineral N from soil is observed [
51]. In most of the Brazilian tropical regions, in well managed pastures, this could be an important strategy for the regrowth cycles at the beginning of the growing season when the first rainfall events occur (
Figure 2, in “A”).
The intermediate regrowth cycles of the growing season are considered the best moment to apply N-fertilizer, due to the stabilization of the tiller population and better climatic conditions for growth [
22]. Effective N nutrition is associated with a high leaf area and the leaf’s photosynthetic potential that, in turn, maximizes leaf growth and herbage accumulation [
77,
78]. The fast rate of litter decay early in the growing season is associated with the decomposition of more soluble compounds [
66].
Then, existing litter mass tends to decrease in the intermediary regrowth cycles of the growing season relatively to the first grazing cycles [
60]. In [
79], it was reported that approximately 50% of the initial litter mass in ‘Tifton 85′ bermudagrass (
Cynodon spp.) pastures were lost along the first 32 d. Therefore, a lower proportion of N is expected to be released from plant litter, particularly if the C:N ratio increases to values above 30 along the growing season.
In this scenario, a shifting between the contribution of litter and external sources, such as excreta and fertilizers, for supplying nutrients to plants is of higher relevance [
17], and N fertilization will probably be required at greater amounts compared with the initial regrowth cycles (
Figure 2, in “B” and “C”). Both [
21], in Mavuno grass, and [
80], in Xaraés palisadegrass, reported that during the intermediate regrowth cycles of the growing season, using the equivalent to 30 kg N ha
−1 after each regrowth cycle was enough for the pastures to attain N sufficiency indexes and sustain maximum growth rates. Moreover, [
81] observed that tillers that sustain higher pasture growth rates during the beginning of the autumn in stockpiled Marandu palisadegrass correspond to the tillers generations that appeared during the summer season (between January and February), highlighting the importance of an adequate N supply at these periods of the year.
Grazing strategies that ensure fast leaf turnover, such as moderate grazing intensities under continuous stocking, frequent defoliation under rotational stocking and N fertilization, increase the N concentration of herbage mass [
79], improve N intake by grazing animals but may also result in higher urinary and fecal N excretion [
17,
69]. According to [
82], peaks of soil NH
4+ normally occur in the first few days after urine deposition, but only from 10 to 14 days after dung deposition. On the other hand, peaks of soil NO
3- can be observed from 23 to 26 days for urine and from 19 up to 50 days after dung deposition. Moreover, [
70,
83] highlighted that manure decomposition rates can be lowered in periods of rainfall shortage along the growing season due to crust formation. In this way, it is expected that, differently than urine, the contribution of dung to soil nutrient input will probably be verified in subsequent regrowth cycles after its deposition [
27].
One of the major challenges to defining more conscious N fertilization practices relies on estimating the nutrient requirement of the different tropical perennial grasses. For example,
Pennisetum purpureum Schum. (Elephant grass) and
Megathyrsus maximus are the most productive tropical perennial grasses largely cultivated in intensive grazing systems. They demand high fertility soils and a higher N supply to sustain their fast growth rates compared with other genera, such as
Urochloa spp. Moreover, a large amount of N from the biological fixation with diazotrophic bacteria on
Urochloa roots can add up to 42 kg N ha
−1 to the soil [
7]. Thus, N fertilization rates for
Urochloa pastures would probably be lower than
Pennisetum spp. and
Panicum spp. even considering intensive pasture grazing systems [
84].
In [
85], the authors reported that N sufficiency based on leaf N content ranged from 21.2 to 31.4 g N kg
−1 dry matter for well managed Marandu palisadegrass pastures during the wet season, but that the values are lower during dry season. Similarly, the leaf N content required to sustain at least 90% of the maximum herbage accumulation (P90%) in Mavuno grass pastures varied from 27.5 to 30.7 g N kg
−1 leaf dry matter during the growing season of pasture establishment [
21], whereas the N rates required to attain P90% progressively decreased along the successive regrowth cycles due to low soil humidity, which prevented plant N uptake.
Despite the values of leaf N content used as a reference for Urochloa pastures during the establishment phase, there is no information currently available considering soil types and for other tropical regions with different climatic conditions. Furthermore, we were also not able to find, in the literature, information on critical N content in Pennisetum purpureum, Megathyrsus maximus and Cynodon spp. pastures under tropical conditions, revealing that there is much more research required before best management practices on N fertilization can be effectively recommended.
Adequate and regular N fertilization has also been recognized for alleviating damage when plants are subjected to drought stress, which is a key factor for pastures during autumn, particularly for those subjected to stockpiling. There is evidence in the literature that higher N levels (or plants with a higher foliar N content) promote turgor maintenance and reduce lipid peroxidation, increasing cell membrane stability as well as enhancing the activity of antioxidant enzymes, decreasing the negative effects of reactive oxygen species and injuries to the tissues under short duration drought events [
86].
Thus, late N fertilizer application is a recommended practice for improving herbage production during autumn, to prolong pasture utilization under grazing [
22] at the end of the growing season (
Figure 2, in “D”) and can also contribute to a deposition of high-quality litter. Moreover, this strategy can also be an important way of maximizing herbage yield for the tactical management of hay or silage cut in some grass species.
For these purposes, N rates varying from 40 up to 90 kg N ha
−1 are normally suggested [
87,
88] for most
Urochloa species. In [
81], the authors showed that in pastures subjected to stockpiling in late summer, aimed at grazing during the dry season (winter), a N rate equivalent to 50 kg N ha
−1 would be enough to sustain pasture growth potential when N is regularly applied during the previous active growth period. In this way, N fertilization protocols based on the principles of the synchronization of N demand with supply [
14,
15] may contribute to a decrease in total fertilizer amounts and costs, minimize negative environmental impacts, and ensure high forage yield with an adequate nutritional value of pastures along the growing season, but also from stockpiled pastures.
For most tropical regions in Brazil, the average temperatures during the transition between summer and autumn, and for the overall dry season, are not restrictive for tropical perennial grasses growth, being that the soil moisture is the main driver of soil N uptake and pasture growth rates, but also of urease activity [
24]. Despite it being expected that urea hydrolysis from late N fertilization would be slow, due to a progressive soil drying process at the transition between summer and autumn, the soil microorganisms’ activity may still be sustained through the maintenance of soil moisture in the rhizosphere by the plant roots.
According to [
89], some plants are able to redistribute water deeper from the soil to the surface in semi-arid conditions, which combined with root exudates, support a large and active microbial community and urease activity, improving soil mineral N content in the rhizosphere during the dry season. The magnitude in which those processes can be verified in tropical perennial grasses is unknown, and the question mark in the
Figure 2, during the dry season, highlights the unpredictable responses in terms of soil N supply.
On the other hand, in terms of N losses [
90], reported significantly lower cumulative NH
3 losses from excreta (dung plus urine) during the dry season compared with the wet season, and the N fraction lost as NH
3 from urea with wet conditions was almost twice that in the dry rainfall conditions. The authors in [
83], studying tropical pastures in Kenya, also reported higher soil N
2O and CO
2 fluxes from excreta in the wet season compared to the dry season. The authors argued that, in addition to the climate effects, particularly low soil moisture, differences in the pasture’s chemical composition during the wet and dry season may exert substantial effects on excreta properties, and then, on soil gases emissions. In [
44], it was observed that there were no apparent effects of increasing N rates on soil N
2O emissions during the dry season in Marandu palisadegrass pastures, and significantly lower soil N
2O emissions were measured during the dry season compared with the wet season.
Late fertilization instalments may also contribute to high quality litter deposition during the dry season. The authors of [
17] observed that by applying instalments with 50 kg N ha
−1 at beginning of the growing season (November), in early-summer (January), and late summer (March), the N deposited in the grass litter was equivalent to 82.1 kg N ha
−1 compared with 57.1 kg N ha
−1 from non-fertilized pastures. The litter deposition rates were higher during the dry period (winter), and were associated with low decomposition rates, which may significantly contribute to N release in the subsequent growing season in regularly fertilized pastures. In this way, by knowing the seasonal trends on soil N mineralization and immobilization and the plant’s demand, the tactical application of N fertilizers, adopting variable N rates, could be identified [
15].
4. Future Perspectives
To express their growth potential, tropical perennial grasses require adequate soil fertility and the adoption of grazing management strategies compatible with their limits of plasticity. Within a sustainable intensification process, and only after ensuring these conditions, N fertilization strategies may be planned to obtain maximum yield, nutritive value, stocking rates, and animal performance.
The relevance of adequate pasture management and conscious N fertilization practices for the establishment of sustainable grazing systems using tropical perennial grasses is well recognized. However, according to [
91], modern approaches to N fertilization should focus on increasing the ability of plants to satisfy their N demand, or at least in part, via a better use of endogenous N soil resources.
Effective advances can only be made through integrated approaches, which consider how and when site-specific soil and climatic conditions affect soil N supply, pasture growth rates and a plant’s N demand. Moreover, with no clear understanding of in-season variations on soil mineralization and immobilization processes, protocols for tactical applications of N fertilizers will not be adequately identified. Within these approaches, the contribution of the animals to nutrient cycling, through urine and dung, and of the targets adopted for pasture management for litter deposition and litter quality, are also key points [
4].
Despite their use in crop production, the EEF’s obtained via coating, encapsulating or by embedding traditional fertilizers, such as urea, with slow or controlled-release agents, using chemical, biological and, most recently, nanotechnological materials, may act to keep N longer in the soil as NH
4+, potentially providing a better synchronization with the plant’s need for N and increase N use efficiency [
31]. However, these technologies are still of restricted use in pasture-based livestock systems, particularly due to their higher costs and uncertainties on the impacts on maximizing forage yield [
40]. Moreover, due to their low use by farmers and the higher costs of other N sources, other than urea, the solution sremains in the identification of site-specific strategic opportunities.
In this way, it is reasonable to consider that the most economic and practical way of ensuring a better use of N fertilizers and reduce N losses, with an emphasis on urea, still remains on being able to identify opportunities for tactical applications. With this purpose, the monitoring of critical N content or the N sufficiency indexes required for sustaining a maximum forage yield, are essential tools for decision making regarding when to apply N fertilizers. However, the N fertilization rates required to attain the indexes for different tropical perennial grasses as well as their possible variations along successive regrowth cycles of the growing season still needs to be properly identified.
Sustainable approaches can be defined by combining the identification of specific regrowth cycles where high N rates are required by plants with the use of EEF’s, and losses could be decreased by identifying and exploring grass species with the potential for biological nitrification inhibition. Reducing nitrification and synchronizing the solubility of different N sources, or the moment of application in each management condition, will represent a great advance in reducing N losses.
There is strong evidence that a plant’s N demand may be partially supplied from soil N mineralization and the N released from plant litter and animal excreta at the beginning of the wet season in well managed and regularly fertilized tropical perennial grasses. Even with the expectation that the magnitude of N being supplied to plants from these sources will be lower in sandy soils, adequate grazing management can ensure plant litter accumulation and build-up of N during the dry season, which is associated with intense tillering and root development during the early wet season and offers opportunities for tactical applications using lower N rates.
In this scenario, using EFF’s, such as polymer-coated or controlled-release fertilizers, appears as an option to decrease N losses and increase N use efficiency. Moreover, fertilizer sources, such as ammonium nitrate and ammonium sulfate, which are less prone to N losses, may also be strategically used.
In the intermediate regrowth cycles of the growing season, during which there is an expected progressive decrease in the contribution of plant litter and excreta, N rates per application should be modulated according to the plant species’ response and prevalent growing conditions. During drought periods followed by short intense rainfall events, delaying N application and strategically using low N rates can reduce NH3 losses and leachable NO3−.
Late fertilization using high N rates can be used to extend the grazing period at the end of the growing season or when the grazing plan predicts using the grazing area as a stockpiling pasture. For tall tussock grasses, such as elephant grass, the last cutting of the growing season can also be used for cutting, and late N fertilization substantially contributes to maximize the harvested herbage amounts. During these periods, using EFF’s can also be an attractive strategy.
Based on the reports of [
15,
56,
92], it is clear that traditional fertilization practices based on flat rates are not capable of ensuring the synchronization of a plant’s N demand and N supply, a higher N use efficiency and decreased N losses. Moreover, the potential variability of a pasture’s responses between years due to the existence of wet or dry spring and summer seasons should be considered, providing flexible strategies for defining N rates. From this point of view, then, the annual amount of N fertilizers effectively used for a given site and grass species should be the combined result of the N rates applied according to the prevailing climatic conditions and potential soil N supply at each regrowth cycle or year.
In view of the above perspectives, reliable and effective protocols can only be proposed through datasets from long-term field experiments that include a range of soils, climatic conditions, and grass species typical of the tropical regions in order to construct robust models and decision support systems, following examples such as those of New Zealand [
92] and Australian grazing systems [
23].
This represents an arduous and difficult task, especially for the pasture-based livestock systems in tropical regions, since the main homework, good pasture management, is far from being accomplished. Due to the complexity that represents the interaction between the components of the grassland ecosystems, research should progress towards solving the big equation for each specific environment, as there is no magic formula that solves everything at the same time. Although those approaches are still being developed, it is expected that an effective reduction in both annual N rates applied, and N losses, may be achievable through the use of multiple and integrated strategies.